Key Points
Pacritinib resulted in superior spleen reduction in absent or low JAK2V617F allele burden MF compared with BAT.
Symptom responses were also superior in low allele burden MF treated with pacritinib.
Abstract
Myelofibrosis (MF) has heterogeneous clinical manifestations, with some patients exhibiting a myelodepletive phenotype characterized by cytopenias and an absent or low JAK2V617F allele burden. Ruxolitinib may be less effective in these patients. We assessed the efficacy of pacritinib, a JAK2/IRAK1 inhibitor, in MF patients with low JAK2V617F allele burden. In this post hoc analysis of the PERSIST-1 and -2 trials, patients with MF randomized to pacritinib or best available therapy (BAT) were stratified by JAK2V617F allele burden quartile for spleen response of ≥35% and improvement in total symptom score of ≥50%. Five hundred thirty-six patients were included. Patients with lower JAK2V617F allele burden had smaller baseline spleens and lower hemoglobin and platelet counts as compared with higher allele burden patients. Among pacritinib-treated patients, spleen responses were observed across all JAK2V617F allele burden quartiles and in JAK2V617F− disease. No spleen responses were observed among BAT-treated patients with allele burden ≤50% or JAK2V617F− disease. The intention-to-treat response rate was significantly higher on the pacritinib arm for JAK2V617F− disease (23.0% vs 0%; P = .033), and for the lowest allele burden quartiles (0%-25%: 20.9% vs 0%, P < .001; 25%-50%: 15.4% vs 0%, P = .020). There were significantly more symptom responders with pacritinib vs BAT in the 0% to 25% and 25% to 50% cohorts. Pacritinib treatment led to superior spleen and symptom burden reduction compared with BAT in patients with absent or low JAK2V617F allele burden, suggesting that pacritinib may be uniquely suited for patients with myelodepletive MF.
Introduction
Myelofibrosis (MF) is a Philadelphia chromosome–negative myeloproliferative neoplasm (MPN) characterized by splenomegaly, constitutional symptoms, bone marrow fibrosis, ineffective hematopoiesis, and resultant cytopenias. MF can either develop de novo, termed primary MF (PMF), or from an antecedent polycythemia vera (PV) or essential thrombocythemia (ET). The pathobiologic hallmark of MF is a constitutively active JAK-STAT signaling pathway, leading to production of proinflammatory cytokines.1 The majority of patients with MF harbor a driver mutation in JAK2, CALR, or MPL.2 In particular, a gain-of-function JAK2V617F is present in ∼60% of patients with PMF.3 Mutations in CALR and MPL similarly lead to constitutive activation of the JAK-STAT signaling pathway, which is central to MPN pathogenesis.4-6
In recent years, there has been increased understanding of the biologic and clinical heterogeneity of MF, which can manifest across a phenotypic spectrum from a myeloproliferative phenotype on one end to a myelodepletive phenotype on the other. Clinically, the myeloproliferative phenotype is characterized by leukocytosis, higher platelet counts, and less severe anemia. Additionally, patients often experience more significant splenomegaly and a greater burden of constitutional symptoms. In contrast, myelodepletive MF is characterized by significant pancytopenia, frequently requiring red blood cell and/or platelet transfusion support, and higher prevalence of primary rather than secondary MF.7 A hallmark of myelodepletive MF is thrombocytopenia, which is associated with poor prognosis, including shorter overall survival.8 A low JAK2V617F allele burden is a key characteristic of the myelodepletive phenotype. In a study of 127 patients with PMF stratified by JAK2V617F allele burden quartile, patients in the lower quartile had more prevalent anemia and leukopenia with less prevalent splenomegaly and constitutional symptoms compared with patients with higher JAK2V617F allele burden. This suggests that low JAK2V6176F allele burden is associated with clinical manifestations consistent with a myelodepletive phenotype. In addition, patients with a low allele burden had inferior overall and leukemia-free survival,9 a finding confirmed in a separate study of 129 patients.10
Ruxolitinib (Jakafi; Incyte) is a JAK1/JAK2 inhibitor that is US Food and Drug Administration (FDA) approved for the treatment of intermediate- or high-risk MF with a platelet count >50 × 109/L based on results of the COMFORT-1 and -2 trials showing improvement in splenomegaly, constitutional symptoms, and quality of life.11-13 However, ruxolitinib does not induce spleen and symptom responses equally across the disease spectrum. Patients with extreme thrombocytopenia are ineligible for ruxolitinib given expected drug-induced cytopenias.14 In addition, patients with low JAK2V617F allele burden have a poor response to ruxolitinib. This was demonstrated in an analysis by Barosi et al of 69 patients treated with ruxolitinib. Spleen response as assessed using the International Working Group Myeloproliferative Neoplasm Research and Treatment (IWG-MRT) criteria was 5.5-fold higher in patients with a JAK2V617F allele burden above 50% as compared with patients with an allele burden <50% or JAK2V617F− disease, even after controlling for other disease features and ruxolitinib dose.15 Therefore, although efficacious in patients with high allele burden, who are more likely to have a myeloproliferative phenotype, ruxolitinib may have a limited benefit in patients with the myelodepletive phenotype of MF.
Pacritinib is a JAK2 inhibitor that also has activity against interleukin-1 receptor-associated kinase 1 (IRAK1).16 The phase 3 PERSIST-1 and PERSIST-2 trials established pacritinib as an effective therapy for patients with MF. In the PERSIST-1 trial, patients with MF irrespective of baseline platelet count and without prior JAK inhibitor exposure were randomized 2:1 to pacritinib or best available therapy (BAT) excluding ruxolitinib. The primary outcome of spleen volume response (SVR) of at least 35% (SVR35%) was met in significantly more patients in the pacritinib group as compared with BAT (19% vs 5%).17 In PERSIST-2, patients with MF and baseline thrombocytopenia (<100 × 109/L) irrespective of prior ruxolitinib therapy were randomized 1:1:1 to pacritinib 400 mg daily, pacritinib 200 mg twice daily, or BAT including ruxolitinib. As in PERSIST-1, the SVR35% end point was achieved in significantly more patients in the pacritinib groups as compared with BAT (18% vs 3%).18 Both of the PERSIST studies demonstrated that pacritinib has clinical activity in patients with severe baseline thrombocytopenia, defined as a platelet count <50 × 109/L. In this subgroup of PERSIST-1, SVR35% occurred in 23% of patients who received pacritinib and 0% of patients who received BAT.17 Similarly, in severely thrombocytopenic patients in PERSIST-2, the SVR35% end point was achieved in 23% of patients who received pacritinib compared with 3% in the BAT group.18 However, the activity of pacritinib in patients based on baseline JAK2V617F allele burden, a biomarker hallmark of the myelodepletive phenotype, remains unknown.
To further assess the efficacy of pacritinib in the myelodepletive setting in which few treatment options exist, we performed a post hoc analysis of PERSIST-1 and PERSIST-2 stratified by baseline JAK2V617F allele burden.
Methods
Patients and study design
Detailed methods for PERSIST-1 and PERSIST-2 have been previously reported.17,18 Briefly, both PERSIST-1 (NCT01773187) and PERSIST-2 (NCT02055781) enrolled patients with primary MF, post-ET MF, or post-PV MF with Dynamic International Prognostic Scoring System (DIPSS) intermediate- or high-risk disease and palpable splenomegaly (≥5 cm below the left costal margin). In PERSIST-1, eligibility was not restricted on the basis of platelet count. In contrast, PERSIST-2 only included patients with baseline platelet counts <100 × 109/L. In addition, PERSIST-2 allowed 1 to 2 prior lines of JAK inhibitor therapy, whereas patients in PERSIST-1 were JAK inhibitor naive.17,18 In PERSIST-1, eligible patients were randomized 2:1 to pacritinib 400 mg once daily (n = 220) or BAT (n = 107) excluding JAK inhibitors. In PERSIST-2, eligible patients were randomized 1:1:1 to pacritinib 400 mg daily, pacritinib 200 mg twice daily, or BAT including ruxolitinib. In both studies, randomization was stratified by DIPSS risk category, platelet count, and geographical region.17,18
Patients were excluded from analysis if they had a missing or unknown baseline JAK2V617F allele burden data.
The study was approved by the institutional review boards at each participating institution in the PERSIST-1 and PERSIST-2 trials.
Evaluations
Baseline JAK2V617F allele burden was quantified by polymerase chain reaction (PCR) on peripheral blood mononuclear cells by central laboratory. Additional mutational data were not available, such as CALR and MPL. The efficacy end point was the percentage of patients achieving an SVR35% (by magnetic resonance imaging or computed tomography scan) at week 24 based on an intention-to-treat (ITT) analysis. In PERSIST-2, the ITT population included patients randomized prior to 7 September 2015. A secondary end point for this analysis was 50% or greater reduction in the 7-symptom version of the Myeloproliferative Neoplasm Symptom Assessment Form Total Symptom Score (MPN-SAF TSS) v2.0 from baseline to week 24. Adverse events were classified and graded according to National Cancer Institute Common Terminology Criteria for Adverse Events v4.03.
Statistical analysis
Baseline characteristics and outcomes were binned by JAK2V617F allele quartile. Comparison of continuous variables and ordinal variables was performed using the Jonckheere-Terpstra test and categorical variables were compared using the Cochran-Armitrage trend test. Confidence intervals of 95% were calculated for treatment differences based on the Agresti-Caffo method and comparisons for SVR35% and total symptom score (TSS) improvement were made using the Fisher's exact test.
Results
Baseline characteristics by allele burden
A total of 536 patients with MF were included in this analysis. Baseline characteristics stratified by JAK2V617F allele burden quartile are shown in Table 1. There was a significant trend toward older age in patients with higher allele burden, although this difference in median age was only 2 years between the lowest and highest quartile. Additionally, there were significant differences in sex distributions across JAK2V617F quartiles. There was no difference in race or performance status at baseline between the 4 quartiles.
Disease characteristics at baseline stratified by JAK2V617F allele burden are described in Table 2. Lower allele burden was associated with smaller spleen size based on both length (by palpation) and volume (by imaging), lower platelet counts, lower hemoglobin, and increased red blood cell transfusion dependence, and there was a trend for association with more hypocellular bone marrow. Taken together, these attributes are consistent with the association between lower allele burden and the myelodepletive phenotype of MF. In addition, low allele burden was associated with PMF, as patients with antecedent PV generally had allele burdens >50%, and on PERSIST-2 higher allele burden was associated with prior JAK inhibitor exposure.
Patients who were negative for JAK2V617F were phenotypically similar at baseline to patients in the lower allele burden quartiles. Specifically, JAK2V617F− patients had low baseline spleen volume, low platelet counts, low hemoglobin, prevalence of red blood cell transfusion dependence, and higher prevalence of PMF similar to patients in the lowest 2 JAK2V617F allele burden quartile. It is not known whether JAK2V617F− patients were triple negative or had an additional driver mutation as further somatic mutational analyses were not available for this cohort.
Response by allele burden
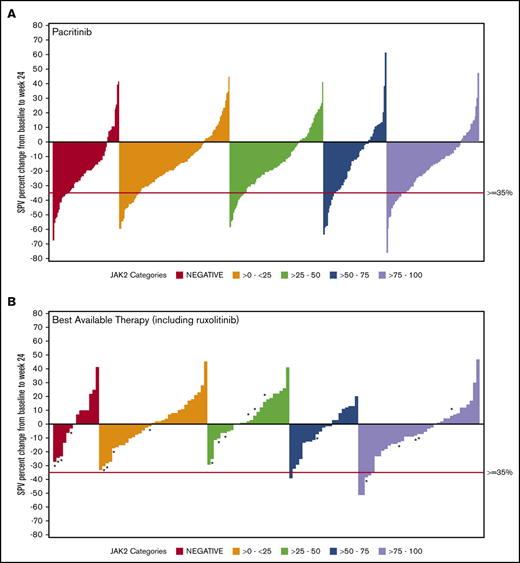
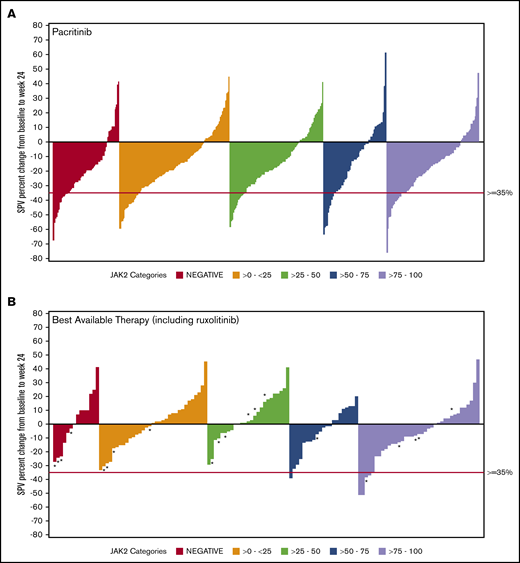
Data on SVR35% at week 24 were available for 369 patients in the combined pacritinib group and 179 in the BAT group (Table 3). Twenty evaluable patients in the BAT arm received ruxolitinib, which was dosed according to label instructions. Among pacritinib-treated patients, SVR35% response rates were similar in JAK2V617F− and JAK2V617F+ patients, as well as across JAK2V617F allele burden quartiles. In contrast, there were no responses observed among BAT-treated patients with allele burden <50% or in patients with JAK2V617F− disease, including those who were treated with ruxolitinib (Figure 1). In the JAK2V617F− cohort, significantly more patients attained an SVR35% in the pacritinib treatment group as compared with BAT (23.0% vs 0.0%; P = .033). Similarly, in the 0% to 25% and 25% to 50% JAK2V617F allele burden quartiles, pacritinib-treated patients were significantly more likely to achieve SVR35% compared with BAT (20.9% vs 0%, P ≤.001 and 15.4% vs 0.0%, P = .020, respectively). Notably, only 1 BAT patient who received ruxolitinib achieved an SVR35%, and this patient was in the >75% quartile. Rates of SVR35% in pacritinib-treated patients were similar in the >0% to 50% allele burden group (18.1%) as the >50% to 100% group (18.4%). However, when compared with BAT, pacritinib was significantly more effective in the >0% to 50% group (P < .001) whereas this difference did not reach significance in the >50% to 100% group (P = .061).
SVR in ITT patients stratified by JAK2V617F allele burden. Waterfall plots for SVR at 24 weeks stratified by JAK2V617F status and allele burden in patients treated with pacritinib (A) and BAT (B) by ITT analysis. Pacritinib produced SVRs irrespective of JAK2V617F allele burden whereas patients who received BAT (including ruxolitinib) had spleen reduction only in the higher JAK2V617F quartiles. *A patient who received ruxolitinib. SPV, spleen volume.
SVR in ITT patients stratified by JAK2V617F allele burden. Waterfall plots for SVR at 24 weeks stratified by JAK2V617F status and allele burden in patients treated with pacritinib (A) and BAT (B) by ITT analysis. Pacritinib produced SVRs irrespective of JAK2V617F allele burden whereas patients who received BAT (including ruxolitinib) had spleen reduction only in the higher JAK2V617F quartiles. *A patient who received ruxolitinib. SPV, spleen volume.
Symptom response assessment was only evaluable in 249 patients in the pacritinib group and 120 in the BAT group as a result of an FDA-mandated change in the version of MPN-SAF TSS from v1.0 to v2.0 during the PERSIST-1 study. Significantly more pacritinib-treated patients had a symptom response compared with BAT-treated patients in the >0% to 25% (25.8% vs 7.78%; P = .038) and 25% to 50% (29.8% vs 8.3%; P = .046) allele burden quartiles, whereas differences were not statistically significant for patients with higher allele burdens or with JAK2V617F− disease.
Safety
Detailed safety information in the pacritinib and BAT for the PERSIST-1 and PERSIST-2 studies has been previously reported.17,18 Given that patients in the low JAK2V617F allele burden quartiles were more cytopenic at baseline, we investigated the prevalence of treatment-emergent cytopenias and hemorrhage among pacritinib-treated patients stratified by allele burden (supplemental Table 1). Similar rates of grade 3/4 neutropenia, anemia, and thrombocytopenia were observed across JAK2V617F allele burden quartiles. High-grade bleeding was similar across quartiles.
Discussion
In this post hoc analysis of the PERSIST-1 and PERSIST-2 trials, pacritinib was effective in achieving SVR across the spectrum of JAK2V617F allele burden. Importantly, MF patients with low allele burden had clinical features consistent with a myelodepletive phenotype: lower spleen volume, more severe cytopenias, more hypocellular marrows, and greater preponderance of primary vs secondary disease. In these patients, who are often ineligible for or intolerant of treatment with ruxolitinib due to cytopenias, pacritinib was significantly more effective in reducing spleen size and improving symptom burden compared with BAT.
Our results encompass one of the largest data sets of patients with MF demonstrating phenotypic differences by allele burden. The findings from this study mirror a prior study of PMF patients, in which low allele burden patients were associated with a smaller spleen, less symptom burden, and more prevalent anemia.9,19 It is therefore notable that pacritinib is able to provide significantly more spleen and symptom responses in this subset of patients.
Patients who were negative for the JAK2V617F mutation in the PERSIST cohort were phenotypically similar to patients in the low allele burden quartiles. Although additional mutational information is not available for these patients, it is possible that many are negative for the CALR mutation as this is associated with an increased platelet count.20 Given that only patients with a platelet count of <100 × 109/L were included in PERSIST-2, the population was also likely enriched for patients negative for all 3 driver mutations, who characteristically have a lower median platelet count.20 Interestingly, there was a higher proportion of male patients in the high vs low JAK2V617F allele burden quartiles. Recent work has demonstrated that male patients have a higher JAK2V617F allele burden in CD34+ cells, although no difference was observed in neutrophil allele burden. Male patients with ET or PV were also more likely to progress to MF in their cohort,21 which may help explain the higher proportion of males in the high allele burden quartiles in which secondary MF is more common.
Ruxolitinib has transformed the treatment of MF by producing meaningful spleen and symptom responses. However, not all patients with MF are eligible for this therapy. The COMFORT studies excluded patients with severe baseline thrombocytopenia, and the populations studied were therefore enriched for high JAK2V617F allele burden disease, with a median allele burden of 84.0%.22 In contrast, the baseline characteristics from patients enrolled in the PERSIST-1 and -2 trials represent the full spectrum of MF patients, and the median allele burden on these studies was only 47%.
The effectiveness of pacritinib in low or absent JAK2V617F allele burden disease suggests that pacritinib may possess a JAK-STAT pathway–independent mechanism for treating MF, perhaps through inhibition of IRAK-1. IRAK-1 has been shown to induce apoptosis in disease-initiating clones and to promote normal hematopoiesis in a preclinical model of myelodysplastic syndrome23 ; absent or deficient miR-146a, a negative regulator of IRAK-1, is associated with development of MF in both mouse models24 and in humans.25 These biologic properties suggest that pacritinib may be uniquely suited to addressing the clinical needs of patients with myelodepletive MF.
This post hoc analysis of the PERSIST-1 and -2 trials demonstrates that low JAK2V617F allele burden is associated with features of myelodepletive MF, and that pacritinib is effective in this patient population with limited treatment options and poor prognosis. Subsequent to the PERSIST studies, the PAC203 dose-finding study in patients with MF who were resistant to or intolerant of ruxolitinib showed efficacy and tolerability of the 200 mg twice daily dose, with the majority of SVR35% responses occurring among patients with severe baseline thrombocytopenia. An ongoing phase 3 study of pacritinib vs Physician’s Choice therapy (including ruxolitinib) in patients with MF and severe thrombocytopenia (NCT03165734) will confirm whether pacritinib is able to meet this area of unmet need.
Data-sharing requests should be sent to John Mascarenhas (john.mascarenhas@mssm.edu).
Acknowledgment
The PERSIST-1 (NCT01773187) and PERSIST-2 (NCT02055781) trials were funded by CTI Biopharma.
Authorship
Contribution: D.T. and J.M. were responsible for study design, data interpretation, and manuscript writing; K.R.-T. provided statistical analysis; S.B. contributed to study design; and R.M., B.S., and S.V. provided data interpretation and critical review of the manuscript.
Conflict-of-interest disclosure: R.M. has received consultancy fees from Novartis, Sierra Oncology, and LaJolla Pharmaceuticals, and has received research funding from Celgene, Incyte, AbbVie, Samus Therapeutics, Genentech, Promedior, and CTI Biopharma. B.S. has received advisory fees from Incyte and Celgene, and research funding from Celgene and Novartis. S.B. and K.R.-T. are currently employed by CTI Biopharma. S.V. has received research funding from Incyte, Roche, NS Pharma, Celgene, Gilead, Promedior, CTI Biopharma, Genentech, Blueprint Medicines, Novartis, Sierra Oncology, PharmaEssentia, AstraZeneca, Italfarmaco, Protagnoist, Constellation Pharmaceuticals, Kartos Therapeutics, Prelude Therapeutics, AbbVie, and Telios Pharmaceuticals. J.M. has received grants and personal fees from Roche, Incyte, Promedior, PharmaEssentia; grants from Kartos, Novartis, Merck, CTI Biopharma, and Janssen; and personal fees from Celgene and AbbVie. D.T. declares no competing financial interests.
Correspondence: John Mascarenhas, Myeloproliferative Disorders Program, Tisch Cancer Institute, Division of Hematology/Oncology, Icahn School of Medicine at Mount Sinai, 1 Gustave L. Levy Pl, Box 1079, New York, NY 10029; e-mail: john.mascarenhas@mssm.edu.
References
Author notes
The full-text version of this article contains a data supplement.