Key Points
Ectopic Lck expression signifies interpatient and intratumoral heterogeneity in CLL.
Lck expression identifies CLL subpopulations with aberrant BCR signaling.
Introduction
Chronic lymphocytic leukemia (CLL) constitutes the commonest hematopoietic malignancy in the western world. Clinical manifestations are highly variable, a fact that is partly explained by diverse molecular expression profiles of CLL cells.1 Although previously believed a homogeneous disease of CD5+ B cells, mutational mapping with advanced sequencing technologies has unraveled a surprisingly high intraclonal heterogeneity in CLL and provided proof of evidence that genetically diverse subclones may be the driving force for clonal evolution of the disease.2-4 CLL cell survival and expansion depends on unremitting signals from the B-cell receptor (BCR).5 Although studies have indicated interpatient heterogeneity of BCR-signaling responses,6-8 there is limited knowledge on the intratumor clonal composition of CLL cells with regard to expression and activation of intracellular signaling mediators. A distinct feature in CLL intracellular signaling is the presence of T-cell–specific proteins, such as CD5, the ζ-associated protein of 70 kDA (ZAP-70), and the lymphocyte-specific protein tyrosine kinase (Lck), a Src family kinase (SFK) member.9-13 Besides CLL, mantle cell lymphomas, T-cell neoplasms, and many lymphomas of germinal center origin have been found to express Lck.14 Lck is indispensable for the phosphorylation of the immunoreceptor tyrosine-based activation motifs (ITAMs) of the T-cell receptor and the consequential triggering of downstream signaling pathways.15 Analogously, it has been shown that Lck colocalizes with the CD79a subunit of the BCR complex and mediates CD79a ITAM phosphorylation upon BCR crosslinking of CLL cells.11-12 Although expression of ZAP-70 has an established prognostic role,16 little is known about the role of Lck in CLL. Though studies have suggested interpatient variability in ectopic Lck,10-13 the potential intratumoral heterogeneity of Lck expression has not been addressed. Moreover, the role of Lck on BCR-signaling behavior and cellular activation within distinct CLL populations has not been clearly defined. This study aims to answer these questions.
Methods
All samples were obtained from newly diagnosed/untreated CLL patients or healthy donor volunteers (HDs) according to institutional review board–approved protocols and in accordance with the Declaration of Helsinki. Diagnosis was made from peripheral blood samples at the FACS Laboratory of the University of Patras by using a Beckman Coulter flow cytometer according to international standards.1 The median absolute lymphocyte number was 48 × 109/L (range, 18-283) and in all cases the lymphocyte pool contained >99% CLL cells identified as CD19+/CD20dim/CD5+. Peripheral blood mononuclear cells (PBMCs) were isolated by Lymphoprep gradient centrifugation (Stemcell Technologies), fixed with PhosFlow Fix Buffer I (BD Biosciences), and permeabilized with saponin buffer (Sigma). Primary antibodies used were: anti-CD5-phycoerythrin (PE) Cy5, anti-CD19–fluorescein isothiocyanate, anti-CD38-PE (Beckman Coulter); anti-CD3-PECy5, anti-Ki67-PE, anti-human CD184 (CXCR4)-allophycocyanin (BD Pharmingen); anti-CD69-PE (Immunotech), anti-Lck-PE (Santa Cruz Biotechnology), anti-phospho Src Family (Tyr416), anti-pCD79a (Tyr182), anti-pAKT (Ser473)–Alexa Fluor 647, Phospho-p44/42 MAPK (Erk1/2)(Thr202/Tyr204)–Alexa Fluor 467 (Cell Signaling Technology). The goat anti-rabbit immunoglobulin G (IgG) Alexa Fluor 647 secondary antibody, mouse IgG2a isotype control–allophycocyanin, and mouse IgG1 isotype control–PE were obtained from Thermo Fischer Scientific. For BCR triggering, cells were incubated for 10 minutes at 37°C with 10 μg/mL F(ab′)2 anti-human IgM/IgG (eBioscience). The selective Lck inhibitors [4-amino-5-(4-phenoxyphenyl)-7H-pyrrolo[3,2-d]pyrimidin-7-yl-cyclopentane] Lck-i (Calbiochem) and Α770041 (Axon Medchem) were used at 1 μM and 5 μM, respectively. Data were acquired on a FACSCalibur (BD Biosciences), analyzed with Flowjo (Tree Star), and compared with the use of GraphPad Prism software by applying appropriate statistical tests as specified.
Results and discussion
Ectopic Lck protein expression was frequent and highly heterogeneous among newly diagnosed CLL patients (n = 43), in line with previous reports.9-13 Nevertheless, Lck expression followed regular patterns that could be categorized in 3 representative groups (Figure 1A). Group A includes patients (16%) with no Lck (Lck−) found in their CLL cells; group B covers cases (26%) where Lck expression follows a drifting tendency toward levels found in the corresponding T cells (Lcklow). Group C includes patients (58%) displaying an unexpected profile: specifically, the CLL cell compartment could clearly be separated in 2 distinct subpopulations based on either low or no Lck expression (Lck−/low) or remarkably high levels of expression, exceeding those found in the corresponding T cells (Lckhi subpopulation) (Figure 1A; supplemental Figure 1A). Surface expression of CD19 and CD5 was similar between the distinct Lckhi and Lck−/low subpopulations (supplemental Figure 1B). The frequency of these distinct Lckhi subsets ranged between 1.2% and 35.7% (median, 7.9%) of CLL cells and their presence has possibly escaped previous studies reporting ectopic Lck expression and activity in CLL due to the experimental approaches used, that is, messenger RNA or western blot analysis of whole cell lysates.9-13 The impact of ectopic Lck expression on the global levels of SFK activity in our samples was evaluated by staining with the anti-pY416 antibody, which recognizes the active form of all SFKs, including Lck17 and was found variable among CLL samples. In line with increased Lck protein expression, the Lckhi subsets contained significantly elevated overall SFK activity compared with Lck−/low subsets or T cells, insinuating that the latter is the result of the former (Figure 1B).
Profiling of ectopic Lck expression patterns in CLL. (A) Interpatient and intratumoral heterogeneity of CLL according to ectopic Lck expression. PBMCs from HDs (n = 15) and from CLL patients (n = 43) were stained with anti-Lck, anti-CD19, and anti-CD3. The gating strategy and additional CD19/CD5 double staining is shown in supplemental Figure 1A. The lymphocyte populations gated to exclusively include CD3+ (representing the T-cell population) and CD19+ (normal B cells or CLL cells) cells are presented as 2-dimensional (2D) fluorescence-activated cell sorting (FACS) plots of Lck vs CD3 staining, to enable a visual comparison of Lck expression between the B/CLL cells (CD19+/CD3−) and T cells (CD3+). CLL cells were gated as Lck−, Lcklow, Lck−/low, and Lckhi relative to the Lck expression in corresponding T cells and used to classify samples in 3 groups A, B, and C (Gp.A, Gp.B, and Gp.C), as indicated. B cells and T cells from HDs were used as negative and positive controls for anti-Lck staining, respectively. Representative FACS plots are shown. (B) Lckhi subsets contain elevated SFK activity and constitutive pERK. PBMCs from HDs and CLL patients were stained with CD3, CD19, Lck, and either pY416 (which measures global SFK activity) or pERK. Graphs display the geometrical mean fluorescence intensity values for Lck, pY416, and pERK staining within gated cell populations as defined in panel A. Data points from individual samples and medians (horizontal lines) are shown. The Mann-Whitney U test was used for comparisons between samples (solid lines) and the Wilcoxon matched-pair test between populations within the same sample (dotted lines). Relevant comparisons are shown. *P < .05; ***P < .0005. CD19 and CD5 expression levels in the different CLL subsets are shown in supplemental Figure 1B. (C) Lck inhibition diminishes constitutive ERK phosphorylation in Lckhi subpopulations. Cells from group C CLL patients were either left untreated or treated with the specific Lck inhibitors Lck-i and A770041. Graphs show the parallel reduction in SFK activity (anti-pY416 staining) and pERK levels (anti-pERK staining) in the Lckhi subset and corresponding T cells after treatment, as indicated (n = 6; Student t test; mean ± standard error of the mean [SEM]; *P < .05; **P < .005). ns, not significant.
Profiling of ectopic Lck expression patterns in CLL. (A) Interpatient and intratumoral heterogeneity of CLL according to ectopic Lck expression. PBMCs from HDs (n = 15) and from CLL patients (n = 43) were stained with anti-Lck, anti-CD19, and anti-CD3. The gating strategy and additional CD19/CD5 double staining is shown in supplemental Figure 1A. The lymphocyte populations gated to exclusively include CD3+ (representing the T-cell population) and CD19+ (normal B cells or CLL cells) cells are presented as 2-dimensional (2D) fluorescence-activated cell sorting (FACS) plots of Lck vs CD3 staining, to enable a visual comparison of Lck expression between the B/CLL cells (CD19+/CD3−) and T cells (CD3+). CLL cells were gated as Lck−, Lcklow, Lck−/low, and Lckhi relative to the Lck expression in corresponding T cells and used to classify samples in 3 groups A, B, and C (Gp.A, Gp.B, and Gp.C), as indicated. B cells and T cells from HDs were used as negative and positive controls for anti-Lck staining, respectively. Representative FACS plots are shown. (B) Lckhi subsets contain elevated SFK activity and constitutive pERK. PBMCs from HDs and CLL patients were stained with CD3, CD19, Lck, and either pY416 (which measures global SFK activity) or pERK. Graphs display the geometrical mean fluorescence intensity values for Lck, pY416, and pERK staining within gated cell populations as defined in panel A. Data points from individual samples and medians (horizontal lines) are shown. The Mann-Whitney U test was used for comparisons between samples (solid lines) and the Wilcoxon matched-pair test between populations within the same sample (dotted lines). Relevant comparisons are shown. *P < .05; ***P < .0005. CD19 and CD5 expression levels in the different CLL subsets are shown in supplemental Figure 1B. (C) Lck inhibition diminishes constitutive ERK phosphorylation in Lckhi subpopulations. Cells from group C CLL patients were either left untreated or treated with the specific Lck inhibitors Lck-i and A770041. Graphs show the parallel reduction in SFK activity (anti-pY416 staining) and pERK levels (anti-pERK staining) in the Lckhi subset and corresponding T cells after treatment, as indicated (n = 6; Student t test; mean ± standard error of the mean [SEM]; *P < .05; **P < .005). ns, not significant.
Our previous work demonstrated that Lck is constitutively active within resting T cells and this preactivated pool is both necessary and sufficient for T-cell receptor–induced triggering of signaling cascades.17 On the other hand, Lck overexpression can cause abnormal signaling in the absence of TCR engagement.18-19 Concordant with this concept, constitutive pERK was exclusively found in the Lckhi subpopulations for all group C patients tested (Figure 1B). Lck−/low cells from group C and groups A and B patients or corresponding T cells did not contain pERK, despite elevated overall SFK activity in certain cases (Figure 1B). Furthermore, treatment with 2 selective Lck inhibitors at concentrations that are not affecting other SFKs11,20 resulted in a reduction of basal pERK levels in Lckhi subsets (Figure 1C). These data couple constitutive pERK to high levels of ectopic Lck expression and imply that elevated SFK activity in the absence of Lck does not suffice to induce constitutive phosphorylation of ERK. In line with this, Muzio et al7 showed that elevated pERK at steady state could not be linked to hyperactive Lyn, the major B-cell SFK. Basal pERK has been previously reported in ∼50% of CLL cases,7 however, the existence of intrasample variability and its correlation with ectopic Lck, as reported here, has never been appreciated.
In addition to constitutively activated ERK (pERK) and in contrast to the Lck−/low subpopulations and corresponding T cells, the Lckhi subsets also contained elevated basal phosphorylation of the BCR-ITAMs (pCD79a) and AKT (Figure 2A). In vitro stimulation of group C cells with anti-IgM/IgG induced activation of pCD79a, pERK, and pAKT only in the Lck−/low cells, indicating a BCR-dependent response in this subset, but failed to further increase phosphorylation of the aforementioned signaling molecules in the Lckhi cells. This failure of the Lckhi subset to respond to in vitro BCR stimulation may in part be explained by the lower expression levels of the BCR complex (CD79a subunit) found in the Lckhi cells compared with the corresponding LcK−/low in some samples (supplemental Figure 1C). Although unresponsiveness to surface immunoglobulin ligation, BCR downregulation, constitutive pERK and Lck expression (in a CLL mouse model) have been characterized as features of an anergy-related profile,7-8,12 we found that the Lckhi subsets invariably correlated with coexpression of activation markers CD69 and CD38 (an indicator of poor prognosis in CLL) and with the nuclear protein Ki-67, which is routinely used to characterize actively proliferating cell subsets21-23 (Figure 2B; supplemental Figure 1D-E). Moreover, anergic CLL cells have been characterized by the absence of constitutive pAKT,7 which is not the case for Lckhi cells (Figure 2A). Thus, elevated basal activation of the BCR pathway in Lckhi subsets does not result in a lethargic cellular state, but rather in positive signaling with upregulation of surface activation markers and enhanced proliferation. Further studies are needed to determine whether there is a functional link between Lck expression and induction of proliferating phenotype. Recently, Märklin et al12 using a mouse CLL model associated Ki67+ expression with ablation of NFAT2, a transcription factor which regulates several genes including Lck.
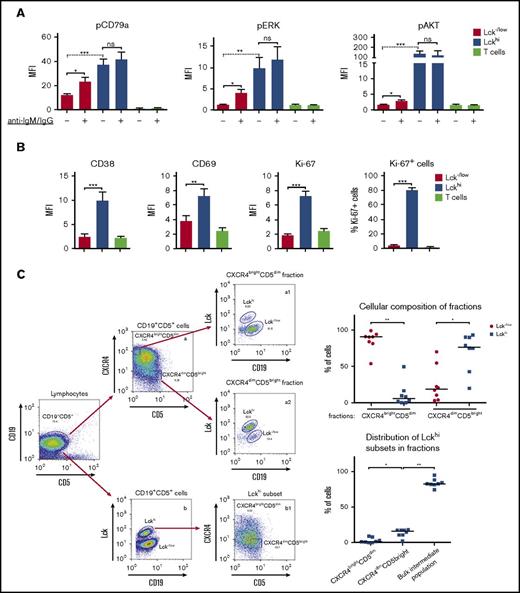
Identification of cellular and signaling signatures of the Lckhi subpopulations. (A) BCR-signaling profiles differ between Lckhi and Lck−/low subpopulations. Cells from group C CLL patients were left unstimulated or triggered with anti-human IgM/IgG. Graphs show pCD79a, pERK, and pAKT levels at steady state (−) and after BCR triggering (+) among Lck−/low, Lckhi, and corresponding T cells, as indicated. The Wilcoxon matched-pair test (dotted line) was used for comparisons between unstimulated samples and the Student t test (solid line) for comparison of responses to BCR crosslinking (n = 8; mean ± SEM; *P < .05; **P < .005; ***P < .0005). Lck−/low cells from group A and B CLL patients respond to BCR crosslinking in a similar fashion as the Lck−/low subpopulation from group C, as shown in supplemental Figure 1C. CD79a total protein expression in different subsets is also shown in supplemental Figure 1C. (B) Lckhi subsets display a hyperactivated phenotype. PBMCs from group C CLL patients were stained with CD3, CD19, pERK and each of Lck, CD38, CD69, and Ki-67. Graphs display the expression levels of CD38, CD69, and Ki-67 in the Lckhi, Lck−/low subsets and T cells as indicated. The right graph shows the percentage of Ki-67+ cells within the Lckhi and Lck−/low subpopulations (n = 11; Student t test; mean ± SEM; **P < .01; ***P < .001). Supplemental Figure 1D-E shows a detailed analysis of a representative experiment. (C) Evaluation of the presence of Lckhi subsets within proliferative (CXCR4dim/CD5bright) and resting (CXCR4bright/CD5dim) CLL fractions. Cells from group C patients were stained for CD19, CD5, CXCR4, and Lck. The CD19+CD5+ populations were analyzed in 2D FACS plots of CXCR4 vs CD5 (a) and of Lck vs CD19 (b). Top rows, The CXCR4dim/CD5bright and CXCR4bright/CD5dim fractions were defined in (a) as 5% of the cells lying on the 2 extremes of the plots, as previously described.24 Each fraction shown as a 2D FACS plot of Lck vs CD19 was used to calculate the relative proportions of Lck−/low and Lckhi subpopulations within the CXCR4bright/CD5dim (a1) and CXCR4dim/CD5bright (a2) compartments, respectively. The graph on the right shows collective data. Bottom row, The Lckhi subset from (b) is represented in a 2D FACS plot of CXCR4 vs CD5 (b1). The gates demarcating the CXCR4bright/CD5dim and CXCR4dim/CD5bright fractions from (a) were applied to (b1) to reveal the distribution of Lckhi cells within the respective fractions and the dominant intermediate population. The graph on the right shows collective data (n = 8; Wilcoxon matched-pair test; *P < .05; **P < .005).
Identification of cellular and signaling signatures of the Lckhi subpopulations. (A) BCR-signaling profiles differ between Lckhi and Lck−/low subpopulations. Cells from group C CLL patients were left unstimulated or triggered with anti-human IgM/IgG. Graphs show pCD79a, pERK, and pAKT levels at steady state (−) and after BCR triggering (+) among Lck−/low, Lckhi, and corresponding T cells, as indicated. The Wilcoxon matched-pair test (dotted line) was used for comparisons between unstimulated samples and the Student t test (solid line) for comparison of responses to BCR crosslinking (n = 8; mean ± SEM; *P < .05; **P < .005; ***P < .0005). Lck−/low cells from group A and B CLL patients respond to BCR crosslinking in a similar fashion as the Lck−/low subpopulation from group C, as shown in supplemental Figure 1C. CD79a total protein expression in different subsets is also shown in supplemental Figure 1C. (B) Lckhi subsets display a hyperactivated phenotype. PBMCs from group C CLL patients were stained with CD3, CD19, pERK and each of Lck, CD38, CD69, and Ki-67. Graphs display the expression levels of CD38, CD69, and Ki-67 in the Lckhi, Lck−/low subsets and T cells as indicated. The right graph shows the percentage of Ki-67+ cells within the Lckhi and Lck−/low subpopulations (n = 11; Student t test; mean ± SEM; **P < .01; ***P < .001). Supplemental Figure 1D-E shows a detailed analysis of a representative experiment. (C) Evaluation of the presence of Lckhi subsets within proliferative (CXCR4dim/CD5bright) and resting (CXCR4bright/CD5dim) CLL fractions. Cells from group C patients were stained for CD19, CD5, CXCR4, and Lck. The CD19+CD5+ populations were analyzed in 2D FACS plots of CXCR4 vs CD5 (a) and of Lck vs CD19 (b). Top rows, The CXCR4dim/CD5bright and CXCR4bright/CD5dim fractions were defined in (a) as 5% of the cells lying on the 2 extremes of the plots, as previously described.24 Each fraction shown as a 2D FACS plot of Lck vs CD19 was used to calculate the relative proportions of Lck−/low and Lckhi subpopulations within the CXCR4bright/CD5dim (a1) and CXCR4dim/CD5bright (a2) compartments, respectively. The graph on the right shows collective data. Bottom row, The Lckhi subset from (b) is represented in a 2D FACS plot of CXCR4 vs CD5 (b1). The gates demarcating the CXCR4bright/CD5dim and CXCR4dim/CD5bright fractions from (a) were applied to (b1) to reveal the distribution of Lckhi cells within the respective fractions and the dominant intermediate population. The graph on the right shows collective data (n = 8; Wilcoxon matched-pair test; *P < .05; **P < .005).
Calissano et al24 have described highly proliferating fractions of CLL cells considered to have recently left the lymphoid tissue, which are phenotypically recognized as CXCR4dim/CD5bright and are enriched, as are the Lckhi subsets, for CD38 and Ki-67 expression. Our analysis revealed that these fractions contained a significantly higher proportion of Lckhi than Lck−/low cells (Figure 2C), implying that Lckhi cells may be early lymph node emigrants. However, evaluation of the cellular distribution of Lckhi subsets revealed that only a small proportion resided in the CXCR4dim/CD5bright fraction, with the vast majority of cells belonging to the dominant intermediate population (Figure 2C). Thus, at this stage, we cannot determine whether the Lckhi cells are indeed early lymph node emigrants. If that is the case, the role of ectopic Lck could be to intensify BCR responses arising from the lymphoid tissue microenvironment. Under this scenario, the fate of Lckhi cells after prolonged residency in the circulation could take 2 directions, they either convert to Lck−/low or perish. Another hypothesis is that Lckhi subsets are not part of a continuum of CLL fractions but they constitute distinct clones. In this case, aberrant Lck may serve as a trigger for autonomous BCR signaling, as insinuated by the constitutive phosphorylation of signaling mediators in the Lckhi subpopulations. Detailed genetic and gene expression analyses, ideally performed in parallel blood and lymph node samples, are needed to define whether the Lckhi subsets arise from the same cell population under the influence of the microenvironment or indeed constitute discrete subclones with unique behavior. Though a recent study could not find any correlation between overall ectopic Lck and disease outcome,13 Lck expression in CLL has been linked with glucocorticoid sensitivity9 and response to chemotherapy.13 Thus, the Lckhi subclones with the distinct BCR-signaling profiles described here may indeed, in follow-up longitudinal studies, prove to have an impact in disease progression and resistance to the BCR-signaling inhibitors clinically used today.
In conclusion, with this study, we extend our understanding of the molecular features that shape interpatient and intratumoral heterogeneity in CLL. This stimulus report provides evidence that CLL is composed of cellular subsets with distinct BCR-signaling and activation profiles, and these differences correlate with the presence or absence of the T-cell kinase Lck. Finally, we showed that Lck inhibition inactivates pathways downstream of the BCR and therefore may be an attractive therapeutic option for CLL in the future.
The full-text version of this article contains a data supplement.
Acknowledgments
This work was supported by grants from the Asklepios Program (Gilead Science Hellas; K.N. and A.S.) and from the “K. Karatheodori” Program (University of Patras; K.N.).
Authorship
Contribution: E.T. and S.A. performed experiments; C.A. and G.F. performed routine diagnostic fluorescence-activated cell sorting; P.C., P.Z., and A.A. contributed to data acquisition and analysis; and K.N. and A.S. conceived and designed the study, supervised the experiments, analyzed data, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Konstantina Nika, Department of Biochemistry, School of Medicine, University of Patras, Panepistimioupoli, 26504 Rio, Greece; e-mail: knika@upatras.gr; and Alexandros Spyridonidis, BMT Unit, University Hospital of Patras, 5th Floor, 26504 Rio, Greece; e-mail: spyridonidis@upatras.gr.
References
Author notes
K.N. and A.S. contributed equally to this study.

![Figure 1. Profiling of ectopic Lck expression patterns in CLL. (A) Interpatient and intratumoral heterogeneity of CLL according to ectopic Lck expression. PBMCs from HDs (n = 15) and from CLL patients (n = 43) were stained with anti-Lck, anti-CD19, and anti-CD3. The gating strategy and additional CD19/CD5 double staining is shown in supplemental Figure 1A. The lymphocyte populations gated to exclusively include CD3+ (representing the T-cell population) and CD19+ (normal B cells or CLL cells) cells are presented as 2-dimensional (2D) fluorescence-activated cell sorting (FACS) plots of Lck vs CD3 staining, to enable a visual comparison of Lck expression between the B/CLL cells (CD19+/CD3−) and T cells (CD3+). CLL cells were gated as Lck−, Lcklow, Lck−/low, and Lckhi relative to the Lck expression in corresponding T cells and used to classify samples in 3 groups A, B, and C (Gp.A, Gp.B, and Gp.C), as indicated. B cells and T cells from HDs were used as negative and positive controls for anti-Lck staining, respectively. Representative FACS plots are shown. (B) Lckhi subsets contain elevated SFK activity and constitutive pERK. PBMCs from HDs and CLL patients were stained with CD3, CD19, Lck, and either pY416 (which measures global SFK activity) or pERK. Graphs display the geometrical mean fluorescence intensity values for Lck, pY416, and pERK staining within gated cell populations as defined in panel A. Data points from individual samples and medians (horizontal lines) are shown. The Mann-Whitney U test was used for comparisons between samples (solid lines) and the Wilcoxon matched-pair test between populations within the same sample (dotted lines). Relevant comparisons are shown. *P < .05; ***P < .0005. CD19 and CD5 expression levels in the different CLL subsets are shown in supplemental Figure 1B. (C) Lck inhibition diminishes constitutive ERK phosphorylation in Lckhi subpopulations. Cells from group C CLL patients were either left untreated or treated with the specific Lck inhibitors Lck-i and A770041. Graphs show the parallel reduction in SFK activity (anti-pY416 staining) and pERK levels (anti-pERK staining) in the Lckhi subset and corresponding T cells after treatment, as indicated (n = 6; Student t test; mean ± standard error of the mean [SEM]; *P < .05; **P < .005). ns, not significant.](https://ash.silverchair-cdn.com/ash/content_public/journal/bloodadvances/2/8/10.1182_bloodadvances.2017015321/3/m_advances015321f1.jpeg?Expires=1767921641&Signature=3LfNoadoH0sAG9I3jGtPWIoi66NJaq13VlH3hcuTAyvfUe-lRUwNFhrbXoF8J7EUfbX-ymcMOU33XxAG9Wk2C5rdfducWrHkM8sMXRJ9hJWcvG5ieruBMjoAZh9BUE5EcmEprzYPmBtBX27GyPEl8ezPYUYOFYaUtUo3npp68akOBsY0uH-RUbCrmhJ4yRKjkc1G13~XftddJaRc~UqYbkqiROGL~5qqSq9mP6FfUXSqqyFkjDRzgefYYEV42NmYFjujKNY6Z5qtoZ-zMP32htRE9MkKp0fSpj2eTQ6VhR-c1zvZGXoTClt0jDXamRmJbZhTKsK09fnUgH2yY~Y~dA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)