Key Points
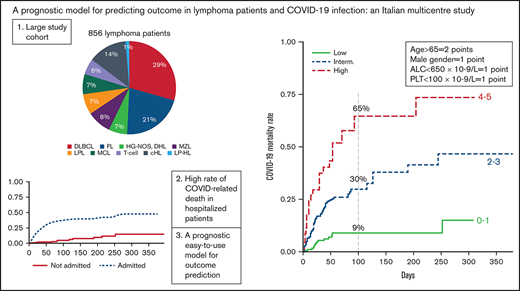
Patients with lymphoma and COVID-19 have mortality rates of 13% (95% CI, 11-15%) and 23% (95% CI, 20-27%) at 30 and 100 days, respectively.
An easy-to-use prognostic model stratifies patients with lymphoma and COVID-19 into 3 groups with extremely different survival expectations.
Abstract
Lymphoma represents a heterogeneous hematological malignancy (HM), which is characterized by severe immunosuppression. Patients diagnosed of coronavirus disease 2019 (COVID-19) during the course of HM have been described to have poor outcome, with only few reports specifically addressing lymphoma patients. Here, we investigated the clinical behavior and clinical parameters of a large multicenter cohort of adult patients with different lymphoma subtypes, with the aim of identifying predictors of death. The study included 856 patients, of whom 619 were enrolled prospectively in a 1-year frame and were followed-up for a median of 66 days (range 1-395). Patients were managed as outpatient (not-admitted cohort, n = 388) or required hospitalization (n = 468), and median age was 63 years (range 19-94). Overall, the 30- and 100-days mortality was 13% (95% confidence interval (CI), 11% to 15%) and 23% (95% CI, 20% to 27%), respectively. Antilymphoma treatment, including anti-CD20 containing regimens, did not impact survival. Patients with Hodgkin’s lymphoma had the more favorable survival, but this was partly related to significantly younger age. The time interval between lymphoma diagnosis and COVID-19 was inversely related to mortality. Multivariable analysis recognized 4 easy-to-use factors (age, gender, lymphocyte, and platelet count) that were associated with risk of death, both in the admitted and in the not-admitted cohort (HR 3.79 and 8.85 for the intermediate- and high-risk group, respectively). Overall, our study shows that patients should not be deprived of the best available treatment of their underlying disease and indicates which patients are at higher risk of death. This study was registered with ClinicalTrials.gov, NCT04352556.
Introduction
Since its first detection in Wuhan, China, in December 2019,1 severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has infected more than 130 million people worldwide, with deaths that are approximating 3 million people. Italy was the first European country where the virus was isolated on 31 January 2020, from 2 Chinese tourists in Rome.2 Since then, in a little more than 1 year, more than 3.5 million cases have been registered in the country.
The clinical spectrum of individuals who are infected with SARS-CoV2 is largely heterogeneous, ranging from mild flu-like symptoms to life-threatening respiratory failure.3 The leading cause of mortality is the acute respiratory distress syndrome, due to the release of proinflammatory mediators, intense immune response, and endothelial damage.4-6 Notwithstanding, comorbidities have been shown to affect disease severity and patient outcomes.7,8 Mortality due to the infection is largely dependent on patients age, with a log-linear increase by age among individuals older than 30 years, with peaks exceeding 8% in older than 80.9 Furthermore, subgroups of patients with coronavirus disease 2019 (COVID-19) have been identified to be at increased risk of morbidity and mortality, including male gender, hypertension, chronic lung disease, diabetes, immunodeficiency, and cancer.10 Patients with solid cancer or with hematological malignancies (HM) often follow a more severe and rapid disease course, with requirement of high-level intensive care and an increased risk of COVID-19 related death11-15 as compared with the general population.12
Lymphomas are a heterogeneous group of cancer, carrying multiple immune dysfunctions of the innate and adaptive immune system, including frequent low immunoglobulin serum levels. Crippled cellular and humoral immunity places these patients at risk of a diverse array of infections, including COVID-19.16 Patients with lymphoma have been under-represented in most cancer or HM series, with just few recent reports addressing specifically their behavior, in relatively small cohorts.17,18 These reports were retrospective, mostly relied on hospitalized patients, and focused on the first wave of infection (spring of 2020).
With this study, the Italian Hematology Alliance on COVID-19 (ITA-HEMA-COV) and the Fondazione Italiana Linfomi collected and analyzed data from adult patients with lymphoma and COVID-19 infection, requiring hospitalization or not, for COVID-19. We report results from a cohort study reporting data of 64 hospitals in Italy, with the aim of improving assistance and prognostication of the disease in such a frail cohort of the entire population affected by COVID-19.
Methods
Study design and inclusion criteria
This multicentre, non-interventional study included a retrospective data review (partly reported in Passamonti et al11 ), and a prospective cohort study, which was implemented in the same centers since 23 June 2020. The present study involved 64 hematology departments in Italy. The ITA-HEMA-COV worked on behalf of all Italian societies dealing with hematology: Società Italiana di Ematologia, Società Italiana di Ematologia Sperimentale, Gruppo Italiano Trapianto Midollo Osseo, Sorveglianza Epidemiologica Infezioni nelle Emopatie, and Fondazione Italiana Linfomi. All consecutive adult patients (aged ≥18 years) with a previous diagnosis of lymphoma, who were registered by single centers between 25 February 2020 and 22 June 2020 (retrospective cohort), and then between 23 June 2020 and 1 February 2021 (prospective cohort), with data cutoff on 1 April 2021 were included in this analysis. Inclusion criteria were a diagnosis of lymphoma according to World Health Organization (WHO)-criteria and laboratory-confirmed SARS-CoV-2 infection, tested by Reverse Transcriptase-Polymerase Chain Reaction on nasopharyngeal swabs following standardized national recommendations.11 The trial was approved by the institutional review board of each hematology unit. Written informed consent was collected from all patients.
Study procedures and definitions
Data on patient characteristics and outcomes were extracted by study investigators from data collection electronic forms obtained from local medical records, and queries were performed to enrich the data on lymphoma patients. Diagnosis of lymphoma subtype was made on the basis of the most recent WHO classification of hematopoietic tumors.19 The histological diagnosis of the lymphoproliferative disorder and stage at diagnosis, the therapeutic history of each patient with number of previous lines of therapy, best response to therapy, disease status at COVID diagnosis, and demographic features at the time of enrollment, including age, sex, Charlson Comorbidity Index, biochemical parameters (hemoglobin, hematocrit, platelets, leukocytes, lymphocytes, clotting tests, serum lactate dehydrogenase, and C-reactive protein), time since diagnosis of lymphoma to COVID-19 diagnosis, time from last lymphoma therapy to COVID-19 diagnosis, and COVID-19 severity including admission to the intensive care unit (ICU) were recorded. Definitions of active disease status (progressive disease or partial remission), active therapy (delivered within the previous 6 months), and severity of COVID-19 as mild, severe, or critical, were previously described.11 This study was registered with ClinicalTrials.gov, NCT04352556.
Outcomes measures and statistical analysis
Characteristics of the study population were described for admitted and nonadmitted patients. The primary outcomes were mortality among patients with lymphoma and COVID-19 (overall survival [OS] from date of COVID-19 diagnosis to date of death for any cause or date of last clinical evaluation) and evaluation of potential predictive parameters of mortality. COVID-19 fatality rate (CFR) was estimated as the proportion of deaths compared with the total number of patients. Secondary outcomes were the characteristics of admitted patients as compared with patients that were managed as out-patient, and the outcome of patients with different lymphoma subtypes or specific treatments. Discrete covariates were summarized by frequencies and percentages, and continuous covariates were summarized by use of standard measures of central tendency and dispersion (median and range). Comparisons between categorical covariates were performed by Fisher’s exact test or χ2 test if appropriate. Comparison between continuous covariates were done using the Mann-Whitney U test. The OS was estimated by means of Kaplan-Meier method, with 95% confidence interval (CI). We compared OS in different cohorts of patients using log-rank test, and the effect of covariate was estimated using the Cox proportional hazard (PH) regression, either in univariable or multivariable analysis.20 Association between covariates and hospital admission was estimated by logistic regression, and the effect of covariate was reported as odds ratio (OR) with 95% CI. The continuous covariates were dichotomized using the cutoff reported in medical literature, except for absolute lymphocyte count (ALC), for which a specific cut-point was identified by modeling the continuous covariate in an explorative Cox PH restricted cubic spline regression, which was stratified by histotype,21 as reported in supplemental Figure 1. All statistical analysis was done with Stata 17SE and all statistical tests were 2-sided.
Histological subtypes and antilymphoma therapy
Patients were grouped into the following histologic categories: (1) large B-cell lymphomas (LBCL), (2) follicular lymphoma (FL), (3) mantle cell lymphoma (MCL), (4) other indolent lymphomas (i-NHL), (5) T-cell lymphomas (T-cell), and (6) Hodgkin lymphoma (cHL).
A list of the included lymphoma subtypes by category is listed in supplemental Material. For the purpose of the present analysis, nodular lymphocyte predominant Hodgkin's lymphoma (LP-HL) were grouped with i-NHL. Patients with chronic lymphocytic leukemia were excluded from the present analysis.
As for therapeutic schemes, we identified the following categories: (1) chemotherapy alone, (2) radiotherapy alone, (3) immunotherapy, (4) immunochemotherapy, (5) biologics (single agents or in combination), and (6) participation to clinical trials. The chemotherapy categories, either in combination with immunotherapy or not, included various combinations of chemotherapy agents; immunotherapy included patients on treatment with monoclonal antibody monotherapy (mostly anti-CD20 and anti-CD30) and/or checkpoint inhibitors (mostly PD-1 inhibitors); biologics included tyrosine kinase inhibitors, immunomodulators, and proteasome inhibitors. For the purpose of this analysis, we further subdivided therapeutic combinations in bendamustine-containing or not and anti-CD20 containing or not.
Prognostic model
The complete cases cohort was used to develop and select the model by means of the likelihood-ratio test. The importance of covariates was evaluated after 1000 bootstrap resamples of the selection model, based on minimum Akaike's information criterion (AIC). The proportionality of risk of the multivariable Cox PH model was checked graphically by means of the scaled Schoenfeld residuals.22 Finally, the model was internally validated using bootstrap techniques to evaluate the discriminant power (C-index, which measures the probability that given a pair of randomly selected patients, the model will correctly predict which patient will experience failure first), shrinkage factor (check for overfitting: the statistical model fitted the noise in the data rather than the relation between covariates and outcome, with loss of generalizability).14 The following categorized covariates were considered: age, ≥65 vs < 65 years; gender, male vs female; hemoglobin (Hb), <12 vs ≥12 g/dL; histology, LBCL/MCL/T-cell vs cHL/FL/i-NHL; disease status, active vs not; Charlson Index, >6 vs ≤6; time lapse between lymphoma diagnosis and COVID-19, <36 vs ≥36 months; platelets (PLT), <100 vs ≥100 10−9/L; and ALC, <650 vs ≥650 10−9/L (the cut-point for ALC is described in supplemental Figure 1) . We assigned a weight to each variable according to its relative importance, derived from the ratio of z-Wald values found in the Cox PH model. The ratio between the z-score for any factor was divided by minimum z-score observed (considered as reference), and the weights were obtained rounding the ratio. Thus, the score was the sum of weights. Finally, from the analysis of the Kaplan-Meier curves, the prognostic score was grouped into 3 risk levels (low, intermediate, and high).
Results
Patients’ characteristics
The study included 856 patients, of whom 237 were included in the previous analysis,11 and 619 were enrolled prospectively since the closure of the initial database. The clinical and laboratory features, and lymphoma histologies of the whole series, then divided according to admission to the hospital or not, are reported in Table 1. Overall, median age was 63 (range 19-94), and 504 (59%) were males. As expected, patients necessitating hospitalization since the initial positive swab, the so-called admitted cohort (n = 468), were in worse clinical conditions than those followed-up as outpatients (n = 388), with 59% and 8% with severe or critical disease, respectively. Therefore, as shown in Table 1, the clinical and laboratory features of the 2 cohorts were significantly different. Not-admitted patients were younger, were more likely to be females, had an inferior comorbidity score, and had a higher hemoglobin level and ALC. Admitted patients instead were more likely to have a recent lymphoma diagnosis and an active disease, while no difference was observed regarding number of previous antilymphoma therapy lines. A logistic regression calculation describing the probability of hospital admission according to different characteristics of patients is shown in supplemental Table 1. The distribution of lymphoma histotypes was in line with the expected prevalence of these diseases in the adult unselected population. Interestingly, patients affected by cHL were more likely to be followed in the outpatient setting.
Overall survival
Overall, 165 patients died after a median follow-up of 66 days (range 1-395), with a CFR of 19.5% and an incidence of 2.45x1000 person-days. Overall, the 30- and 100-days mortality was 13% (95% CI, 11% to 15%) and 23% (95% CI, 20% to 27%), respectively, as shown in Figure 1A. The vast majority of deaths were due to COVID-19 infection or complications related to the infection (91%, vs 15 unrelated, 9%). Unrelated deaths were due to lymphoma progression in 13 of the 15 cases. As expected, patients admitted to the hospital had significantly worse OS than patients not admitted (P < .0001), as shown in Figure 1B. The CFR of admitted vs not-admitted patients was 33.4% and 3.8%, respectively. The mortality incidence of admitted and not-admitted patients was 4.03 and 0.50x1000 person-days, respectively. The 30-days mortality for mild, severe, and critical COVID-19 infection, which is a parameter strictly related to hospitalization, were 4% (95% CI, 3% to 7%), 22% (95% CI, 17% to 28%) and 45% (95% CI, 33% to 57%), respectively. The 100-days rates were 9% (95% CI, 6% to 13%), 38% (95% CI, 31% to 46%), and 75% (95% CI, 61% to 87%) for the mild, severe, and critical groups, respectively, as shown in Table 2. The OS for status of COVID-19 showed the same pattern in prospective or retrospective cohort (test for trend, P < .001 for both). Factors that were associated with significantly impaired OS in univariate analysis are shown in Table 2.
Overall survival for enrolled patients. Kaplan-Meyer curves for overall survival in all enrolled patients (A), and according to admission to the hospital (B), lymphoma histotype (C).
Overall survival for enrolled patients. Kaplan-Meyer curves for overall survival in all enrolled patients (A), and according to admission to the hospital (B), lymphoma histotype (C).
No difference in OS was observed between the prospective and retrospective cohorts (3-months OS 76% vs 75%, respectively, P = .205). Possibly due to selection bias, the retrospective series had higher rate of hospitalization and lower rate of mild COVID compared with the prospective series (74% vs 44%, P < .001; 54% vs 68%, P = .001, respectively).
Overall, 474 patients were treated anytime for lymphoma, with 274 patients (33%) that were on active treatment at the time of the COVID-19 diagnosis, and 200 (24%) that had completed their therapy more than 6 months before infection. The mortality rate of patients who had recent therapy (≤ 6 months) or previous therapy (> 6 months) was 25% (95% CI, 20% to 33%), and 29% (95% CI, 22-38), respectively (P = .996). Patients who had received antilymphoma treatment (yes or no, any time) had worse OS than patients that were never treated (n = 357), as shown in Table 2. When comparing patients that had received bendamustine (n = 61) with patients that had received different therapies, we observed no significant difference in terms of OS (P = .23, supplemental Figure 2). Nevertheless, bendamustine mortality curve raised sharply in the initial 50 days from COVID-19 infection, suggesting that a detrimental effect of the drug cannot be excluded in our relatively small sample. When comparing patients that had received anti-CD20 immunotherapy (n = 163) with patients treated without these drugs, no difference was observed in terms of OS (P = .65, supplemental Figure 2), with mortality rates that were quite superimposable in the initial 100 days from infection. The absence of a detrimental acute impact of anti-CD20 on mortality was confirmed in the 16 FL patients that underwent or were undergoing maintenance therapy with anti-CD20 in our series (data not shown). Of lymphoma histologies, cHL was associated with significantly better OS than others (P = .001), while low-grade lymphomas, including both FL and i-NHL, were borderline (P = .049, Figure 1C). Indeed, when we considered the impact of age, gender, and the frequency of admission to the hospital of patients with cHL, which was different from other histologies, as expected, this advantage was weakened (log-rank test stratified by age >65, gender, and hospital admission, P = .318).
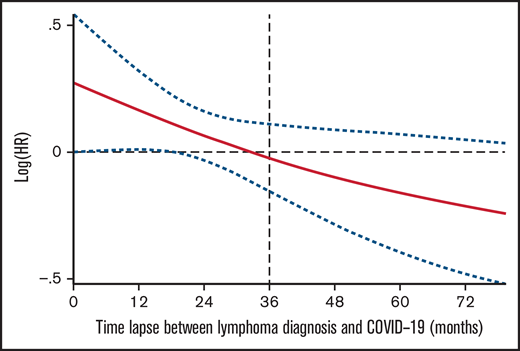
Interestingly, the time interval between lymphoma diagnosis and COVID-19 infection was inversely related to mortality, with 36 months as cutoff, meaning that the longer the interval the inferior the probability of death. As shown in Figure 3, the risk of death declined over time in a linear fashion as we moved away from the time of lymphoma diagnosis.
Predictive model for survival
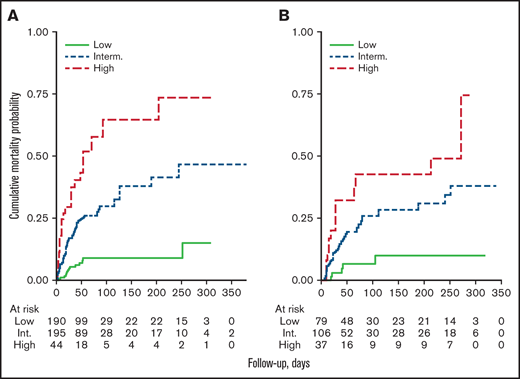
The model was built from 429 patients with complete data, where multivariable analysis revealed that age >65 years old, male gender, absolute lymphocyte count less than 650 × 10−9/L, and platelets less than 100 × 10−9/L were the strongest factors associated with inferior OS (Table 3). The relative importance for inclusion into the model of each factor is reported in supplemental Table 2. Given weight 2 for age and 1 for male gender, lymphopenia and thrombocytopenia, respectively, a score ranging from 0 to 5 was obtained that grouped 3 risk categories by Kaplan-Meier curves: low (0-1), intermediate (2-3), and high (4-5) (Table 4; Figure 2A). In the cohort of 429 patients, the 100-days mortality for low, intermediate and high risk were 8% (95% CI, 5% to 13%), 29% (95% CI, 23% to 36%), and 54% (95% CI, 41% to 68%), respectively. The HR between intermediate and low risk was 3.79 (95% CI, 2.09-6.85, P < .001) and 2.33 (95% CI, 1.43-3.83, P = .001) between high and intermediate risk. In 193 patients without complete data, but in which it was possible to compute the score, the prognostic index showed a comparable property (Table 4; Figure 2B). Moreover, the prognostic model could reliably separate the 3 levels of risk either for hospitalized or not-hospitalized patients (log rank, P < .001 for both) and had comparable discrimination ability in the prospective and retrospective cohorts: C-Harrell 0.692 (95% CI, 0.600-0.711) and 0.654 (95% CI, 0.629-0.754), respectively. Finally, when we calculated the respective prognostic index according to lymphoma histology (International Prognostic Index, Follicular Lymphoma International Prognostic Index, and Mantle Cell Lymphoma International Prognostic Index) in 340 patients with available data, our model for COVID-19 worked properly both in low- and high-risk groups, as shown in supplemental Figure 3.
Overall survival by the prognostic model. Kaplan-Meyer curves for overall survival assigned by the prognostic model in the cohorts of patients with complete data (n = 429) (A), or without complete data (n = 193) (B).
Overall survival by the prognostic model. Kaplan-Meyer curves for overall survival assigned by the prognostic model in the cohorts of patients with complete data (n = 429) (A), or without complete data (n = 193) (B).
COVID related signs and symptoms, COVID management
The most common clinical findings at presentation among hospitalized patients with lymphoma were pneumonia (80%) and fever (76%), followed by dyspnoea (62%). As expected, these findings were less represented among not-admitted patients (10%, 53%, and 12%, respectively). Fever represented the relatively most common sign or symptom in not-admitted patients. Of hospitalized patients, 60% were admitted in various departments of internal medicine care, 29% at the infectious disease departments, and 11% in ICU. The characteristics of patients admitted to the hospital, either in ICU or not, and of those not admitted in terms of signs and symptoms, divided by severity of COVID-19 infection, are reported in supplemental Tables 3 and 5.
Medications that were delivered to admitted patients, both in terms of oxygen supplementation, inotropes, antibiotics, or antiviral drugs, are described in detail in supplemental Table 4. Differences in the administration of drugs or ventilation according to disease severity are also listed. Overall, hydroxychloroquine was administered to 34% of admitted patients, lopinavir in 34%, remdesivir in 41%, and antibiotics in 74%. Deep-vein thrombosis prophylaxis was delivered to 71% of patients, and 6% had tocilizumab.
Discussion
In the present study, we evaluated the impact of COVID-19 on survival of Italian lymphoma patients. To the best of our knowledge, this is the largest series of patients reported so far and has some unique characteristics that may impact clinical practice: (1) it includes a large prospective cohort (n = 619), (2) it has the longest follow-up to date, and (3) it includes both hospitalized and not-hospitalized patients. Overall, we could draw conclusions on a population with a wide age distribution, affected by lymphoid malignancies with variable disease status, and with several lymphoma histologies that were well represented. With the present study, we can conclusively say that fatality rate of hospitalized patients with lymphoma is high (33.4%), which was in line with smaller retrospective series from the literature on lymphoma patients17,23 or on HM in general.12-16,24 Furthermore, we could establish for the first time the relatively low mortality rate (3.8%) of patients that were managed as outpatients. Our analysis confirmed that the demographic characteristics of the patients are of main importance in the COVID-19–and–cancer scenario. Male gender and advanced age (>65 years old) by themselves conferred a significant higher risk of death to patients with lymphoma and COVID-19, with hazard ratios in the range of 2 and 3.5, respectively. When demographic characteristics were paired by platelet and lymphocyte count, we could build an easy-to-use prognostic model that identified patients with ∼50% probability of death in the initial 2 months after COVID-19 infection (Table 4; Figure 2). Our prognostic model successfully applied both to admitted and not-admitted patients. The detrimental role of lymphopenia in our model may be related to the impaired humoral and cell-mediated response, associated to the reduced seroconversion related to lymphopenia. Thrombocytopenia instead may be related to the degree of acute inflammation, when proinflammatory mediators are released, and systemic endothelial damage occurs. Of note, the reason for death was registered as a prerequisite in our database to avoid overestimation bias in the rate of death due to comorbidities or other reasons that were not COVID related. Indeed, 91% of the registered and computed deaths were reported as COVID related. We acknowledge that the performance of our model was not verified in independent series, although it was validated internally (Figure 2), and therefore we need to consider our findings as hypothesis-generating for future external validation. Overall, these findings may assist hematologists and national health commissions in their decision-making processes regarding preventive measures and treatment in this patient population. Vaccination has been shown to be not as effective in lymphoma patients as in the normal setting, making this population more vulnerable to COVID-19.25-28 A substantial subset of vaccinated lymphoma patients may be at high risk of breakthrough COVID-19 infection.28 Adapted vaccination schedules for lymphoma patients, especially when exposed to anti-CD20–including regimens, are urgently needed, as this highlights the need for continuous, careful monitoring of this frail population.
Our work may facilitate a change in some common thinking about the impact of delivered antilymphoma treatment on fatality rate. We observed no differences in survival for patients on active antilymphoma treatment (≤6 months) as compared with all others (Table 2). This observation confirms other observations on lymphoma patients14,17,23 but differs from what generally reported in solid cancer patients, where active treatment has been associated with increased risk of death,29,30 although not uniformly.13,31 Anti-CD20 containing therapy, as already observed by others,16 did not impact the short-term outcome, as was the case of the number of previous antilymphoma lines of therapy. However, this aspect will need to be clarified further, in view of the recently described strong association between anti-CD20 containing therapy and impaired immune response to vaccination.25-28 The use of bendamustine was not statistically associated with impaired survival in our series, but larger samples will be needed to confirm this observation. Instead, based on our findings, withholding specific treatments does not seem to be justified, since the presence of active disease was uniformly associated with higher risk of death in ours and other series.17,18
Our analysis showed that an initial lymphoma diagnosis of 3 or more years prior to COVID-19 infection was significantly associated with better clinical behavior and inferior fatality rate. This observation, which to our knowledge has not been previously reported, may facilitate the screening of patients that may be candidates to early vaccination strategies (Figure 3).
Risk of death related with the time from lymphoma diagnosis to COVID infection. Restricted cubic spline Cox PH regression describing the relationship between time lapse between lymphoma diagnosis and COVID infection, and risk of death.
Risk of death related with the time from lymphoma diagnosis to COVID infection. Restricted cubic spline Cox PH regression describing the relationship between time lapse between lymphoma diagnosis and COVID infection, and risk of death.
In the COVID-19 literature, only a few retrospective series specifically describing patients with lymphoma have been published so far.16,17 We observed that patients with cHL had a lower rate of admission to the hospital and the lowest mortality rate among all subtypes. This was consistent with several other reports, with the limit of the low number of cHL patients included in the other series.16 We acknowledge that patients with cHL were significantly younger than other histologies (median 43 vs 65 years, P ≤ .0001), which conferred them already the most relevant survival advantage according to our Cox PH regresion analysis. Nevertheless, our prognostic model was equally effective in discriminating survival of any lymphoma subtype. Low-grade lymphomas experienced a relatively better survival as compared with aggressive histotypes, but this may reflect the intrinsic longer expected survival of patients with indolent histologies. However, our model was predictive in each subgroup of the prognostic indexes related to lymphoma histotype both in aggressive and indolent histologies (International Prognostic Index, Follicular lymphoma International Prognostic Index, and Mantle Cell Lymphoma International Prognostic Index; supplemental Figure 3).
All patients that were not admitted to the hospital (n = 388) were enrolled prospectively by centers. This makes this cohort informative regarding the ability of our model to predict survival of not-hospitalized patients. Indeed, the vast majority of lymphoma patients included in previous reports were admitted to the hospital (85% and 100% in the Spanish and French reports, respectively16,17 ). The signs and symptoms related to COVID-19 infection in this population were obviously milder than hospitalized patients: fever, pneumonia, or cough were registered in 53%, 10%, and 36% of not-admitted patients, respectively, as compared with 76%, 80%, and 53% for hospitalized patients. Analyzing the effectiveness of the various COVID-19 treatments to improve final outcome represents a crucial objective of scientific research in the COVID-19 era, but it was outside the aims of the present report. Furthermore, longitudinal observation of our patients may inform us about the impact of long COVID-19 and how long-lasting signs or symptoms may have affected the subsequent treatment of underlying lymphoma. This aspect warrants further investigations.
In conclusion, we have reported a high mortality rate in patients with lymphoma and COVID-19, which can be easily predicted both in hospitalized and not-hospitalized patients by demographics or hematological ready-to-use variables. Our study, which was largely based on a prospectively enrolled cohort, may represent the basis for future comparison in such an intriguing field of modern translational medicine.
Acknowledgments
The authors thank all the patients and families who were included in this study, as well as all the health workers that took care of their patients in Italy; the charity Associazione italiana contro le leucemie, linfomi e mieloma–Verona Onlus; and Emilia Elizbieta Florea for the precious administrative assistance. This study received no specific financial support.
Authorship
Contribution: All of the investigators and their research teams collected data. L.B. confirmed the accuracy of the data and compiled the data for analysis. C.V., L.M., and V.B. performed statistical analysis and contributed to the conception of the study. C.V., C.G.-P., F.P., and F.M. supervised the research. All authors reviewed the manuscript and agreed with manuscript submission.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
A list of the ITA-HEMA-COV investigators appears in “ Appendix.”
Correspondence: Carlo Visco, Department of Medicine, Section of Hematology, University of Verona, 37100 Verona, Italy; e-mail: carlo.visco@univr.it.
Appendix: ITA-HEMA-COV investigators
Investigators Francesco Passamonti,1,2 Alessandra Romano,3 Marco Salvini,2 Francesco Merli,4 Matteo Giovanni Della Porta,5 Riccardo Bruna,6 Elisa Coviello,7 Ilaria Romano,8 Roberto Cairoli,9 Roberto Lemoli,10 Francesca Farina,11 Adriano Venditti,12 Alessandro Busca,13 Marco Ladetto,14 Massimo Massaia,15 Antonio Pinto,16 Luca Arcaini,17 Agostino Tafuri,18 Francesco Marchesi,19 Nicola Fracchiolla,20 Monica Bocchia,21 Daniele Armiento,22 Anna Candoni,23 Mauro Krampera,24 Mario Luppi,25 Valeria Cardinali,26 Sara Galimberti,27 Chiara Cattaneo,28 Elettra Ortu La Barbera,29 Roberto Mina,30 Francesco Lanza,31 Giuseppe Visani,32 Pellegrino Musto,33 Luigi Petrucci,34 Francesco Zaja,35 Enrico Derenzini,36 Monia Marchetti,37 Anna M. Scattolin,38 Alessandro Corso,39 Patrizia Tosi,40 Filippo Gherlinzoni,41 Carlo G. Passerini,42 Michele Cavo,43 Carmen Fava,44 Mauro Turrini,45 Carlo Visco,24 Paolo Antonio Grossi,1,2 Lorenza Bertù,2 Livio Pagano,46 Patrizia Zappasodi,17 Michele Merli,2 Barbara Mora,1,2 Alessandro M. Vannucchi,8 and Paolo Corradini,47
Affiliation of ITA‐HEMA‐COV investigators: 1Dipartimento di Medicina e Chirurgia, Università dell’Insubria, Italy; 2ASST Sette Laghi, Ospedale di Circolo of Varese, Italy; 3Università degli Studi di Catania, Catania, Italy; 4Hematology, AUSL‐IRCCS, Reggio Emilia, Italy; 5Humanitas Clinical and Research Hospital – IRCCS and Department of Biomedical Sciences, Humanitas University, Milan, Italy; 6Department of Translational Medicine, University of Eastern Piedmont and Ospedale Maggiore della Carità, Novara, Italy; 7Ospedale Policlinico San Martino‐IRCCS, Genoa, Italy; 8University of Florence and AOU Careggi, Florence , Italy; 9ASST Grande Ospedale Metropolitano Niguarda, Milan, Italy; 10University of Genoa, Genoa, Italy; 11San Raffaele, Scientific Institute, Milan, Italy; 12Ematologia, Dipartimento di Biomedicina e Prevenzione, Università Tor Vergata, Rome, Italy; 13Stem Cell Transplant Center, AOU Citta' della Salute e della Scienza of Turin, Italy; 14Dip di Medicina Traslazionale, Università del Piemonte Orientale ed AO SS Antonio e Biagio e Cesare Arrigo, Alessandria, Italy; 15Santa Croce Hospital, Cuneo, Italy; 16Istituto Nazionale Tumori IRCCS “Fondazione G. Pascale”, Naples, Italy; 17Division of Hematology, Fondazione IRCCS Policlinico San Matteo & Department of Molecular Medicine, University of Pavia, Italy; 18University Hospital Sant'Andrea, Department of Clinical and Molecular Medicine, Sapienza, Univ. of Rome, Rome, Italy; 19Hematology and Stem Cell Transplant Unit, IRCCS Regina Elena National Cancer Institute, Rome, Italy; 20Fondazione IRCCS Ca' Granda ‐ Ospedale Maggiore Policlinico, Milan, Italy; 21Azienda Ospedaliera Universitaria Senese, University of Siena, Italy; 22Unit of Hematology, Stem Cell Transplantation, University Campus Bio‐Medico, Rome, Italy; 23Division of Hematology, University Hospital of Udine ‐ASUFC, Udine, Italy; 24Department of Medicine, University of Verona, Italy; 25Department of Medical and Surgical Sciences, University of Modena and Reggio Emilia, Modena, Italy; 26University of Perugia, Italy; 27Università di Pisa, Pisa, Italy; 28ASST‐Spedali Civili, Brescia, Italy; 29Ospedale Santa Maria Goretti, Latina, Italy; 30Università di Torino, Azienda Ospedaliera Città della Salute e della Scienza, Turin, Italy; 31Santa Maria delle Croci, Ravenna, Italy; 32Azienda Ospedaliera Ospedali Riuniti Marche Nord, Pesaro, Italy; 33Department of Emergency and Organ Transplantation,"Aldo Moro" University School of Medicine and Unit of Hematology and Stem Cell Transplantation, AOU Consorziale Policlinico, Bari, Italy; 34Division of Hematology Department of Translational and Precison Medicine, Sapienza University of Rome, Italy; 35Università di Trieste, Trieste, Italy; 36Oncohematology Division, IEO European Institute of Oncology IRCCS, Milan, Italy; Department of Health Sciences, University of Milan, Italy; 37Azienda Ospedaliera SS Antonio e Biagio e Cesare Arrigo, Alessandria, Italy; 38Ospedale dell’Angelo di Mestre, Mestre, Italy; 39ASST Ovest Milanese, Legnano, Italy; 40Ospedale degli Infermi di Rimini, Rimini, Italy; 41Ospedale Ca' Foncello, Vicenza, Italy; 42Università degli Studi di Milano‐Bicocca, Italy; 43IRCCS Azienda Ospedaliero‐Universitaria, Bologna, Italy e Istituto di Ematologia “Seràgnoli”, Dipartimento di Medicina Specialistica, Diagnostica e Sperimentale, Università degli Studi, Bologna, Italy; 44University of Turin, Turin, Italy; 45Ospedale Valduce, Como, Italy; 46University Cattolica del Sacro Cuore, Rome, Italy; 47University of Milan & Fondazione IRCCS Istituto Nazionale dei Tumori, Milan, Italy.
References
Author notes
F.P. and F.M. contributed equally to this study.
For data sharing, contact the corresponding author: carlo.visco@univr.it.
The full-text version of this article contains a data supplement.