TO THE EDITOR:
Outcomes of older and unfit adults with acute myeloid leukemia (AML) differ significantly from their younger counterparts, owing to their adverse disease biology (complex karyotype, antecedent myelodysplastic syndrome) and age-related comorbidities.1 Recently, venetoclax-based lower intensity therapies were approved for newly diagnosed (ND) AML in older (aged ≥75 years) adults and/or those not eligible for intensive chemotherapy.2,3 However, given the lack of consensus on the definition of eligibility criteria for intensive chemotherapy in older age, intensive chemotherapy continues to be used under the assumption that lower intensity therapies may sacrifice efficacy for tolerability.4 We evaluated 248 older and unfit patients with ND non–core-binding-factor AML treated on 2 clinical trials investigating double nucleoside-analog therapy (DNT) alternating with decitabine and demonstrated a composite complete remission (CRc) rate of 66% with a median overall survival (OS) of 12.5 months. The 4- and 8-week mortality rates were 2% and 11%, respectively. In a longer follow-up, we confirm that low-intensity DNT regimens led to higher response rates, durable remissions, and improved OS compared with our historical experience with hypomethylating agents.5
In a recent prognostic model incorporating karyotypic abnormalities and 7 recurrently mutated genes in AML, the Acute Leukemia French Association (ALFA) 1200 study investigators identified and validated a genomic score that effectively classified patients with ND AML aged ≥60 years into 3 distinct prognostic groups based on their outcomes with intensive chemotherapy.6 Itzykson and colleagues defined “go-go” as the group with favorable outcome, with a predicted 2-year OS of 66%, “no-go” as the adverse prognostic group with a 2-year OS of 3%, and a “slow-go” proceed-with-caution group with a 2-year OS of 39% with respect to the use of intensive therapy in AML.6 The ALFA study decision tool is restricted to those older fit patients treated with intensive chemotherapy-based approaches. The predictive accuracy of this tool in patients treated with alternative agents including DNT regimens is unknown. Therefore, we sought to validate the predictive accuracy of the ALFA study tool in our 248 older patients treated on a clinical trial with DNT alternating with decitabine regimen and compare outcomes to intensively treated patients and validate the performance of the ALFA model.
Between October 2008 and April 2018, 248 patients aged 60 years or older or unfit with ND non–core-binding-factor AML were enrolled on DNT alternating with decitabine regimen, the details of which has already been published. Both protocols (NCT00778375 and NCT01515527) were approved by the MD Anderson Cancer Center’s institutional review board; patients signed a written informed consent before enrollment in accordance with the Declaration of Helsinki.5 Mutational testing was available for a subset of patients as next-generation sequencing became available (ASXL1 [n = 68], FLT3-ITD [n = 237], NPM1 [n = 237], RAS [n = 237], TP53 [n = 109]). FLT3 allelic ratio was available in all patients; KRAS and NRAS mutations were grouped as RAS mutations. Because there were no patients with favorable karyotype included in the DNT trials, patients were categorized to nonpoor (diploid and nondiploid intermediate-risk karyotype), and poor (noncomplex adverse, and complex karyotype with ≥3 chromosomal abnormalities) karyotypes. The 3-tier ALFA oncogenetic decision model included nonpoor cytogenetics and either NPM1 mutation by itself or with 1 other mutation in FLT3-ITD, DNMT3A, ASXL1, or NRAS in the go-go group; adverse risk cytogenetics and either a mutation in KRAS or TP53 in the no-go group and the rest in the slow-go group.7 We applied the ALFA study decision tool to our DNT cohort. Because we did not have complete mutation data on all our patients, 35 patients with diploid karyotype and wild-type FLT3, NPM1, and RAS mutations were assigned to the go-go group (subset analysis of these 35 patients demonstrated 100% CRc rate and a 2-year OS of 61%). Among patients in whom TP53 mutational testing was not available, monosomy chromosome 17 or deletion of 17p (with adverse karyotype) were used as a surrogate marker for TP53 mutation and were categorized among the no-go group. Twenty-seven patients with chromosome 17 abnormalities had a complete remission rate of 44%, and a 2-year OS of 4%, without significant difference from patients with poor cytogenetic risk with TP53 mutation (complete remission = 52%, 2-year OS = 2.8%) in the ALFA cohort.6 Survival outcomes were not censored for hematopoietic stem cell transplantation (HSCT).
Baseline characteristics were well matched between the DNT and comparison ALFA cohort. In the DNT cohort, the median age was 69 years with 41% aged ≥70 years. There were no patients with favorable risk cytogenetics, and adverse risk karyotype was significantly more common (44% vs 18% [ALFA], P < .0001) (Table 1). The DNT cohort was enriched with mutations in TP53 (23%), RAS (15%), NPM1 (13%), and FLT3-ITD (10%). In the intermediate cytogenetic risk group, 81% achieved CRc compared with 56% patients with poor-risk cytogenetics (P < .0001).
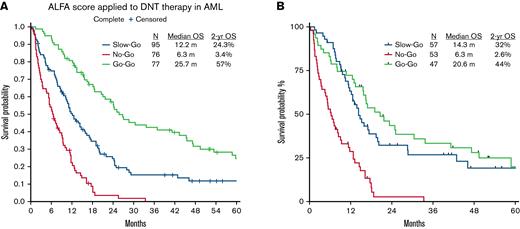
Based on the to the ALFA decision tool, the DNT cohort was stratified into go-go (31%), slow-go (38%), and no-go (31%) groups. Compared with the ALFA cohort, significantly more patients from the DNT cohort were categorized as no-go (31% vs 8%, P < .0001). CRc rates in the go-go, slow-go, and no-go groups were 100%, 54%, and 47%, respectively. Response rates of the go-go group in the DNT cohort compared favorably with the ALFA cohort. Two-year OS estimates were 57%, 24.3%, and 3.4% in the go-go, slow-go, and no-go groups, respectively (Figure 1A). Statistically significant differences in OS were seen between all decision tiers (all P < .0001). The outcomes of each of the groups are summarized in Table 2. Outcomes of the lower intensity DNT cohort were similar to the ALFA1200 cohort and the published validation cohorts (Hauts-de-France, AML Study Group, and Study Alliance Leukemia group) using 7 + 3.
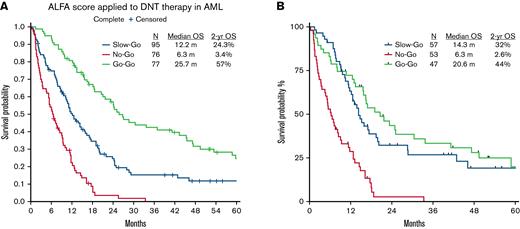
Kaplan-Meier plots (not censored for hematopoietic stem cell transplantation) showing overall survival in the (A) entire cohort and (B) cohort with complete mutation data.
Kaplan-Meier plots (not censored for hematopoietic stem cell transplantation) showing overall survival in the (A) entire cohort and (B) cohort with complete mutation data.
To precisely match the ALFA model, we excluded cases without complete molecular data and analyzed a subset in which complete cytogenetic and mutational data were available (N = 157). Per the ALFA decision tool, the DNT subset was stratified into go-go (47, 30%), slow-go (57, 36%), and no-go (53, 34%) groups. CRc rates in the go-go, slow-go, and no-go groups were 77% (n = 36), 77% (n = 44), and 47% (n = 25), respectively. Response rates of all tiers in the DNT cohort were in line with the ALFA cohort. Two-year OS estimates were 44%, 32%, and 2.6% in the go-go, slow-go, and no-go groups, respectively. Statistically significant differences in OS were seen only between no-go and other decision tiers (all P < .0001) but not between the go-go and the slow-go groups (Figure 1B).
Among the 164 responding patients (n = 248, 66%), 36 underwent HSCT (34%), of which 12 were from go-go (n = 77, 16%), 11 from slow-go (n = 51, 22%), and 13 from no-go (n = 36, 36%) groups. Among the 128 responding patients who did not undergo HSCT, 99 patients were included in the landmark analysis and 29 patients were excluded who either relapsed (n = 6), died in remission (n = 3), patient/physician choice (n = 19), or were lost to follow-up (n = 1) within 4.4 months of AML diagnosis (ie, the median time to HSCT). The outcomes of patients who underwent SCT was significantly better across in the slow-go (no response vs 17 months, P = .03) than the go-go (43.3 vs 28.7 months, P = .09) or the no-go (11.7 vs 11.7 months, P = .63) groups.
In conclusion, the ALFA1200 decision tool reliably discriminated the no-go group from the go-go and slow-go groups within the DNT cohort. There was less discrimination between the slow-go and go-go groups in our cohort, likely from the lack of patients with core-binding factor AML. Overall response and survival outcomes of the DNT cohort were in line with the intensively treated ALFA cohort. The DNT cohort is distinguished by its older unfit patient group and predominantly adverse risk inherent to this age group. In the poor cytogenetic risk group, neither intensive nor DNT affects the outcome, highlighting the need for novel therapies for this subset. Of note, the ALFA 1200 decision tool distinguished the prognostic subgroups among patients treated with DNT, a lower intensity treatment regimen in whom the outcomes were comparable to those treated with 7 + 3. The prognostic ability of ALFA 1200 decision tool needs to be further evaluated among older patients treated with hypomethylating agents + venetoclax-based regimens.
Acknowledgments: The study has been supported in part by the Anderson Cancer Center Support Grant P30 CA016672 and Award Number P01 CA049639, and by the “Charif Souki Cancer Research Fund.”
Contribution: S.V. and T.M.K designed the study; S.V., H.K., G.B., N.D., C.D.D., N.P., N.J.S., H.A.A., G.G.-M., M.K., F.R., and T.K collected data; S.V. and T.K. analyzed the data; S.V. wrote the first draft of the manuscript; and all authors provided critical input.
Conflict-of-interest disclosure: N.D. has received research funding from Daiichi-Sankyo, Bristol-Myers Squibb, Pfizer, Gilead, Sevier, Genentech, Astellas, Daiichi-Sankyo, Abbvie, Hanmi, Trovagene, FATE therapeutics, Amgen, Novimmune, Glycomimetics, Trillium, and ImmunoGen and has served in a consulting or advisory role for Daiichi-Sankyo, Bristol-Myers Squibb, Arog, Pfizer, Novartis, Jazz, Celgene, AbbVie, Astellas, Genentech, Immunogen, Servier, Syndax, Trillium, Gilead, Amgen, Shattuck labs, and Agios. F.R. has received research support from BMS/Celgene, Amgen, Xencor, Syros, Abbvie; Taiho, Astex, Macrogenics, Biomea Fusion, and consultancy/advisory board/honoraria from BMS/Celgene, Novartis, Syros, Taiho, Agios, Jazz, and AstraZeneca. C.D.D. has received research funding from Abbvie, Agios, Astex, Immune onc, BMS/Celgene, Daiichi Sankyo, Calithera, and Loxo; consultant fees from Abbvie, Agios, Immune onc, BMS/Celgene, Daiichi Sankyo, and Cleave; and honoraria from Abbvie, Agios, Immune onc, BMS/Celgene, Daiichi Sankyo, Novartis, Medimmune, Takeda, Foghorn, and Aprea. N.P. has received honoraria from Incyte, Novartis, LFB Biotechnologies, Stemline Therapeutics, Celgene, AbbVie, MustangBio, Roche Diagnostics, Blueprint Medicines, DAVA Oncology, and Springer Science + Business Media LLC; grants from Novartis, Stemline Therapeutics, Samus Therapeutics, AbbVie, Cellectis, Affymetrix, Daiichi Sankyo, and plexxikon; is a consultant to Pacylex Pharmaceuticals, ImmunoGen, Bristol Myers Squibb, and Blueprint Medicines. M.Y.K. has received grants from National Institutes of Health, National Cancer Institute, Abbvie, Genentech, Stemline Therapeutics, Forty-Seven, Eli Lilly, Cellectis, Calithera, Ablynx, AstraZeneca; consulting/honoraria from AbbVie, Genentech, F. Hoffman La-Roche, Stemline Therapeutics, Amgen, Forty-Seven, Kisoji; clinical trial support from Ascentage; stocks/royalties in Reata Pharmaceutical. G.G.-M. has received research support from grant or research support from Amphivena, Helsinn, Novartis, Abbvie, BMS, Astex, Onconova, H3 Biomedicine, Merck, Curis, Janssen Genentech, Forty Seven, Aprea; and honoraria from BMS, Astex, Helsinn, and Genentech. S.V., G.B., N.J.S., H.A.A., and T.M.K. declare no competing financial interests.
Correspondence: Tapan M. Kadia, Department of Leukemia, The University of Texas MD Anderson Cancer Center, 1515 Holcombe Blvd, Unit 428, Houston, TX 77030; e-mail: Tkadia@mdanderson.org.
References
Author notes
Requests for data sharing may be submitted to Tapan M. Kadia (Tkadia@mdanderson.org).