Key Points
Somatic GATA2 mutation is associated with immunodeficiency and pulmonary alveolar proteinosis in a patient with myeloproliferative neoplasm.
Introduction
GATA2 is a zinc-finger transcription factor integral to hemopoietic stem cell regulation, mononuclear development, and alveolar macrophage activity.1 Heterozygous germ line GATA2 mutations are associated with mycobacterial infection, monocytopenia, and deficiency of B/natural killer cells, and pulmonary alveolar proteinosis (PAP). GATA2 mutations confer a 90% lifetime risk of developing myelodysplastic syndrome (MDS)/acute myeloid leukemia (AML) with mutations detected in 7% to 15% of MDS in young adults and children.2-3 GATA2 mutations can be present in germ line or arise somatically, and, in either case, heterozygous mutations can prove clinically consequential. The clinical features of GATA2 mutation are largely known through studies of germ line mutation, which may be inherited or arise de novo.2-6 Somatic mutations have been reported as drivers of myeloid neoplasia, including myeloproliferative neoplasms (MPN), and are well described in leukemia arising in the context of bi-allelic CEBPA mutation.7 However, features such as mononuclear cytopenia, immunodeficiency, and pulmonary alveolar proteinosis (PAP) have not been reported.
PAP is a rare syndrome characterized by the abnormal accumulation of surfactant in the alveoli and terminal airways resulting in respiratory failure.8 Neutralizing anti–granulocyte macrophage colony-stimulating factor (anti–GM-CSF) antibodies are detected in the majority of patients with primary PAP. Secondary PAP (sPAP), which is associated with hematologic malignancies (chiefly MDS/AML), results from intrinsic dysregulation of alveolar macrophages.9-10 Median survival is <50% at 2 years, and mortality is mainly due to PAP itself. Twenty-five percent of sPAP patients have germ line GATA2 mutations, and one-fifth of germ line GATA2-mutated individuals develop PAP.1,3,9-10
We report the first case of a patient with proven acquired/somatic GATA2 mutation who developed typical immunodeficiency features of germ line GATA2 mutation, including monocytopenia, probable mycobacterial infection, and PAP, on a background of MPN/MDS.
All data and samples were collected after informed consent in accordance with the Declaration of Helsinki.
Case description
A 58-year-old woman was investigated for incidental detection of thrombocytosis in 2006. Blood results showed hemoglobin 111 g/L, white blood cell count 9.6 × 109/L, platelets 727 × 109/L, and mean corpuscular volume 103.1 fL. A provisional diagnosis of essential thrombocythemia or MDS was made after exclusion of secondary thrombocytosis. In 2009, she underwent a bone marrow (BM) biopsy, and a diagnosis of MPN/MDS was confirmed on the basis of hypercellular marrow, dyserythropoiesis, and grade 2/4 reticulin. She had normal hearing, no evidence of human papillomavirus papillomatosis, hypogammaglobulinemia, or lymphedema and remained well until 2012.
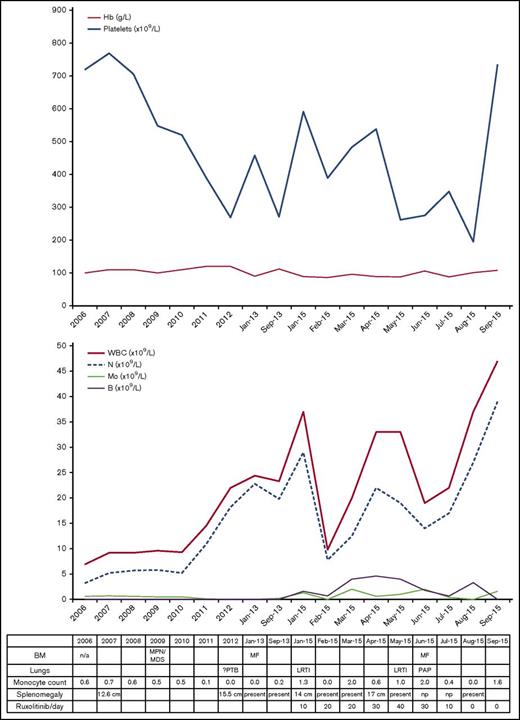
In December 2012, she developed night sweats and weight loss with persistent respiratory symptoms and radiologic findings consistent with mycobacterial disease. She was refractory to standard antibiotics, and although bronchoalveolar lavage (BAL) was smear and culture negative, empirical antimycobacterial therapy led to clinical and radiographic resolution. At this time, she developed a leukoerythroblastic blood film with circulating blasts and intermittent monocytopenia (Figure 1). She lacked mutations of JAK2, CALR, and MPL genes and in 2013 was diagnosed with secondary myelofibrosis based on BM biopsy findings.
Blood and BM investigations and clinical course (2006-2015). Progress of hemoglobin (Hb) and platelet count (top). Progress of white blood cell count (WBC), neutrophils (N), monocytes (Mo), and blasts (B) in the blood (middle). Table listing diagnostic details, absolute monocyte counts, and clinical events (bottom). LRTI, lower respiratory tract infection; MF, myelofibrosis.
Blood and BM investigations and clinical course (2006-2015). Progress of hemoglobin (Hb) and platelet count (top). Progress of white blood cell count (WBC), neutrophils (N), monocytes (Mo), and blasts (B) in the blood (middle). Table listing diagnostic details, absolute monocyte counts, and clinical events (bottom). LRTI, lower respiratory tract infection; MF, myelofibrosis.
In October 2014, she had new chest infiltrates associated with respiratory symptoms, responding to antibiotics. In January 2015, she developed worsening fatigue, splenomegaly, and blood counts showing monocytopenia. Allogeneic stem cell transplant (SCT) was discussed and ruxolitinib commenced. Within 1 month, her symptoms and blood counts improved. Over the next 2 months, the dose of ruxolitinib was increased; her blood counts fluctuated, but splenomegaly resolved in June 2015 (Figure 1).
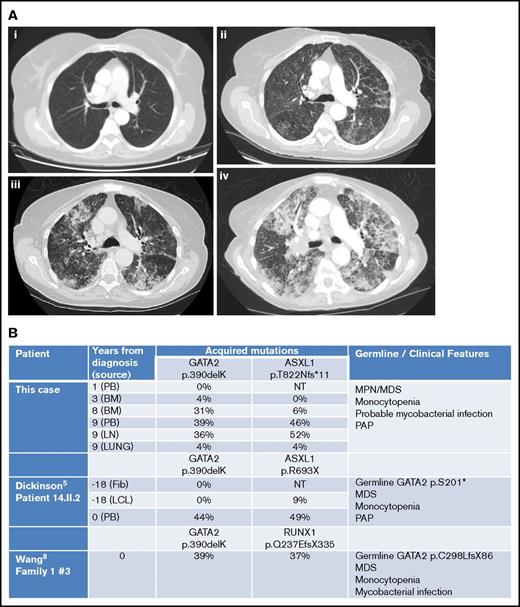
However, by then, she reported weight loss, worsening cough with breathlessness, and dystrophic nails consistent with fungal infection. Chest computed tomography revealed progressive changes with interstitial thickening and air space shadowing throughout both lungs, plus small volume necrotic mediastinal lymphadenopathy (Figure 2A). Sputum, BAL, and endobronchial ultrasound biopsies of her central lymphadenopathy were culture negative; lymph node histology showed acute necrotic changes. BAL contained branching hyphae consistent with Aspergillus species. BAL galactomannan was positive at 1.58, and voriconazole was commenced.
Imaging and molecular investigations. (A) Serial computed tomography chest images (2007-2015). (i) June 2007: no respiratory symptoms. Normal imaging. (ii) January 2015: no respiratory symptoms but evidence of progressive MPN/MDS. Early interstitial changes are present. (iii) June 2015: cough and breathlessness. Progressive interstitial changes plus air space shadowing are present. (iv) September 2015: respiratory failure secondary to PAP. “Crazy paving” changes plus consolidation characteristic of PAP visible. (B) Comparison of somatically acquired c.1168_1170delAAG p.390delK GATA2 mutations. Three patients have been reported with somatically acquired p.390delK mutation in GATA2. Germ line mutations in GATA2 and other cooperating mutations in ASXL1 or RUNX1 were also present in two previous cases. Fib, fibroblast; LCL, lymphoid cell line; LN, lymph node; NT, not tested; PB, peripheral blood.
Imaging and molecular investigations. (A) Serial computed tomography chest images (2007-2015). (i) June 2007: no respiratory symptoms. Normal imaging. (ii) January 2015: no respiratory symptoms but evidence of progressive MPN/MDS. Early interstitial changes are present. (iii) June 2015: cough and breathlessness. Progressive interstitial changes plus air space shadowing are present. (iv) September 2015: respiratory failure secondary to PAP. “Crazy paving” changes plus consolidation characteristic of PAP visible. (B) Comparison of somatically acquired c.1168_1170delAAG p.390delK GATA2 mutations. Three patients have been reported with somatically acquired p.390delK mutation in GATA2. Germ line mutations in GATA2 and other cooperating mutations in ASXL1 or RUNX1 were also present in two previous cases. Fib, fibroblast; LCL, lymphoid cell line; LN, lymph node; NT, not tested; PB, peripheral blood.
Repeat BM biopsy showed myelofibrosis with hypolobated nuclei and 5% to 10% blasts. Hydroxycarbamide was substituted for ruxolitinib; her pulmonary symptoms worsened despite anti-infective treatment, and a thoracoscopic surgical biopsy of the lung confirmed a diagnosis of PAP. Anti–GM-CSF antibodies were negative. Her symptoms and radiology progressed in line with PAP, and she died in September 2015 of respiratory failure.
She was investigated for the presence of GATA2 mutation. BM DNA was extracted from paraffin blocks using the QIAamp DNA FFPE Tissue Kit (56404; QIAGEN) according to the manufacturer’s instructions. Using Sanger sequencing, the patient was found to harbor a mutation in exon 8 of GATA2 (c.1168_1170delAAG; p.390delK) and a mutation of ASXL1 (c.2464_2465insA; p.T822Nfs*11). The GATA2 mutation was predicted to be disease-causing using the MutationTaster prediction program6 (Mutation Surveyor software version 4.0.8). On retrospective analysis of her previous DNA samples and estimation of variant allele frequency from electrophoretograms, this GATA2 mutation was not detected in 2006, increased in abundance from 2009 to 2015, and was only found at 4% in lung tissue in 2015, suggesting that it was an acquired somatic mutation (Figure 2B; supplemental Figures). ASXL1 mutation was observed emerging at later time points, probably as a subclone.
Results and discussion
In summary, we present details of an adult with MPN/MDS evolving to myelofibrosis over 7 years. She acquired a somatic GATA2 mutation 3 years after initial diagnosis. Three years later, she developed possible mycobacterial infection and myelofibrosis. For a further 2 years, she had intermittent respiratory symptoms with progressive pulmonary infiltrates from 2014 onwards, and PAP was eventually diagnosed. The presence of PAP with negative GM-CSF antibodies led to the identification of GATA2 mutation in our patient.
Somatic mutations in GATA2 occur rarely in MPN and other myeloid neoplasms,9,11-13 with the exception of MDS/AML associated with biallelic CEBPA mutations.7 Our patient had a trinucleotide deletion c.1168_1170delAAG; p390delK, identical to that previously reported as a second somatic mutation in 2 other patients with coexisting germ line GATA2 mutations (Figure 2B). In our previous patient (14.II.2),5 p390delK was confirmed as somatic by serial sampling and sequencing of fibroblast and LCL DNA. In the other reported patient, p390delK was proven to be biallelic with the germ line GATA2 mutation by TA-cloning.12 In all three, cooperating ASXL1 or RUNX1 mutations were observed at similar variant allele frequency to the somatic GATA2 variant. The present case remains unique in that an isolated somatic GATA2 mutation was responsible for the GATA2-related disease.
Discrete episodes of pulmonary symptoms occurred from 3 years after first detection of her GATA2 mutation, around the time of transformation to myelofibrosis. The initial episode was consistent with probable mycobacterial disease and resolved with specific treatment of this. In June 2015, she developed possible Aspergillus lung infection, though we believe that PAP was responsible for her death. The pulmonary episodes through 2014 may have represented the early stages of PAP. GATA2-associated PAP has a poor prognosis9 but can be successfully treated with SCT.10,14-15 A multidisciplinary approach by hematologists, pulmonologists, and radiologists is recommended to achieve an early diagnosis of sPAP, definitive treatment, and improved outcomes.
The origin of alveolar/tissue macrophages and the relative contribution of yolk sac versus hemopoietic stem cells in the development and maintenance of alveolar macrophages10 is unclear in humans. Certainly, alveolar macrophages persist after many years of monocytopenia in GATA2 mutation.16 Although this suggests that they can be self-maintaining in the absence of inflammation, their dysfunction in the case of somatic GATA2 mutation and the successful response of PAP to SCT14-15 both indicate that alveolar macrophages can arise from hemopoietic stem cells if required. Our patient with PAP associated with a somatic mutation demonstrates that somatic mutation in myeloid progenitors can lead to disruption of tissue/alveolar macrophage function.
To conclude, the combination of monocytopenia, probable mycobacterial infection, and PAP in the context of myeloid malignancy is well documented in germ line GATA2 mutation,2-6 but in our patient, these conditions are united by a somatic mutation. To our knowledge, this is the first report of somatic GATA2 mutation in a myeloid malignancy leading to fatal PAP. The case suggests that the immune and hematologic consequences of the somatic mutation can closely resemble those observed with the germ line mutation.
The full-text version of this article contains a data supplement.
Acknowledgments
Polymerase chain reaction and Sanger sequencing of BM and blood DNA was performed by the NHS Northern Molecular Genetics Service and Source Bioscience (https://www.sourcebioscience.com/services/genomics/sanger-sequencing-service/). Lung tissue for analysis was kindly provided by Martin Young, Consultant Histopathologist and Cytopathologist, Royal Free London NHS Trust.
Authorship
Contribution: M.S. was involved in the care of the patient, collected and analyzed data, and wrote the paper; D.L. was involved in the care of the patient and analysis of the data and contributed to the writing of the paper; R.P. collected the data and wrote the initial draft of the paper; C.M. was involved in the care of the patient and procurement of samples; T.M. reviewed all bone marrow histology; R.D. undertook all molecular genetic analysis of samples; M.C. was involved in planning and interpretation of molecular analysis, interpretation, discussion of clinical and laboratory data and writing of the paper; and M.L. was involved in the care of the patient, collection and analysis of data and writing the paper.
Conflict-of-interest disclosure: M.S. has received research funding and honoraria from Novartis. The remaining authors declare no competing financial interests.
Correspondence: Mallika Sekhar, Department of Haematology, Royal Free London NHS Trust, Pond St, London NW3 2QG, United Kingdom; e-mail: mallika.sekhar@nhs.net.
References
Author notes
M.C. and M.L. contributed equally to this study.