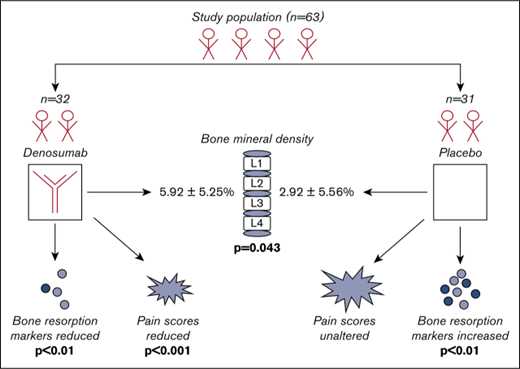
Key Points
Denosumab given twice per year in patients with TDT increased the bone mineral density of the L1-L4 more efficiently than placebo.
Denosumab resulted in a significant reduction of bone resorption and pain scores after 12 months, which was not observed in placebo group.
Abstract
Denosumab (DNM) is a fully human monoclonal antibody against the receptor activator of nuclear factor kappa-B ligand (RANKL) that has been licensed for the treatment of different types of osteoporosis. However, the prospective data for the evaluation of DNM efficacy on transfusion-dependent thalassemia (TDT)–induced osteoporosis are rather limited. Thus, we conducted a randomized, placebo-controlled, double-blind, phase 2b clinical trial to evaluate DNM in TDT osteoporosis. Patients were assigned to receive either 60 mg DNM (n = 32) or placebo (n = 31) subcutaneously on day 0 and 180 during a total of 12 months of follow-up. The percentage increase of L1-L4 bone mineral density was higher in the DNM group than the placebo group (5.92% ± 5.25% vs 2.92% ± 5.56%, respectively; P = .043), whereas the advantage of DNM regarding wrist bone mineral density was much higher compared with placebo (−0.26% ± 5.31% vs −3.92% ± 8.71%, respectively; P = .035). No grade 3 or 4 toxicity was observed. DNM reduced pain scores that remained unaltered in the placebo group. DNM showed a significant reduction of soluble RANKL (sRANKL), sRANKL/osteoprotegerin ratio, C-telopeptide of collagen type I, tartrate-resistant acid phosphatase isoform-5b, and bone-specific alkaline phosphatase between baseline and the 12th month (P < .01 for all comparisons) without changes in dickkopf-1, sclerostin, and osteocalcin. On the contrary, placebo patients showed an increase in sRANKL, osteoprotegerin, dickkopf-1, sclerostin, C-telopeptide of collagen type I, tartrate-resistant acid phosphatase isoform-5b, and bone-specific alkaline phosphatase during the study period (P < .01 for all comparisons). In conclusion, DNM increased lumbar spine and wrist bone mineral density and reduced pain and bone remodeling markers, and thus it is another valuable option for the management of TDT-induced osteoporosis. This trial was registered at www.clinicaltrials.gov as #NCT02559648.
Introduction
As treatment with transfusion programs and chelation therapy have significantly prolonged survival in patients with transfusion-dependent thalassemia (TDT), osteopenia and osteoporosis significantly add to the morbidity burden in young adults.1,2 The reported incidence ranges between 40% and 50%.3,4 Thalassemia-induced osteoporosis is multifactorial, and thus its management is rather puzzling. Both genetic (collagen type Ia1 [COLIA1] gene, vitamin D receptor polymorphisms) and acquired factors including bone marrow expansion, iron overload and iron chelators, endocrine dysfunction, nutritional deficiencies, and renal involvement seem to play a key role in the development of low bone mass in these patients.2,5 The therapeutic challenge lies in the fact that despite the normalization of hemoglobin levels, adequate hormone replacement, and effective iron chelation, bone turnover remains deregulated, and the increased resorptive phase results in seriously reduced bone mineral density.6-8 The increased bone resorption observed in patients with thalassemia-induced osteoporosis has led to the use of bisphosphonates in this subset of patients, as bisphosphonates are potent inhibitors of osteoclastic bone resorption.9-11
The emergence of novel biomarkers of bone remodeling has elucidated the underlying pathophysiology of the disease. In parallel to the well-described osteoblast dysfunction, accumulating evidence suggests the increased osteoclast activation as another major pathogenic mechanism for osteoporosis in TDT.12 The receptor activator of nuclear factor kappa-B (RANK)/RANK ligand (RANKL)/osteoprotegerin (OPG) molecular pathway seems to be of great importance for the activation and proliferation of osteoclast precursors. Previous studies have shown that circulating RANKL, the most potent osteoclast activator, is elevated in patients with TDT and is associated with low bone mineral density.9,13,14
Denosumab (DNM) is a fully human monoclonal antibody that binds RANKL with high affinity and specificity and inhibits its action. DNM has shown efficacy in both men and postmenopausal women with osteoporosis,15-17 as well as in bone disease attributed to solid tumors18-20 and multiple myeloma.18,21
To our knowledge, there are only limited data regarding the effect of DNM in TDT-induced osteoporosis.22 Thus, the aim of this prospective, randomized, placebo-controlled, double-blind, phase 2b clinical trial was to evaluate the efficacy and safety of DNM in patients with TDT and osteoporosis and provide an insight into surrogate biomarkers of bone turnover.
Methods
Study design
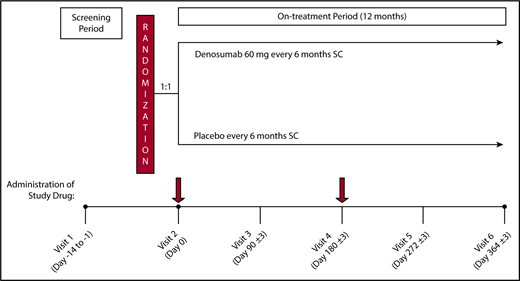
This was a single-site, randomized, placebo-controlled, double-blind phase 2b clinical trial. Patients with a TDT and bone mineral density T score between −2.5 and −4.0 in at least 1 of the 3 examined sites (lumbar spine, femoral neck, or wrist bone) participated in this study and were treated with DNM or placebo. On enrolment in the study, patients were randomly assigned in a 1:1 fashion to receive either 60 mg DNM or placebo administered subcutaneously (sc) every 6 months for 12 months, for a total of 2 doses (day 0 ± 3 and day 180 ± 3). The dosage of DNM was based on a randomized, double-blind, placebo-controlled, phase 3 trial (FREEDOM) demonstrating that DNM treatment reduced the incidence of new vertebral fractures, new nonvertebral fractures, and hip fractures when compared with placebo in postmenopausal women with osteoporosis.15 The overall study design and plan are depicted in Figure 1.
Study schema. On enrolment in the study after eligibility assessment, patients were randomly assigned in a 1:1 fashion to receive either 60 mg DNM or placebo sc, every 6 months for 12 months for a total of 2 doses (Day 0 ± 3 and Day 180 ± 3). Patients were followed every 3 months for clinical and laboratory evaluation.
Study schema. On enrolment in the study after eligibility assessment, patients were randomly assigned in a 1:1 fashion to receive either 60 mg DNM or placebo sc, every 6 months for 12 months for a total of 2 doses (Day 0 ± 3 and Day 180 ± 3). Patients were followed every 3 months for clinical and laboratory evaluation.
This trial was conducted at Thalassemia Reference Centre at Laiko General Hospital (Athens, Greece). The protocol was approved by the independent ethics committee and the institutional review board. The study was conducted according to International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use–Good Clinical Practice guidelines for clinical trials, the Declaration of Helsinki, and local rules and regulations of the country. It is registered with ClinicalTrials.gov (NCT02559648). All patients provided written informed consent for participation in the study.
Eligibility criteria
Patients were deemed eligible for inclusion if they had a diagnosis of TDT and low bone mineral density (T score between −2.5 and −4.0) in at least 1 of the 3 examined sites; namely, lumbar spine, femoral neck, and wrist bone. Only adults >30 years of age described as skeletally mature subjects were included. Patients with bone mineral density T score less than −4.0 in 1 of the 2 studied sites (lumbar spine, femoral neck) or those with previous administration of DNM or bisphosphonates, fluoride, and strontium ranelate within 1 year of study enrolment were excluded. Additional exclusion criteria are provided in supplemental Methods.
Randomization and masking
This was a double-blind clinical trial; neither the patient nor the investigator knew which patient belonged to the test group (DNM group) or the control group (placebo group). Details of the randomization and masking procedures are provided in supplemental Methods.
Procedures
Interventions
After successful enrolment and randomization, patients received either sc DNM 60 mg (Prolia, Amgen, Colorado) or sc placebo every 6 months for 12 months, for a total of 2 doses (day 0 ± 3 and day 180 ± 3). Participants were also provided daily supplements of calcium (≥1000 mg elemental calcium/day) and vitamin D (≥400 IU/day if screening level was >20 ng/mL or ≥800 IU if screening level was 12-20 ng/mL) throughout the study.
Clinical and laboratory follow-up
The patients were evaluated at screening and at each visit thereafter. A detailed description of the assessments is provided in supplemental Methods.
Measurement of bone mineral density
Measurement of bone mineral density with dual-energy X-ray absorptiometry (LUNAR, PRODIGY version 8.60.006/SYSTEM GE medical system LUNAR USA 726; Heartland Trail, Madison, WI) at 3 body sites (lumbar spine; L1-L4, femoral neck, and wrist bone) was conducted during the screening period (day −28 to −1) and at the end of the study (day 364 ± 3).
Evaluation of bone pain
Bone pain was quantified according to Huskisson’s visual analog scale (0 cm, no pain; 10 cm, worst pain possible).23 Patients were asked to evaluate this scale before entering the trial and then 12 months after DNM or placebo administration. The McGill–Melzack scoring system, a verbal scale with 6 levels ranging from 0 to 5 (0, no pain; 1, mild pain; 2, troublesome pain; 3, severe pain; 4, very severe pain; 5, excruciating pain) was also measured at the times mentioned.24
Measurement of markers of bone remodeling
Four categories of markers of bone remodeling were assessed on day 0 and then every 3 months up to 12 months (every patient had 5 measurements), using commercially available enzyme-linked immunosorbent assays: bone resorption markers including C-terminal crosslinking telopeptide of type I collagen and tartrate-resistant acid phosphatase isoform-5b; bone formation markers including bone-specific alkaline phosphatase and osteocalcin; osteoclast regulators including sRANKL and OPG; and osteoblast inhibitors including dickkopf-1 and sclerostin. Details of commercial kits used are provided in supplemental Methods.
Study endpoints
The primary objective was to evaluate the effect of DNM (plus vitamin D and calcium) (DNM group) on lumbar spine bone mineral density in patients with TDT and osteoporosis as compared with control (placebo plus vitamin D and calcium; placebo group) at 12 months.
Secondary objectives of this study were to evaluate the effect of treatment (DNM group) on femoral neck and wrist bone bone mineral density as compared with control (placebo group) at 12 months, to evaluate the effect of DNM on pain scores, to evaluate the effect of DNM on markers of bone remodeling, and to evaluate the safety profile of DNM in patients with TDT and osteoporosis.
Statistical analyses
Analyses of efficacy were based on the intention-to-treat principle. All P values were 2-sided, the level of significance was equal to .05, and confidence intervals referred to 95% boundaries. Statistical analyses were performed using the SAS/STAT statistical package. Further details are provided in supplemental Methods.
Role of the funding source
The funder of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Results
Patient characteristics
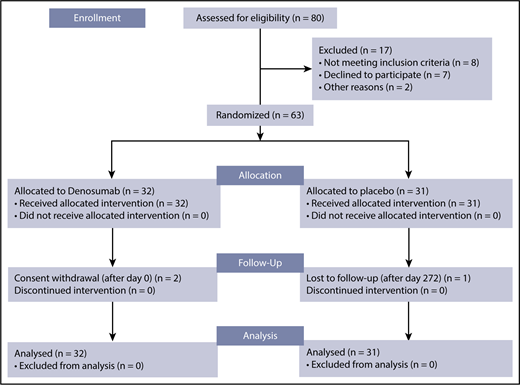
Sixty-three patients with TDT-induced osteoporosis were deemed eligible for inclusion in the clinical trial from 18 September 2014 to 2 December 2015. Thirty-two patients were randomly assigned to the DNM group, and 31 to the placebo group. All patients received the allocated treatment and were included in the analyses. In total, 3 discontinuations occurred; specifically, 1 patient from the placebo group was lost to follow-up after day 272 and 2 patients from the DNM group withdrew consent after day 0. The corresponding consort flow diagram is shown in Figure 2.
Consort flow diagram. Among the 80 patients assessed for eligibility, 63 were eventually randomized: 32 were allocated to and received DNM, whereas 31 were allocated to and received placebo. One patient from the placebo group was lost to follow-up, whereas 2 patients from the DNM group withdrew consent. The final analysis was made on an intent-to-treat basis.
Consort flow diagram. Among the 80 patients assessed for eligibility, 63 were eventually randomized: 32 were allocated to and received DNM, whereas 31 were allocated to and received placebo. One patient from the placebo group was lost to follow-up, whereas 2 patients from the DNM group withdrew consent. The final analysis was made on an intent-to-treat basis.
Baseline patient characteristics are provided in Table 1. Study population had no differences in the distribution of sex or hypogonadism among patient groups. Median (range) age was 52.5 (34-70) years for the DNM group and 56.0 (36-78) years for the placebo group (P = .254). Median (range) elapsed time from first osteoporosis diagnosis to study entry was 1.0 (0-25.8) year for the DNM group and 0.5 (0-19.7) years for the placebo group (P = .908).
No statistically significant differences between DNM and placebo group were observed regarding the mean values of hemoglobin, platelets, white blood cells, glucose, urea, creatinine, uric acid, AST, ALT, γGT, total bilirubin, direct bilirubin, LDH, total protein, albumin, ferritin, iron, phosphate, potassium, sodium, magnesium, 25-hydroxy-vitamin D, fT3, or fT4. The mean value of alkaline phosphatase was lower in the placebo group compared with the DNM group (68.48 IU/L vs 85.45 IU/L, respectively; P = .013).
None of the study patients had a history of spontaneous fractures. All study participants had TDT; thus, they had a history of blood transfusions. The median (range) values for the number of transfusions in the 12-month period before study enrolment was 15 (8-60) for the DNM and 11 (8-48) for the placebo group. No significant difference between the 2 groups was noted (P = .714). All patients had received at least 8 transfusions per year for the last 3 years before study enrolment. All included patients had a history of iron chelation treatment. Median (range) liver iron concentration values were 5.5 mg/g/dw (1.0-24.4) and 4.4 mg/g/dw (1.1-15.4) for the DNM and placebo group, respectively (P = .887). However, iron chelation therapy was taken by 36 (58.1%) study patients (n = 19 in the DNM group and n = 17 in the placebo group; P = .829) during the study period. Patients with acceptable liver iron concentration values and ferritin levels did not receive iron chelation treatment and were under close monitoring, per institutional clinical practice. Overall, there were no statistically significant differences in medical history between the 2 study groups.
Bone mineral density and markers of bone remodeling at baseline
Bone mineral density was measured with dual-energy X-ray absorptiometry during the screening period in the femoral neck, lumbar spine, and the wrist bone. The ranges of the T score for those 3 sites were −3.20 to −0.50 and −3.30 and −0.40, respectively, for the femoral neck; −4.00 and −0.90 and −4.00 and −0.90 for lumbar spine; and −11.7 and −1.10 and −8.70 and −0.10 for the wrist bone in the DNM and the placebo group. No significant differences between the 2 groups were noted in any of the examined sites, as shown in Table 1.
The bone absorption marker C-terminal crosslinking telopeptide of type I collagen was measured at baseline with no statistically significant differences between the 2 groups (mean value, 0.16 ng/mL for the DNM group and 0.14 ng/mL for the placebo group; P = .905). Regarding the tartrate-resistant acid phosphatase isoform-5b marker, mean value was significantly higher in the DNM group (0.42 IU/L vs 0.16 IU/L in the placebo group; P = .026). No statistically significant differences between the 2 groups were observed in the values of the bone formation markers (bone-specific alkaline phosphatase and osteocalcin), markers of osteoclast activation (sRANKL, OPG, sRANKL/OPG), and markers of osteoblast inhibition (sclerostin, dickkopf-1) under investigation (Table 1).
Bone mineral density after DNM or placebo administration
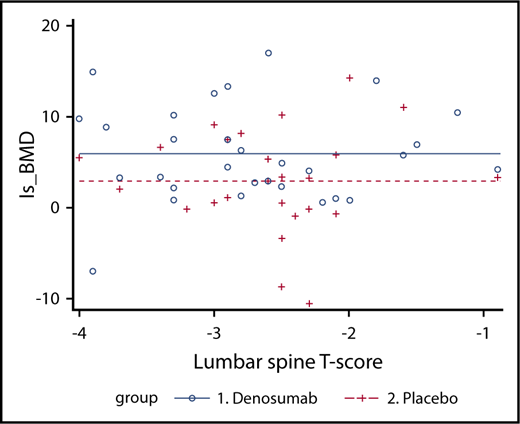
As stated earlier, the primary objective was to evaluate the effect of DNM (DNM group) compared with placebo (placebo group) on lumbar spine bone mineral density in patients with TDT-induced osteoporosis. At 12 months, the mean (standard deviation [SD]) percentage increase of lumbar spine bone mineral density was 5.92% (5.25%) in the DNM group and 2.92% (5.56%) in the placebo group compared with baseline. The difference was statistically significant (P = .043). Furthermore, an analysis of covariance model was used to evaluate the effect of DNM as compared with control. Treatment was the main effect, and covariates included the level of baseline T score. A significant difference on the percentage change (from baseline to 12-month visit) between the 2 groups was observed, after adjusting for the baseline T score (P = .0428; Figure 3). Finally, to further investigate the association between treatment and lumbar spine bone mineral density, the effect of the interaction between baseline T score and treatment was evaluated; however, no evidence of interaction was observed.
Analysis of covariance for the bone mineral density percentage change, defined as the dependent variable, with treatment group as a factor, defined as the independent variable consisting of 2 levels (DNM and placebo), and first baseline T score as covariate or nuisance variable. A significant difference on the percentage change (from baseline to 12-month visit) between the 2 groups was observed, after adjusting for the baseline T score (P = .043). DNM induced a significantly greater increase in bone mineral density compared with placebo.
Analysis of covariance for the bone mineral density percentage change, defined as the dependent variable, with treatment group as a factor, defined as the independent variable consisting of 2 levels (DNM and placebo), and first baseline T score as covariate or nuisance variable. A significant difference on the percentage change (from baseline to 12-month visit) between the 2 groups was observed, after adjusting for the baseline T score (P = .043). DNM induced a significantly greater increase in bone mineral density compared with placebo.
Regarding the first secondary objective, the treatment effect on wrist bone and femoral neck bone mineral density between the 2 groups was assessed. At 12 months, compared with the baseline, an average decrease in wrist bone bone mineral density, which was significantly larger in the placebo group, was observed (−0.26% [SD, 5.31] and −3.92% [SD, 8.71], respectively; P = .035). On the contrary, femoral neck bone mineral density showed an increase of 4.08% and 1.96% in DNM and the placebo group, respectively; however, the difference between the 2 groups was not significant (P = .870).
Bone pain at baseline and after DNM or placebo
Patients at baseline had mild to moderate pain according to pain scoring systems used. There were no differences in pain scores among the 2 studied groups at baseline (Tables 2 and 3). Patients of the DNM group had a significant reduction of pain score after 12 months of DNM administration (P < .001 for both scoring scales; Table 2). On the contrary, patients of the placebo group showed no alteration in bone pain during the study period (Table 3).
Markers of bone remodeling after DNM or placebo administration
In terms of the third secondary objective, changes in markers of bone remodeling were evaluated in the 2 study groups. Three months after the first sc injection of DNM or placebo, the average percentage change of the bone absorption marker C-terminal crosslinking telopeptide of type I collagen was −31.6% (SD, 17.74) in the DNM group and +16.51% (SD, 44.22) in the placebo group compared with baseline (P < .001). Furthermore, patients in the DNM group showed a significant reduction of sRANKL, sRANKL/OPG ratio, C-terminal crosslinking telopeptide of type I collagen, tartrate-resistant acid phosphatase isoform-5b, bone-specific alkaline phosphatase between baseline and 12th month (P < .01 for all comparisons) without changes in dickkopf-1, sclerostin, and osteocalcin. On the contrary, patients in placebo group showed an increase in sRANKL, OPG, dickkopf-1, sclerostin, C-terminal crosslinking telopeptide of type I collagen, tartrate-resistant acid phosphatase isoform-5b, and bone-specific alkaline phosphatase during the study period (P < .01 for all comparisons), along with a slight increase of osteocalcin that did not reach statistical significance (Tables 2 and 3).
Safety evaluation and adverse events
Seventeen cases of adverse events were reported in 14 different patients during the study period. Fourteen of the 17 adverse events were classified as mild (grade 1). Three of 14 mild adverse events concerned the placebo group. Moreover, the majority of the mild adverse events (11/14) concerned abnormalities on blood or biochemical testing, and only 3 of them a clinical symptom; specifically, headache, diarrhea, and fever. Only 3 months after the first DNM administration, the number of patients with an adverse event was greater in the DNM group compared with the placebo group (5 patients in the DNM group vs no patient in the placebo group; P = .018).
The 3 serious adverse events reported during the study duration occurred in the DNM group. The diagnoses for them with the corresponding severity grade were pleural effusion (grade 3), supraventricular tachycardia (grade 4), and atrial fibrillation (grade 3). All 3 events occurred more than 3 months after the first DNM injection and before the second. As per investigator’s assessment, the causal relationship of study treatment to the event was defined as unrelated to study drug.
Discussion
In this single-site, randomized, placebo-controlled, double-blind phase 2b clinical trial, we sought to evaluate the effect of DNM on bone mineral density in patients with TDT and osteoporosis. The DNM group showed a statistically significant increase in the lumbar spine bone mineral density compared with placebo (5.9% vs 2.9%) at 12 months compared with at baseline. Our results are consistent with a single-group study that administered DNM in 30 patients with TDT-induced osteoporosis on the same dosing schedule as in the present clinical trial.22 A significant increase in the lumbar spine bone mineral density of 9.2% (95% confidence interval, 8.2%-10.1%) at 12 months compared with baseline was observed. A significant increase in the femoral neck bone mineral density of 6.0% (95% confidence interval, 5.2%-6.7%) was also reported, which was not replicated in our trial. However, it should be noted that the study population also included osteopenic patients (T score less than −1.0); thus, the interpretation and generalization of the results should be cautious. On the contrary, the randomized, placebo-controlled, double-blind design of the present clinical trial, along with the exclusive inclusion of osteoporotic patients (T score less than −2.5) and the adequate power in terms of the low patient discontinuation rate, provided robustness to our outcomes.
Furthermore, we found an average decrease in wrist bone bone mineral density in both groups, which was significantly more pronounced in the placebo group (−3.92% vs −0.26%). Regarding femoral neck bone mineral density, the mean increase was not significantly different between the 2 patient groups (4.08% and 1.96% in the DNM and placebo groups, respectively). These results coincide with another clinical trial evaluating the effect of zoledronic acid in thalassemia-induced osteoporosis. Although a significant increase in the lumbar spine bone mineral density was observed after treatment, there was not a similar increase in the femoral neck and wrist bone bone mineral density.10 Bone microarchitecture and mechanics may explain the reason why changes in the bone microenvironment are reflected early in lumbar spine bone mineral density, whereas significant variations in the femoral neck and wrist bone bone mineral density may require a longer follow-up period.25
Importantly, the DNM group had a significant reduction in bone pain (by more than 80%), which was not observed in the placebo group in this blinded study. DNM has been reported to reduce bone pain in patients with osteoporotic fractures vs alendronate,24 whereas DNM also reduced bone pain by more than 55% in patients with osteoporotic fractures resulting from the chronic use of glucosteroids.25 Our group of patients had no fractures at baselines, and possibly this is the reason for the marked reduction of bone pain observed. In contrast, the placebo group showed no significant reduction of pain, although they had an increase of their lumbar spine bone mineral density, which is attributed to calcium and vitamin D supplementation.
DNM specifically binds RANKL and counteracts the bone resorption state that is prevalent in patients with thalassemia. The RANK/RANKL/OPG molecular pathway has been identified as a central regulator of the interplay between osteoclasts and osteoblasts in thalassemia-related bone disease.26 It has been repeatedly shown that serum RANKL levels are elevated among patients with thalassemia compared with healthy control patients.9,10,13,14,27 The subsequent disturbance of the RANKL/OPG ratio ultimately favors osteoclast-mediated bone loss.13,27 The abovementioned data, along with efficacy results of DNM administration in patients with bone disease resulting from osteoporosis15-17 and malignancies18-20 have provided a strong rationale for the present study.
In this context, we found that the average percentage change of the bone absorption marker C-terminal crosslinking telopeptide of type I collagen was −31.6% in the DNM group and +16.51% in the placebo group at 3 months compared with baseline. This significant finding was evident even after the first dose of the drug, and it is in accordance with the study by Yassin et al reporting very early reduction of C-terminal crosslinking telopeptide of type I collagen after a single dose of DNM.22 Bone resorption markers including C-terminal crosslinking telopeptide of type I collagen, urinary levels of N-telopeptide cross-linked type 1 collagen, pyridinoline, and deoxypyridinoline are found elevated in patients with TDT-related osteoporosis and may be useful markers for response to treatment.10,12,13,28,29 This early effect of DNM on bone remodeling may have profound implications on bone fragility and the risk for fracture in the long term,30 considering also the impaired osteoblastic activity in patients with thalassemia.26
Furthermore, we provided evidence for a differential effect of DNM on biomarkers of bone remodeling compared with placebo at 12 months. In the DNM group, both bone resorption markers, namely, C-terminal crosslinking telopeptide of type I collagen and tartrate-resistant acid phosphatase isoform-5b, as well as the RANKL to OPG ratio, were significantly reduced. On the contrary, C-terminal crosslinking telopeptide of type I collagen, tartrate-resistant acid phosphatase isoform-5b, and serum RANKL levels were significantly increased in the placebo group. However, it should be noted that the interpretation of the results regarding tartrate-resistant acid phosphatase isoform-5b should be cautious because of the baseline difference between the 2 groups. In the placebo group, there was also a significant increase in the osteoblast inhibitors dickkopf-1 and sclerostin. Both markers have been associated with thalassemia-induced osteoporosis and have been proposed as potential therapeutic targets.31,32 It has to be noted that these markers remained unchanged in the DNM group. Therefore, it may be postulated that even after DNM administration, there is a residual effect preventing the normal osteoblast function. Treatment with zoledronic acid has also failed to reduce circulating sclerostin levels in patients with osteoporosis with thalassemia32 or postmenopausal women.33 Regarding markers of bone formation, bone-specific alkaline phosphatase was reduced in the DNM group and was increased in the placebo group, whereas osteocalcin did not show significant changes in any of the 2 groups. Although these findings may be partially attributed to compensating mechanisms, the exhaustion of osteoblasts in patients with thalassemia should be also taken into consideration.34 Iron poisoning is a well-established contributing factor for osteoblast dysfunction,34,35 and all our patients had a history of blood transfusions; more than half of them were receiving iron chelation therapy. Overall, the net effect of DNM therapy was against bone resorption, and it was reflected on the significantly greater average increase of lumbar spine bone mineral density compared with placebo.
Although bisphosphonates are currently the mainstay of treatment in patients with thalassemia with osteoporosis,36,37 DNM may provide a more favorable efficacy and tolerability profile. In contrast to oral bisphosphonates, sc administration of DNM bypasses the gastrointestinal tract, and thus, prevents the gastrointestinal adverse effects, whereas it is associated with better pharmacokinetics.38,39 Moreover, recent studies have revealed the superiority of DNM compared with zolendronic acid in different clinical settings, including postmenopausal women with osteoporosis40,41 and oncology patients.21,42 In patients with thalassemia-induced osteoporosis, there has been currently no study providing a direct comparison between DNM and bisphosphonates. In this context, a clinical trial is ongoing that aims to compare DNM vs zoledronic acid in patients with TDT-induced osteoporosis in terms of C-terminal crosslinking telopeptide of type I collagen reduction and dual-energy X-ray absorptiometry scan absorptiometry improvement (NCT03040765).
Regarding the safety profile of DNM, there was some evidence of an increased number of adverse events in the DNM group compared with placebo, although all of them were considered unrelated to study drug. However, both in the original report of FREEDOM study as well as the report from the FREEDOM Extension study including patients with postmenopausal osteoporosis with up to 8 years of DNM treatment, there was no difference in the number of total adverse events between the DNM and the placebo-treated group.15,16 Therefore, longer follow-up is warranted to establish safety of DNM among patients with TDT-induced osteoporosis.
One of the limitations of our study may pertain to the evaluation method of osteoporosis. Bone mineral density measurement is a widely available noninvasive means of identifying individuals with osteoporosis and, possibly, those at high risk for fracture. However, there is accumulating evidence indicating that changes in bone mineral density do not correlate sufficiently with the probability of fracture risk among postmenopausal women receiving osteoporosis therapy.43 It is also true that bone mineral density is only 1 of several contributors to bone strength and fracture risk reduction. Two principal aspects of bone strength should be considered: bone quantity consisting of density and size and bone quality encompassing structure, material characteristics, and bone turnover.30 As a consequence, there are supplemental measures of osteoporosis treatment efficacy as candidate variables for future evaluation in clinical trials.43 Another possible limitation may pertain to the heterogeneity of study participants in terms of transfusion dependency. All patients had received 8 or more transfusions annually for the last 3 years before study enrolment, but the range was relatively high (8-60). Although all participants were transfusion dependent, both thalassemia major and thalassemia intermedia patients could be included as long as they were treated as TDT according to their clinical presentation. However, it should be underlined that there was no significant difference in the number of transfusions between the 2 treatment groups.
In addition to the above, the implementation of general lifelong measures starting from early childhood, such as dietary modifications and regular physical activity, reduce fracture risk and prevent disability.5 Those may represent confounding factors that need to be accounted for in future studies, which may identify subsets of patients with TDT-related osteoporosis who would derive benefit from DNM treatment.
In conclusion, DNM administration was associated with a significantly greater increase in the lumbar spine bone mineral density in patients with TDT-associated osteoporosis compared with placebo. DNM seemed also to reduce biomarkers promoting bone resorption. However, there was evidence for a higher number of adverse events in the DNM group compared with placebo. Subsequently, more research is needed to clarify the effect of DNM on other surrogate endpoints, to control for confounding factors, and to evaluate safety with a longer follow-up period.
The full-text version of this article contains a data supplement.
Acknowledgments
This study as a whole is dedicated to the thalassemia patients of Laikon General Hospital, whose participation made it possible.
This study was supported by Amgen.
Authorship
Contribution: E.V. and E.T. designed the study; E.V. and E.T. provided patients or study materials; I.N.-S., A.P., D.C., M.D., K.R., A.P., and M.P. participated in the collection and assembly of data and in data analysis and interpretation; D.C. participated in the statistical analysis; A.P. and E.T. performed bone markers measurements; E.V., I.N.-S., and E.T. wrote the first draft of the manuscript; and all authors participated in the critical review and revision of this manuscript and provided approval of the manuscript for submission.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Evangelos Terpos, Department of Clinical Therapeutics, Medical School, National and Kapodistrian University of Athens, Alexandra General Hospital, 80 Vas. Sofias Ave, 11528 Athens, Greece; e-mail: eterpos@med.uoa.gr or eterpos@hotmail.com.