Key Points
sCR and non-sCR in acute myeloid leukemia are associated with different prognoses.
Three lineage blood cell recoveries have independent impacts on prognosis.
Abstract
Stringent complete remission (sCR) of acute myeloid leukemia is defined as normal hematopoiesis after therapy. Less sCR, including non-sCR, was introduced as insufficient blood platelet, neutrophil, or erythrocyte recovery. These latter characteristics were defined retrospectively as postremission transfusion dependency and were suggested to be of prognostic value. In the present report, we evaluated the prognostic impact of achieving sCR and non-sCR in the Danish National Acute Leukaemia Registry, including 769 patients registered with classical CR (ie, <5% blasts in the postinduction bone marrow analysis). Individual patients were classified as having sCR (n = 360; 46.8%) or non-sCR (n = 409; 53.2%) based on data from our national laboratory and transfusion databases. Survival analysis revealed that patients achieving sCR had superior overall survival (hazard ratio [HR], 1.34; 95% confidence interval [CI], 1.10-1.64) as well as relapse-free survival (HR, 1.25; 95% CI, 1.03-1.51) compared with those with non-sCR after adjusting for covariates. Cox regression analysis regarding the impact of the stringent criteria for blood cell recovery identified these as significant and independent variables. In conclusion, this real-life register study supports the international criteria for response evaluation on prognosis and, most importantly, documents each of the 3 lineage recovery criteria as contributing independently.
Introduction
Acute myeloid leukemia (AML) is a heterogeneous malignant clonal disorder, with acquired genetic lesions of hematopoietic stem cells.1,2 In Denmark, approximately 250 patients are diagnosed with AML each year, which corresponds to an incidence rate of 5.4 per 100 000 persons per year.3,4 The cumulative 5-year survival is 17%.4 Despite AML being a highly malignant disease, it is potentially curable if patients can tolerate and respond to intensive treatment. Curative treatment requires initial induction chemotherapy, with the goal of achieving complete remission (CR). The prognostic value of achieving CR is well known.5,6
By international standards, since 2003, stringent CR (sCR) has been defined as <5% blasts in the bone marrow, absence of blasts with Auer rods, and absence of extramedullary disease combined with neutrophil counts >1.0 × 109/L, platelet counts >100 × 109/L, and independence from red cell transfusions.5,7,8 Patients with <5% blasts in the bone marrow who do not fulfill all the criteria for CR are categorized as having non-sCR. Non-sCR is similar to CR but with incomplete recovery (CRi), defined as CR with residual neutropenia or thrombocytopenia.5,8 However, CRi does not include the possibility of a patient having anemia and a persistent need for red blood cell transfusions; hence, the term non-sCR is used in this report.
Several studies show that sCR is associated with better outcomes in terms of both overall survival (OS) and relapse-free survival (RFS) compared with CRi.7,9-12 Such findings suggest that distinguishing between sCR and other degrees of CR is of clinical value when evaluating treatment response. However, most studies are limited by excluding independence from red cell transfusions as a criterion for achieving sCR, and therefore, the prognostic impact of sCR is unclear.
The Danish National Acute Leukaemia Registry (DNLR) includes all Danish patients diagnosed with AML since January 1, 2000. It contains information regarding prognostic factors, treatment regimens, and outcomes, including patients’ remission status after induction therapy. In parallel, we have national patient-centered databases regarding blood values and transfusions identified by civil registration numbers. The goal of the present study was to confirm the prognostic impact of sCR compared with non-sCR in a nationwide cohort of patients with AML.
Materials and methods
Registries: an epidemiological source of high quality
A keystone of this study was the use of population-based registries. The study population was generated from the DNLR. This registry and the quality of its data have been described in detail in a prior study.3 In brief, the DNLR includes 99% of patients with AML diagnosed in Denmark and covers information on intention of treatment (palliation or remission induction), type and dose of chemotherapy, treatment response, and outcome (ie, vital status). We combined information from different registries using the civil registration numbers of study participants. The civil registration number is a unique personal identification number given to all Danish inhabitants at birth or immigration, which unambiguously records linkage.13,14 In 1997, the Danish National Pathology Registry was established. It contains data on all pathological tests performed in Denmark (eg, dates and results of bone marrow biopsies). The information in the registry is reported by all departments of pathology and is mandatory. As for the other registries, the data are linked to the civil registration numbers.15,16 The Danish Transfusion Registry contains information concerning transfusion data for all patients receiving transfusions in Denmark. Information includes the date and type of transfusion and the civil personal registration number of the recipient.17
Study population and classification of CR
Patients fulfilling the following criteria were included in this study: age ≥18 years; diagnosed with AML, excluding acute promyelocytic leukemia, from 1 January 2000 to 31 December 2010; and selected for intensive therapy, defined as a remission-induction regimen with a backbone of standard to high dose of cytarabine (≥200-400 mg/m2 per day) in combination with an anthracycline or anthracycline-related compound, with achievement of classical CR after first or second induction therapy. We evaluated the quality of the CR as proposed by the International Working Group.5,8 Patients fulfilling all CR criteria (ie, <5% blasts in the bone marrow, absence of blasts with Auer rods, and absence of extramedullary disease combined with neutrophil counts >1.0 × 109/L, platelet counts >100 × 109/L, and independence from red cell transfusions5,7,8 ) were categorized as sCR. Patients with <5% blasts in the bone marrow but not fulfilling all criteria (ie, having insufficient blood platelet, neutrophil, or erythrocyte recovery) were categorized as non-sCR. Evaluation of pathology was based on data gathered from the Danish National Pathology Registry. Transfusion history data were acquired from the Danish Transfusion Registry, and neutrophil and platelet count data were collected from the various departments of hematology and departments of clinical biochemistry in Denmark using primarily LABKA (laboratory information system).18
We defined day 0 as the date of bone marrow aspiration (ie, the day of treatment evaluation) and defined a time period in which the patients with sCR did not receive red blood cell transfusions from day +1 until the first day of the next treatment cycle. Patients not receiving red blood cell transfusions from day +1 and forward were classified as being transfusion independent. We strived to collect data concerning blood neutrophil and platelet counts at day 0; however, this was not possible for all patients, so we expanded the timespan for these values from day −3 to day +7.
Statistical analysis
The distributions of demographic and clinical characteristics as well as postremission characteristics of the assigned sCR and non-sCR groups were compared using Pearson’s χ2 test and Fisher’s exact test for categorical variables. Mann-Whitney U test was used for continuous variables.
RFS for each patient was defined as the time from the classical CR response to AML relapse, death resulting from any cause, or censoring for patients alive without relapse at the time of last follow-up and registration. OS was defined as the time from CR response to death resulting from any cause or censoring. RFS and OS curves were computed using the Kaplan-Meier method, and differences between groups were tested using the log-rank test. The association between groups (sCR and non-sCR) and outcomes (OS and RFS) were evaluated using crude and adjusted Cox proportional hazards regression analyses, including 769 patients in the multivariable analyses. Adjustment were performed for age, sex, World Health Organization performance score at diagnosis, leukocyte count at diagnosis, CR as response after first or second induction therapy, and cytogenetic risk group. Hazard ratios (HRs) with 95% confidence intervals (CIs) were calculated. Throughout the analyses, 95% CIs were reported and used if estimated results were statistically significant. All data were analyzed using Stata/MP 13.1 (StataCorp, College Station, TX).
Ethics
This study was approved by the Danish Data Protection Agency (2008-58-0028) and the Danish Health Authority.
Results
Patient characteristics and outcome assignment
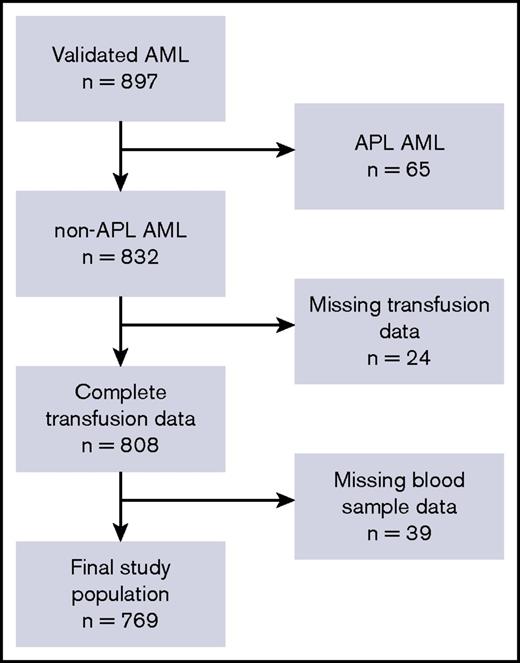
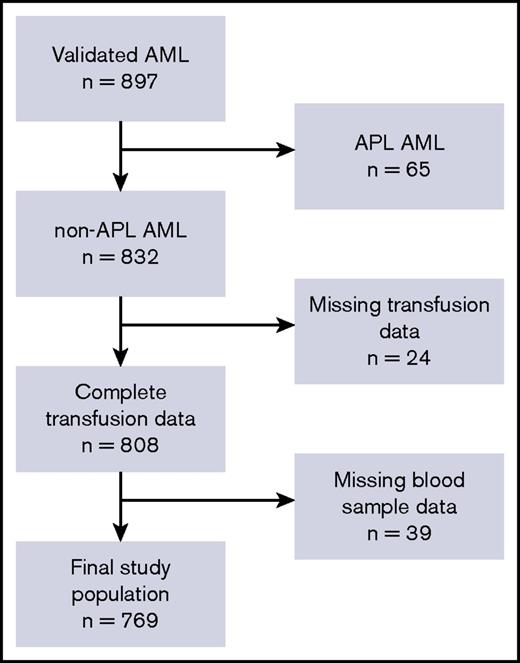
In total, 832 patients with AML fulfilled the inclusion criteria, as illustrated in Figure 1. We retrieved transfusion data from the Danish Transfusion Registry for 808 patients (97.6%) and blood platelet and neutrophil counts from LABKA for 769 patients (92.4%).
The patient cohort and defined study population. Flowchart describing the postinduction, classical CR cohort and final study population. In total, 832 patients met all inclusion criteria. Because of missing data regarding blood samples in LABKA and patients’ transfusion history, the final study population included 769 patients who were included in the population analyzed in the present study. APL, acute promyelocytic leukemia.
The patient cohort and defined study population. Flowchart describing the postinduction, classical CR cohort and final study population. In total, 832 patients met all inclusion criteria. Because of missing data regarding blood samples in LABKA and patients’ transfusion history, the final study population included 769 patients who were included in the population analyzed in the present study. APL, acute promyelocytic leukemia.
Table 1 lists baseline information regarding patient characteristics and classification. A total of 360 (46.8%) of 769 patients were classified as having sCR and 409 (53.2%) of 769 as having non-sCR.
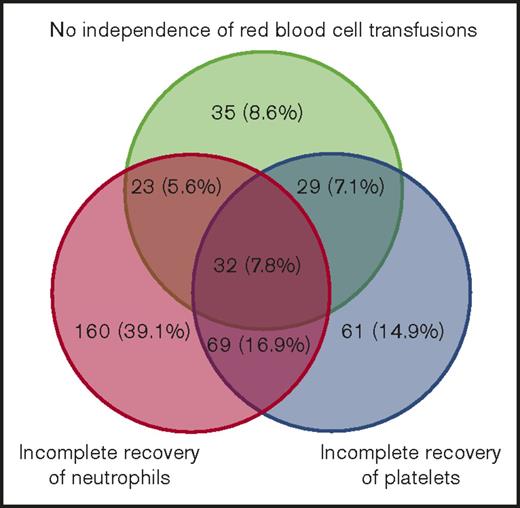
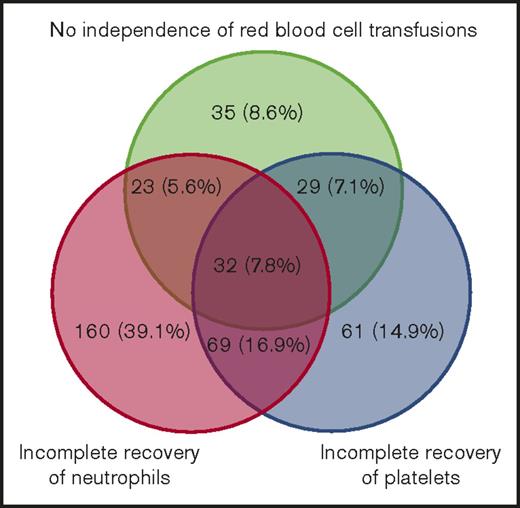
We observed that 678 patients with AML (88.2%) were in CR after their first induction regimen, of whom 310 (45.7%) were classified as achieving sCR and 368 (54.3%) as achieving non-sCR. A total of 91 patients (11.8%) were in CR after the second induction regimen, of whom 50 (54.9%) were classified as achieving sCR and 41 (45.1%) as achieving non-sCR. In total, 525 patients (68.4%) died after CR, of whom 221 (42.1%) had achieved sCR and 304 (57.9%) had achieved non-sCR. The median OS was 3.4 years (range, 1.3-7.7 years) for those achieving sCR and 2.0 years (range, 0.9-7.3 years) for those achieving non-sCR. Furthermore, 333 patients experienced postremission relapse, of whom 152 (45.7%) were classified as achieving sCR and 181 (54.3%) as achieving non-sCR on day 0. Assignment of non-sCR according to each of the 3 lineage recovery criteria is presented as a Venn diagram in Figure 2, illustrating heterogeneous classifications. Red blood cell transfusions alone classified 35 patients (8.6%), incomplete neutrophil recovery alone classified 160 patients (39.1%), and incomplete platelet recovery alone classified 61 patients (14.9%). The remaining patients were classified as non-sCR as a result of a combination of ≥2 criteria not fulfilled.
Absolute and relative numbers of patients with insufficient 3-lineage recovery. Venn diagram illustrating analysis of international consensus criteria for CR in patients who did not fulfill sCR criteria (non-sCR patients).
Absolute and relative numbers of patients with insufficient 3-lineage recovery. Venn diagram illustrating analysis of international consensus criteria for CR in patients who did not fulfill sCR criteria (non-sCR patients).
OS and RFS
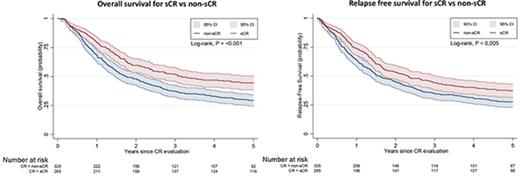
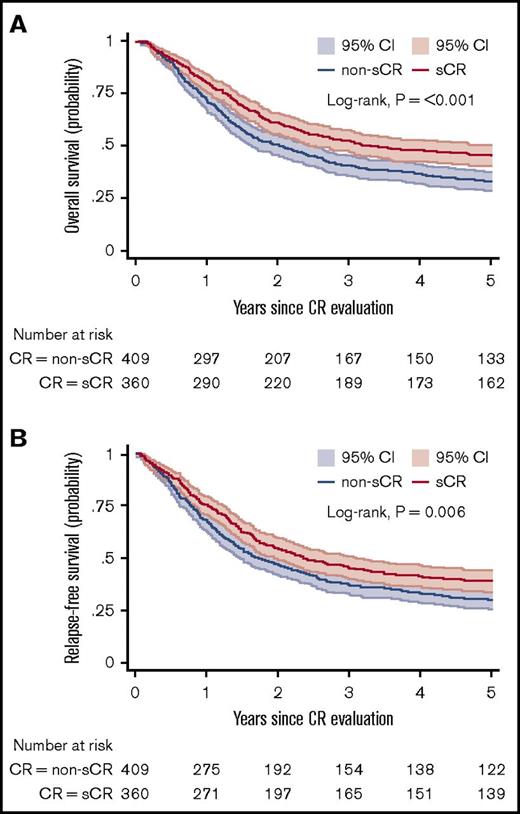
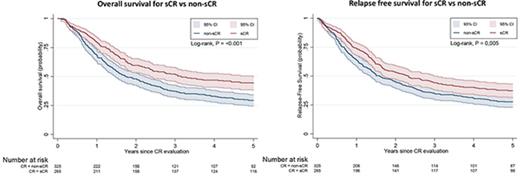
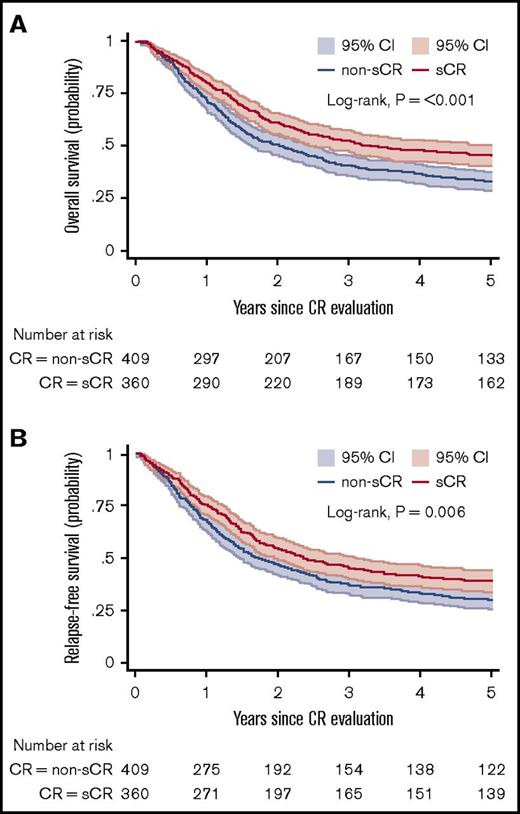
Figure 3A-B shows the OS and RFS Kaplan-Meier curves for the sCR and non-sCR groups. Patients who achieved sCR had significantly better outcomes compared with those with non-sCR. As summarized in Table 2, the adjusted HR was 1.34 (95% CI, 1.10-1.64) for OS and 1.25 (95% CI, 1.03-1.51) for RFS for sCR compared with non-sCR. Incomplete platelet, neutrophil, and erythrocyte (red blood cell transfusions) recovery were each independent risk factors for inferior outcomes (Table 3). That is, each of the consensus criteria was associated with a statistically significantly higher risk of death or relapse of AML. This association persisted after further adjustment for AML classification (ie, AML de novo, secondary AML, or therapy-related AML; data not shown). Secondary analysis (data not shown) showed no significant difference between the sCR and non-sCR groups regarding the frequency of undergoing hematopoietic stem-cell transplantation.
OS and RFS. Kaplan-Meier estimates for OS (A) and RFS (B) presented with 95% CIs and log-rank test for 769 patients at risk. (A) Probability of OS in patients who achieved sCR or non-sCR. (B) Probability of RFS of patients who achieved sCR or non-sCR.
OS and RFS. Kaplan-Meier estimates for OS (A) and RFS (B) presented with 95% CIs and log-rank test for 769 patients at risk. (A) Probability of OS in patients who achieved sCR or non-sCR. (B) Probability of RFS of patients who achieved sCR or non-sCR.
Discussion
The present study demonstrates and supports that patients with AML who achieve sCR have statistically significantly higher OS and RFS compared with patients who do not achieve sCR (ie, those achieving non-sCR).7,9,11,12 Moreover, our study validates the significance of each of the 3 lineage recovery criteria for achieving sCR, providing evidence for the use of all these criteria in clinical practice. Several studies have shown that patients with AML who achieve sCR have a better prognosis than patients who do not.7,9,11,12 However, these studies differ by various definitions of the study populations and criteria for CR, which limits comparison.
We investigated the impact of each single criterion on the prognostic outcome at the time that patients achieved <5% blasts in bone marrow and documented that each criterion was associated with OS and RFS. This finding is in agreement with a study by de Greef et al,19 in which the CR criteria as proposed by Cheson et al5 were verified. Both platelet and neutrophil recovery had a high impact on prognosis regarding OS (HR, 2.17; P = .002 and HR, 1.41; P = .090, respectively), comparable to our results. We found that all sCR criteria had a high impact on prognosis (Table 3). Incomplete recovery of neutrophils was the criterion most likely to interact with the remaining criteria, as shown in the Venn diagram (Figure 3). Unfortunately, de Greef et al19 did not include information regarding red blood cell transfusion dependency.
To the best of our knowledge, no prior study has documented the expected prognostic value of red blood cell transfusion dependency in the evaluation of CR quality. Our study shows that patients who are independent of red cell transfusions, in addition to achieving platelet and neutrophil recovery, have an improved prognosis.
Achievement of sCR is an important goal after induction chemotherapy for AML. We found a positive association between CR quality and risk of relapse, which indicates that inferior OS related to non-sCR may be due to the relapse of AML and is possibly caused by residual disease. The association persisted after adjustment for type of AML (de novo, secondary, or therapy-related AML). Furthermore, the association was not explained by difference in rate of allogeneic bone marrow transplantation among patients in sCR compared with non-sCR. We performed adjustment for cytogenetic risk group, which did decrease the association between degree of CR and RFS, supporting that cytogenetic risk group may influence the chance of sCR; however, cytogenetic risk group is probably not the only explanation, because the association persisted (Table 3). We propose extended studies to enumerate the level of minimal residual leukemic bone marrow blasts and define sCR using advanced assays, with counting accuracy and high specificity at the level of sensitivity.
The response criteria for patients with AML were developed based on recovery of normal hematopoiesis by blood cell regeneration and bone marrow assessment, which are relatively insensitive analyses. Given the high rates and varying depths of CRs seen with new treatment approaches, there is a clinical need to define new response categories that can identify deeper responses. Recent attempts have focused on identifying residual tumor cells in the bone marrow using flow cytometry or specific gene expression.20-22 Combining these new methods may define new response categories of minimal residual disease negativity, which will allow for uniform reporting in the precision medicine era.
Our large nationwide population-based cohort study covers nearly every patient with AML in Denmark fulfilling the inclusion criteria. We were unable to retrieve a complete data set regarding blood cell transfusion, platelets counts, or neutrophil counts on the date of response evaluation (day 0) in only 63 (7.6%) of 832 patients (all excluded). We do not think that missing information was associated with the classification of sCR or non-sCR, and therefore, we do not think that this biased our results.
In summary, the present survival analysis confirms that patients achieving sCR have superior OS as well as RFS compared with those with non-sCR when adjusted for covariates. Cox regression analysis identified blood counts and transfusions as independent variables. Therefore, red blood cell transfusion must not be ignored when evaluating treatment response. Using a prospective approach, there is a clinical need to define sCR by exact values for minimal residual leukemic blasts in the postinduction therapy bone marrow analysis.
Presented in abstract form at the Congress for Medical Student Research 2016, Nyborg, Denmark, 17-20 March 2016.
Acknowledgments
This study was supported by research funding from the KE Jensen Foundation to H.E.J., M.B., and K.D. A.K.Ø. was supported by the Research Foundation of North Denmark Region. A.O. was supported by a scholarship from the Danish Cancer Society.
Authorship
Contribution: A.K.Ø., A.O., M.D.B., H.E.J., and M.T.S. validated and analyzed data and wrote the draft manuscript; A.O., H.E.J., and M.T.S. designed the protocol; M.D.B. and M.B. provided statistical analysis support; A.K.Ø., A.O., M.D.B., J.B., P.J., L.S.L., I.M., T.B.M., D.W., G.E., C.S., J.Q.T., L.S.G.Ø., O.J.N., and K.D. collected data; and all authors approved the final manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Marianne T. Severinsen, Department of Haematology and Department of Clinical Medicine, Aalborg University Hospital and Aalborg University, DK-9000 Aalborg, Denmark; e-mail: m.severinsen@rn.dk.