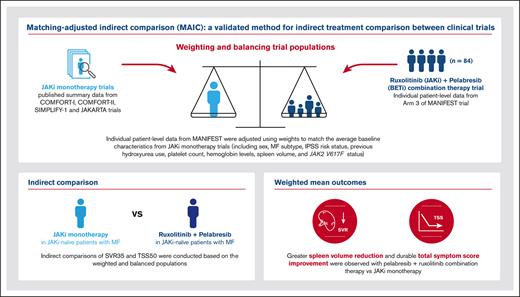
Key Points
Pelabresib plus ruxolitinib combination has potential for higher clinical efficacy than JAKi monotherapy in patients with MF.
Greater SVR and durable TSS improvement was observed with pelabresib plus ruxolitinib vs JAKi monotherapy.
Abstract
Janus kinase inhibitors (JAKis) ruxolitinib, fedratinib, and pacritinib are the current standard of care in symptomatic myelofibrosis (MF). However, progressive disease and toxicities frequently lead to JAKi discontinuation. Preclinical data indicate that combining JAK and bromodomain and extraterminal (BET) domain inhibition leads to overlapping effects in MF. Pelabresib (CPI-0610), an oral, small-molecule BET1,2 inhibitor (BETi), in combination with ruxolitinib showed improvements in spleen volume reduction (SVR35) and total symptom score reduction (TSS50) from baseline in the phase 2 MANIFEST study (NCT02158858) in patients with MF. Given the absence of a head-to-head clinical comparison between JAKi monotherapy and JAKi with BETi combination therapy, we performed an unanchored matching-adjusted indirect comparison analysis to adjust for differences between studies and allow for the comparison of SVR35, TSS50, and TSS measured at several timepoints in arm 3 of MANIFEST (pelabresib with ruxolitinib in JAKi treatment–naive patients with MF), with data from the following JAKi monotherapy studies in JAKi treatment–naive patients: COMFORT-I and COMFORT-II (ruxolitinib), SIMPLIFY-1 (ruxolitinib and momelotinib), and JAKARTA (fedratinib). Response rate ratios >1 were observed for pelabresib with ruxolitinib vs all comparators for SVR35 and TSS50 at week 24. Improvements in TSS were observed as early as week 12 and were durable. These results indicate that pelabresib with ruxolitinib may have a potentially higher efficacy than JAKi monotherapy in JAKi treatment–naive MF.
Introduction
Myelofibrosis (MF) is a life-threatening myeloproliferative neoplasm characterized by genetically transformed hematopoietic stem cells (clonal myeloproliferation), which leads to local and systemic bone marrow inflammation and accumulation of fibrous tissue in the bone marrow.3,4 The hallmarks of MF are cytopenias, enlarged spleen, disease symptoms, and bone marrow fibrosis.5-7 At all disease stages, MF is associated with significant clinical burden and impact on quality of life.6,8 MF can occur as a primary condition or secondary to polycythemia vera (PV) or essential thrombocythemia (ET).4,6,8
A number of inflammatory signaling pathways have been implicated in the pathophysiology of MF, including the Janus kinase (JAK), signal transducer and activator of transcription, and nuclear factor κB (NF-κB) pathways.9,10 JAK inhibition is a proven therapeutic strategy in MF. Two JAK inhibitors (JAKis), ruxolitinib11 and fedratinib,12 have been approved by the US Food and Drug Administration and European Medicines Agency for the treatment of adults who have intermediate- or high-risk MF.13-16 A third JAKi pacritinib is approved by the US Food and Drug Administration for patients with platelets <50 × 109/L.17 Momelotinib is a JAKi in clinical development as a potential treatment for relapsed/refractory MF with anemia.18
Approved JAKis have demonstrated splenic responses and symptomatic improvement in the following pivotal phase 3 trials: ruxolitinib in the COMFORT-I19 and COMFORT-II studies (NCT00952289 and NCT00934544, respectively),15 fedratinib in the JAKARTA study (NCT01437787),16 and pacritinib in the PERSIST-1 (NCT01773187)20 and PERSIST-2 (NCT02055781)21 studies. Despite promising results with JAKi therapy, progressive disease and toxicity frequently lead to JAKi discontinuation.16,22,23
Pelabresib (CPI-0610) is an investigational, oral, small-molecule bromodomain and extraterminal (BET) domain inhibitor.1,2 BET proteins regulate transcription of specific genes integrating an array of oncogenic signals, including NF-κB pathway activation.9 Inhibiting BET may modify critical MF pathways, including megakaryocyte differentiation and proliferation.9 For instance, bromodomain-containing protein 4 results in the reduction of proinflammatory cytokine expression via the NF-κB signaling pathway, which plays a key role in the proinflammatory state in MF.24 Preclinical data have indicated that the combination of JAK and BET inhibition can lead to overlapping effects in the treatment of MF.9 The combination of pelabresib with ruxolitinib showed a 68% response rate for ≥35% spleen volume reduction from baseline (SVR35) and a 56% response rate for ≥50% total symptom score reduction from baseline (TSS50) and was generally well tolerated in JAKi treatment–naive patients with intermediate- or high-risk MF in arm 3 of the open-label phase 2 MANIFEST study (NCT02158858).25 On this basis, it is of clinical interest to compare the combination of pelabresib with ruxolitinib with JAKi monotherapy in the MF setting.
To date, there are no available data directly comparing pelabresib with ruxolitinib vs JAKi monotherapy, and indirect comparisons between studies are subject to bias because of differences in study design, baseline patient characteristics, or outcome definitions.26 Matching-adjusted indirect comparison (MAIC) has been proposed as a validated method for indirect treatment comparison because of its potential to adjust for differences in baseline characteristics between studies. A robust comparison is achieved by matching individual patient-level data (IPLD) from clinical trials of 1 treatment with published aggregate summary statistics from trials of another treatment.26 There have been several recent examples of MAIC analyses across oncology trials,27-30 including a comparison of fedratinib vs ruxolitinib, which found that a higher proportion of patients treated with fedratinib achieved SVR35 at 24 weeks than with ruxolitinib in patients with MF who were ruxolitinib treatment naive.31
Here, we report the results of a MAIC designed to compare SVR and TSS improvements achieved with pelabresib and ruxolitinib in MANIFEST arm 3 with those from historical JAKi monotherapy studies in JAKi treatment–naive patients. Pacritinib studies were not included in the MAIC because of nonoverlapping baseline platelet count (<100 × 109/L) requirements vs those in MANIFEST arm 3.20,21 SVR35 and TSS50 are standard established end points used in clinical trials in MF; however, for symptom improvement, the 50% cutoff for TSS50 response may not accurately reflect the clinical benefit in patients with a TSS reduction of <50% or achieve TSS50 but could benefit from further symptomatic improvement.32 To overcome the limitations of using TSS50 alone, we also present a percentage change in TSS as a continuous end point from baseline at weeks 12, 24, 36, and 48 from MANIFEST arm 3 and performed indirect comparisons of the percentage change in TSS at 24 weeks observed in MANIFEST arm 3 with that of the historical JAKi trials.
SVR35 and TSS50 data for ruxolitinib monotherapy were obtained from the COMFORT-I and COMFORT-II studies15,19 and the ruxolitinib arm of SIMPLIFY-1, which was a phase 3 randomized trial of momelotinib vs ruxolitinib.33 Data for fedratinib monotherapy were derived from the JAKARTA trial,16 and data for momelotinib monotherapy were derived from the SIMPLIFY-1 trial.33 For TSS, data were derived from COMFORT-I (ruxolitinib),19 SIMPLIFY-1 (ruxolitinib and momelotinib),33 and JAKARTA (fedratinib 400 mg).16
Materials and methods
Data sources
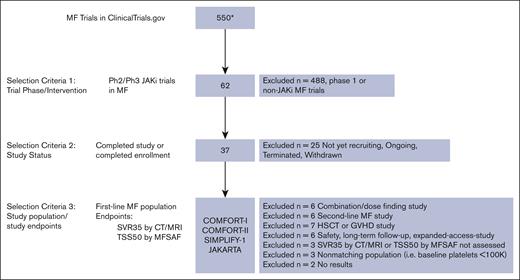
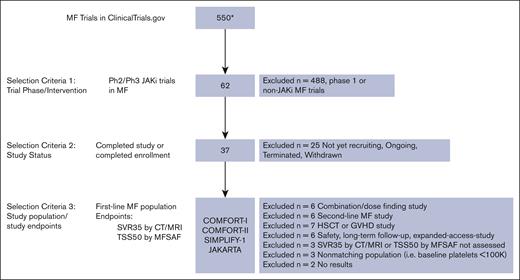
To identify clinical trials to include in the MAIC analysis, the initial search via clinicaltrials.gov at the time of analysis identified 550 MF trials. Of these, 62 were phase 2 or 3 trials investigating JAKis (ruxolitinib, fedratinib, momelotinib, pacritinib, and itacitinib), of which 37 were completed. These were further reviewed to identify and include trials conducted in populations of treatment–naive patients with MF with similar baseline characteristics and those that used similar tools to assess SVR35 and TSS50 end points as in MANIFEST arm 3. See Figure 1 for the selected studies flowchart.
MAIC study selection flowchart. ∗Indicates clinicaltrials.gov results yielded from search conducted on 12 August 2022. CT, computed tomography; GVHD, graft-versus-host disease; HSCT, hematopoietic stem cell transplantation; MFSAF, myelofibrosis symptom assessment form; MRI, magnetic resonance imaging; Ph, phase.
MAIC study selection flowchart. ∗Indicates clinicaltrials.gov results yielded from search conducted on 12 August 2022. CT, computed tomography; GVHD, graft-versus-host disease; HSCT, hematopoietic stem cell transplantation; MFSAF, myelofibrosis symptom assessment form; MRI, magnetic resonance imaging; Ph, phase.
IPLD from MANIFEST arm 3 (JAKi treatment–naive patients with MF treated with pelabresib and ruxolitinib) and published summary data from COMFORT-I, COMFORT-II, JAKARTA, and SIMPLIFY-1 studies were used to estimate the weights to perform the MAIC analysis. For the percentage change in TSS at 24 weeks, IPLD were available for MANIFEST arm 3. Percentage changes in TSS at 24 weeks for COMFORT-I, SIMPLIFY-1, and JAKARTA (fedratinib 400 mg arm) were extracted from published waterfall plots using WebPlotDigitizer.34 COMFORT-I was a randomized, double-blind, phase 3 trial comparing twice daily, oral ruxolitinib (n = 155) with placebo (n = 154) in patients with primary MF, post-PV MF, or post-ET MF.19 COMFORT-II was a randomized, phase 3 trial comparing ruxolitinib (n = 146) with the best available therapy (n = 73) in patients with primary MF, post-PV MF, or post-ET MF.15 JAKARTA was a double-blind, randomized, phase 3 study comparing once daily, oral fedratinib 400 mg (n = 96) or 500 mg (n = 97) with placebo (n = 96) in patients with intermediate-2 or high-risk primary MF, post-PV MF, or post-ET MF.16 SIMPLIFY-1 was a randomized, phase 3, noninferiority trial comparing momelotinib 200 mg once daily (n = 215) with ruxolitinib 20 mg twice daily (n = 217) in patients with high-, intermediate-2, or symptomatic intermediate-1–risk MF.33 The primary end point for COMFORT-I, COMFORT-II, SIMPLIFY-1, and JAKARTA was SVR35 at 24 weeks, as measured by magnetic resonance imaging or computed tomography.15,16,19,33 Definitions and end point assessment methods were reviewed to determine the comparability of the study end points between all studies (supplemental Table 1). All reviewed studies were approved by the institutional review boards of the respective institutions before patient enrollment and were conducted in accordance with the principles of the Declaration of Helsinki. All patients provided written informed consent.
MAIC analysis
Given the absence of a connected network of treatment arms, an unanchored MAIC analysis was performed, following the approach proposed by Signorovitch et al.35 IPLD from MANIFEST were extracted and adjusted using weights to match the average baseline characteristics for each comparator arm using the propensity score method. The weights were used to calculate the effective sample size (ESS) achieved after matching patients (∑wi)2 / (∑wi2), in which wi represents weights for the ith patient.36 The ESS indicates the number of independent nonweighted individuals that would be required to give an estimate with the same precision as the weighted sample estimate.37 A low ESS indicates high variability in the weights because of a lack of overlap between the study populations, meaning that only a small proportion of patients may be used to drive the treatment effect. A lower ESS also results in a loss of statistical significance.
Indirect comparisons were then conducted based on the weighted or balanced populations. The matching process included relevant prognostic and effect-modifying baseline characteristics reported in the published data that could affect relative treatment effects. Effect modifiers adjusted for in the unanchored MAIC analysis were sex, MF subtype, International Prognostic Scoring System (IPSS) risk status, previous hydroxyurea use, platelet count, hemoglobin levels, spleen volume, and JAK2 V617F status. The continuous variables in the comparator arms were dichotomized based on their median values so that each group had 50% of the patients.
COMFORT-I, COMFORT-II, and JAKARTA studies did not include patients with IPSS intermediate-1 risk, whereas SIMPLIFY-1 and MANIFEST arm 3 did. Therefore, we performed 2 MAICs:
Main analysis population: this population consisted of all patients from MANIFEST arm 3 who received at least 1 dose of the study drug but excluded patients with IPSS intermediate-1 risk. Patients with IPSS intermediate-2 risk in comparator studies were matched with patients with IPSS intermediate-2 risk in MANIFEST, and those with IPSS high risk in the comparator arms were matched with patients with IPSS high risk in MANIFEST.
Sensitivity analysis population: this population included patients with IPSS intermediate-1 risk, grouped together with patients with IPSS intermediate-2 risk. Patients from MANIFEST with IPSS intermediate-1 and -2 risk were matched with patients from comparator arms with IPSS intermediate-2 risk, and patients with IPSS high risk in MANIFEST were matched with those from comparator arms with IPSS high risk.
Patients in MANIFEST arm 3 were also excluded if any of the following baseline data were missing: sex, MF subtype, IPSS risk category, platelet count, hemoglobin, spleen volume, or transfusion dependency (n = 4; supplemental Table 2). Patients with missing baseline characteristics in the comparator studies were distributed in the nonmissing subcategories based on the distribution of the subcategories. Because IPLD baseline from comparator studies were not available, this was done to include all relevant information in the analysis.
Statistical analysis was performed using Statistical Analysis System version 9.4 (SAS Institute Inc, Cary, NC). Any missing end point values from MANIFEST arm 3 were imputed as if the patient did not reach the end point goal (ie, nonresponder).
Outcomes of interest
Treatment effect outcomes for SVR35 and TSS50 at week 24 were compared between 2 arms in terms of the response rate ratio (RRR; defined as the ratio of the response rate [RR] in MANIFEST arm 3 to that in the comparator arm). Weighted mean outcomes for these end points were estimated together with their 95% confidence interval (CI), using robust sandwich estimators for variance. An RRR of >1 demonstrates that the SVR35 and/or TSS50 outcome was improved with pelabresib and ruxolitinib in MANIFEST arm 3 vs the JAKi monotherapy comparator. TSS and its subdomains (fatigue, night sweats, itching, abdominal discomfort, pain under ribs, feeling of fullness, and bone pain) were evaluated in terms of the percentage change from baseline at weeks 12, 24, 36, and 48. For percentage change in TSS at 24 weeks as a continuous end point, weighted and unweighted treatment effects are presented in terms of difference in least squares means with their 95% CIs, using robust sandwich estimators for variance. Although studies used different versions of the TSS questionnaire, comparisons demonstrate reliable cross-sectional validity.38
Results
Baseline characteristics before matching
Baseline characteristic data for pelabresib with ruxolitinib were extracted from MANIFEST arm 3 (n = 84), with 80 patients included in the sensitivity analysis set (including patients with IPSS intermediate-1-risk status). Four patients were excluded from both the main and sensitivity analyses, including 3 patients with missing MF subtype data and 1 patient with missing platelet count data. Two of the 4 patients achieved SVR35 response, and 1 achieved TSS50 response (supplemental Table 2). Data were analyzed according to the treatment regimen: ruxolitinib from COMFORT-I (n = 155), COMFORT-II (n = 146), and SIMPLIFY-1 (n = 217); fedratinib 400 mg (n = 96) and fedratinib 500 mg (n = 97) from JAKARTA; and momelotinib (n = 215) from SIMPLIFY-1. Patient baseline characteristics from all included studies are summarized in Table 1. Compared with patients from both COMFORT studies and both treatment arms from JAKARTA, patients analyzed in the arm 3 of MANIFEST presented with a higher median platelet count, lower median hemoglobin level (except for that vs COMFORT-II, which did not report this), and lower spleen volume.
Matching of baseline characteristics
IPLD from MANIFEST arm 3 were adjusted to match the data for patients in COMFORT-I, COMFORT-II, JAKARTA, and SIMPLIFY-1. The distributions of weights before and after rescaling to the ESS are shown in supplemental Figures 1 and 2. Supplemental Figure 2 shows the proportion of patients excluded from the rescaled weighted analysis (rescaled weight = 0). The exclusion of these patients resulted in a smaller ESS. After adjusting for the weights, complete balance was achieved for 8 prognostic baseline variables between MANIFEST arm 3 and the comparator arms. Baseline characteristics before and after adjusting (unweighted vs weighted) for comparator studies are shown in supplemental Figure 3. The ESS for the main and sensitivity populations from all comparator studies are summarized in Table 2. Excluding the patients with IPSS intermediate-1 risk resulted in a smaller ESS in the main analysis than that in the sensitivity analysis.
SVR35
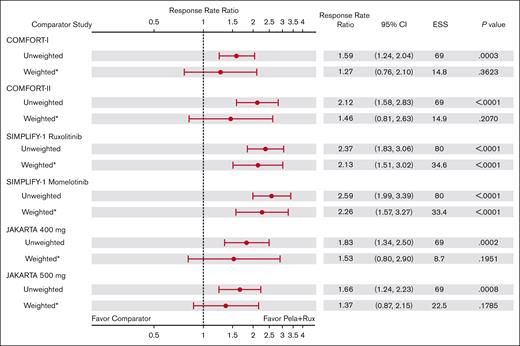
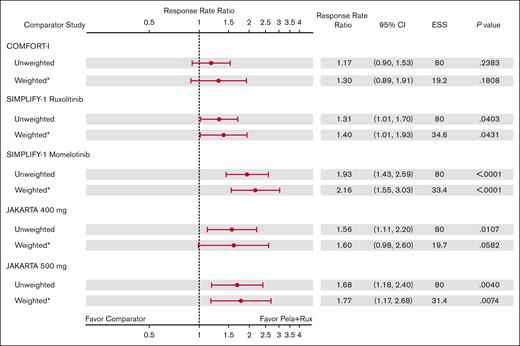
Excluding patients with IPSS intermediate-1 risk in the main analysis population resulted in a smaller ESS than that in the sensitivity analysis population, leading to larger standard errors, CIs, and P values. The RRR in the main analysis population for SVR35 at week 24 for pelabresib with ruxolitinib remained numerically higher (>1) than that of all comparator study arms. The RRR was 1.27 (95% CI, 0.76-2.10) vs COMFORT-I (ruxolitinib); 1.46 (95% CI, 0.81-2.63) vs COMFORT-II (ruxolitinib); 2.13 (95% CI, 1.51-3.02) vs SIMPLIFY-1 (ruxolitinib); 2.26 (95% CI, 1.57-3.27) vs SIMPLIFY-1 (momelotinib); 1.53 (95% CI, 0.80-2.90) vs JAKARTA 400 mg; and 1.37 (95% CI, 0.87-2.15) vs JAKARTA 500 mg (Figure 2); however, statistically significant RRR advantage with pelabresib plus ruxolitinib was only observed vs the ruxolitinib (P < .0001) and momelotinib (P < .0001) arms of SIMPLIFY-1. Comparisons vs COMFORT-I, COMFORT-II, and JAKARTA fedratinib 400 mg and 500 mg did not reach statistical significance.
RRRs of SVR35 at week 24 with pelabresib + ruxolitinib in MANIFEST arm 3 vs comparator arms: main analysis population (excluding patients with IPSS intermediate-1 risk). ∗Indicates that CIs and P values are calculated using the robust sandwich estimation of variance. RRR = (RR in Pela+Rux)/RR in the comparator arm. Pela, pelabresib; Rux, ruxolitinib.
RRRs of SVR35 at week 24 with pelabresib + ruxolitinib in MANIFEST arm 3 vs comparator arms: main analysis population (excluding patients with IPSS intermediate-1 risk). ∗Indicates that CIs and P values are calculated using the robust sandwich estimation of variance. RRR = (RR in Pela+Rux)/RR in the comparator arm. Pela, pelabresib; Rux, ruxolitinib.
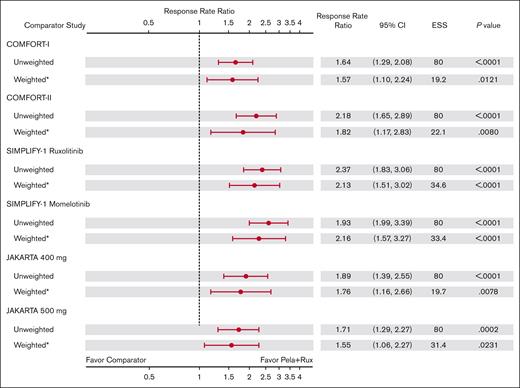
In the sensitivity analysis, MAIC-estimated weighted values for SVR35 at week 24 were statistically significantly higher in MANIFEST arm 3 than that in all comparators (Figure 3). RRR for SVR35 was 1.57 (95% CI, 1.10-2.24; P = .012) vs COMFORT-I, 1.82 (95% CI, 1.17-2.83; P = .008) vs COMFORT-II, 2.13 (95% CI, 1.51-3.02; P < .001) vs SIMPLIFY-1 (ruxolitinib), 2.26 (95% CI, 1.57-3.27; P < .001) vs SIMPLIFY-1 (momelotinib), 1.76 (95% CI, 1.16-2.66; P = .008) vs JAKARTA (fedratinib 400 mg), and 1.55 (95% CI, 1.06-2.27; P = .023) vs JAKARTA (fedratinib 500 mg). supplemental Figure 4 shows the SVR35 RRs in the sensitivity analysis population.
RRRs of SVR35 at week 24 with pelabresib + ruxolitinib in MANIFEST arm 3 vs comparator arms: sensitivity analysis population (including patients with IPSS intermediate-1 risk). ∗Indicates that CIs and P values are calculated using the robust sandwich estimation of variance. RRR = (RR in Pela+Rux)/RR in comparator arm.
RRRs of SVR35 at week 24 with pelabresib + ruxolitinib in MANIFEST arm 3 vs comparator arms: sensitivity analysis population (including patients with IPSS intermediate-1 risk). ∗Indicates that CIs and P values are calculated using the robust sandwich estimation of variance. RRR = (RR in Pela+Rux)/RR in comparator arm.
TSS50
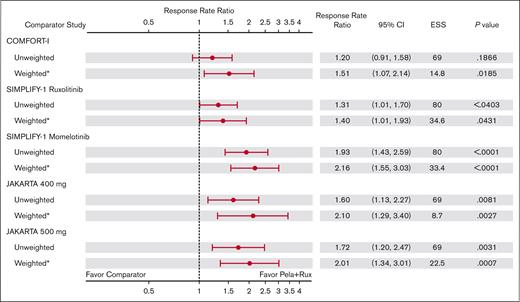
In the main analysis population, the MAIC-estimated weighted RRR values for TSS50 at week 24 were statistically significant and favored pelabresib with ruxolitinib therapy in MANIFEST arm 3 vs all comparator arms (Figure 4). RRR for TSS50 was 1.51 (95% CI, 1.07-2.14; P = .019) vs COMFORT-I (ruxolitinib); 1.40 (95% CI, 1.01-1.93; P = .043) vs SIMPLIFY-1 (ruxolitinib); 2.16 (95% CI, 1.55-3.03; P < .0001) vs SIMPLIFY-1 (momelotinib); 2.10 (95% CI, 1.29-3.40; P = .003) vs JAKARTA (fedratinib 400 mg); and 2.01 (95% CI, 1.34-3.01; P = .001) vs JAKARTA (fedratinib 500 mg). Because TSS50 was not evaluated in the COMFORT-II study, no comparative analysis for COMFORT-II was computed.
RRRs of TSS50 at week 24 with pelabresib + ruxolitinib in MANIFEST arm 3 vs comparator arms: main analysis population (excluding patients with IPSS intermediate-1 risk). ∗Indicates that CIs and P values are calculated using the robust sandwich estimation of variance. RRR = (RR in Pela+Rux)/RR in comparator arm.
RRRs of TSS50 at week 24 with pelabresib + ruxolitinib in MANIFEST arm 3 vs comparator arms: main analysis population (excluding patients with IPSS intermediate-1 risk). ∗Indicates that CIs and P values are calculated using the robust sandwich estimation of variance. RRR = (RR in Pela+Rux)/RR in comparator arm.
The RRR for pelabresib with ruxolitinib in MANIFEST arm 3 vs comparator JAKi monotherapy arms for TSS50 at week 24 in the sensitivity analysis population are shown in Figure 5. All RRRs were numerically higher (>1) for pelabresib with ruxolitinib in MANIFEST arm 3, and the RRR reached statistical significance for the comparisons with ruxolitinib (RRR, 1.40; 95% CI, 1.01-1.93; P = .043) and momelotinib (RRR, 2.16; 95% CI, 1.55-3.03; P < .0001) in SIMPLIFY-1 and fedratinib 500 mg in JAKARTA (RRR, 1.77; 95% CI, 1.17-2.68; P = .007). Supplemental Figure 5 shows the TSS50 RRs in the sensitivity analysis population.
RRRs of TSS50 at week 24 with pelabresib + ruxolitinib in MANIFEST arm 3 vs comparator arms: sensitivity analysis population (including patients with IPSS intermediate-1 risk). ∗Indicates that CIs and P values are calculated using the robust sandwich estimation of variance. RRR = (RR in Pela+Rux)/RR in comparator arm.
RRRs of TSS50 at week 24 with pelabresib + ruxolitinib in MANIFEST arm 3 vs comparator arms: sensitivity analysis population (including patients with IPSS intermediate-1 risk). ∗Indicates that CIs and P values are calculated using the robust sandwich estimation of variance. RRR = (RR in Pela+Rux)/RR in comparator arm.
Percentage change in TSS from baseline
Data for TSS as a continuous end point were analyzed in the following treatment arms: ruxolitinib with pelabresib in MANIFEST arm 3 (n = 84), ruxolitinib in COMFORT-I (n = 145), ruxolitinib (n = 211) and momelotinib (n = 211) in SIMPLIFY-1, and fedratinib 400 mg in JAKARTA (n = 91). Patients with missing TSS values at week 24 were excluded from the continuous end point analysis: n = 5 from MANIFEST arm 3, n = 0 from COMFORT-I, n = 21 from SIMPLIFY-1 (ruxolitinib), n = 37 from SIMPLIFY-1 (momelotinib), and n = 23 from JAKARTA.
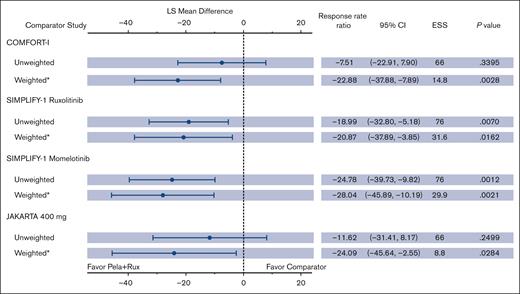
At baseline, TSSs were comparable between ruxolitinib with pelabresib from MANIFEST arm 3 (16.1), ruxolitinib from COMFORT-I (18.2), ruxolitinib (17.9) and momelotinib (19.4) from SIMPLIFY-1, and fedratinib 400 mg from JAKARTA (17.5). In MANIFEST arm 3, a median reduction in TSS from baseline of >50% was observed; this reduction remained consistent from week 12 to week 48 (supplemental Figure 6). TSS distribution in MANIFEST arm 3 at week 24 showed greater median and mean reductions from baseline vs all comparator JAKi monotherapy study arms (supplemental Figure 7). Figure 6 shows the least squares mean percentage change in TSS from baseline to week 24 for MANIFEST arm 3 and comparator studies in the weighted and unweighted analyses. In the weighted analysis, the reduction observed in MANIFEST arm 3 was >20% greater than that observed for all JAKi monotherapy studies, and P values for all comparisons were statistically significant.
Change from baseline in TSS as a continuous end point: Naive and MAIC comparison. ∗Indicates that CIs and P values are calculated using the robust sandwich estimation of variance. LS mean difference = (LS mean for pela + rux) – (LS mean for comparator arm). LS, least squares.
Change from baseline in TSS as a continuous end point: Naive and MAIC comparison. ∗Indicates that CIs and P values are calculated using the robust sandwich estimation of variance. LS mean difference = (LS mean for pela + rux) – (LS mean for comparator arm). LS, least squares.
Discussion
Preclinical data indicate the combination of JAK inhibition and BET inhibition can lead to overlapping beneficial effects in the treatment of MF.9 The combination of pelabresib with ruxolitinib resulted in SVR35 in 68% and TSS50 in 56% of JAKi treatment–naive patients with intermediate- or high-risk MF in arm 3 of the open-label, phase 2 MANIFEST study.39 Thus, it is of clinical interest to compare the combination of pelabresib with ruxolitinib with JAKi monotherapy in the MF setting. This analysis used MAIC methodology to facilitate a valid comparison with MF therapies in the absence of available comparative data. Data from MANIFEST arm 3 (pelabresib with ruxolitinib) were compared with ruxolitinib treatment arms in COMFORT-I, COMFORT-II, and SIMPLIFY-1; the momelotinib arm in SIMPLIFY-1; and fedratinib 400 mg and 500 mg arms in JAKARTA. The results showed considerable improvements with the combination of pelabresib and ruxolitinib vs ruxolitinib, momelotinib, or fedratinib monotherapy in SVR35 and TSS50 rates at week 24 as well as durable improvements in TSS observed as early as week 12.
The MAIC approach is well established and enables reliable comparisons to be made between treatments when indirect comparisons based only on published aggregate data are limited by cross-trial differences.26 The purpose of the MAIC approach was to address potential bias because of differences in baseline characteristics to facilitate a balanced comparison of outcome data between the studies. Before weighting, observed differences included the following: in the comparison with SIMPLIFY-1, a lower percentage of patients with primary MF in arm 3 of MANIFEST but higher than that in ruxolitinib vs momelotinib; IPSS high-risk status was less common in arm 3 of MANIFEST than in COMFORT-I and COMFORT-II but more common than comparators in other studies; the platelet count in arm 3 of MANIFEST was higher than that in comparator arms across all studies; hemoglobin level in arm 3 of MANIFEST was lower than that in comparator arms across all studies; and spleen volume was lower in arm 3 of MANIFEST than that in COMFORT-I, COMFORT-II, and JAKARTA.
By using IPLD available only for 1 comparator treatment, MAICs may substantially increase the availability and reliability of comparative evidence compared with naive indirect comparison.26 Bias due to potential unmeasured differences in patient populations between trials in MAIC is a limitation and cannot be excluded; however, efforts were made to decrease the chance of bias by including relevant prognostic and effect-modifying baseline characteristics that are well characterized and studied for MF. A considerable number of prognostic factors were used in this study, making the matching robust. It is not possible to use established methods for checking the fit and calibration of the propensity score model because of the availability of only aggregate data for the comparator trials. Exclusion of IPSS intermediate-1 from the main analysis population led to a lower ESS (especially for comparisons with COMFORT-I and -II and JAKARTA 400 mg). This resulted in larger standard errors and CIs. Although in some instances statistical significance was not achieved because of this limitation, a numerically larger treatment benefit with respect to both SVR and TSS was observed in all cases for patients treated with pelabresib + ruxolitinib compared with in those treated with ruxolitinib alone. The limitation of a smaller ESS was overcome in the sensitivity analysis by including all patients, for whom statistical significance and positive treatment effect was observed in all comparisons with respect to SVR and TSS.
Although this analysis was focused on an indirect comparison of efficacy outcomes between pelabresib in combination with ruxolitinib and JAKi monotherapies, data from arm 3 of MANIFEST indicated that pelabresib in combination with ruxolitinib was well tolerated.25 The safety profile of combination therapy is consistent with that of ruxolitinib monotherapy reported in the COMFORT-I19 and COMFORT-II15 studies.
In conclusion, this analysis, performed using established MAIC methodology, indicates that pelabresib in combination with ruxolitinib has the potential to achieve clinically meaningful improvements in SVR35 and TSS50 rates at week 24 vs ruxolitinib, momelotinib, or fedratinib monotherapy. A randomized, double-blind, phase 3 study (MANIFEST-2; NCT04603495) comparing the safety and efficacy of pelabresib in combination with ruxolitinib vs ruxolitinib with placebo in JAKi treatment–naive patients is ongoing.
Acknowledgments
Writing support was provided by Laura Travers, of LiNK Medical, with funding from Constellation Pharmaceuticals Inc, a MorphoSys company.
Authorship
Contribution: D.D. conceived and designed the study and acquired and analyzed the data; and all authors were involved in drafting and critically revising the content, interpretation of results, and final approval of the version to be published, and agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Conflict-of-interest disclosure: V.G. received consulting fees from AbbVie, Biopharma, Bristol Myers Squibb (BMS)/Celgene, Constellation Pharmaceuticals Inc, a MorphoSys company, Incyte, Novartis, Pfizer, and Sierra Oncology; payment honoraria from BMS/Celgene, Constellation Pharmaceuticals Inc, a MorphoSys company, and Novartis; and participates on a data safety or advisory board for AbbVie, BMS/Celgene, Incyte, Pfizer, and Roche. J.M. received consulting fees from BMS/Celgene, Constellation Pharmaceuticals Inc, a MorphoSys company, Cell Therapeutics Inc (CTI) BioPharma, Galecto, Geron, GlaxoSmithKline (GSK), Incyte, Kartos, Karyopharm, Novartis, PharmaEssentia, Prelude, Roche, and Sierra Oncology, and research funding from Biopharma, CTI Biopharma, Geron, Incyte, Kartos, Merck, Novartis, PharmaEssentia, and Roche. M.K. received consulting fees from Constellation Pharmaceuticals Inc, a MorphoSys company, CTI BioPharma, Incyte, and Protagonist Therapeutics, and research funding from Astellas, Astex, BMS, Chimerix, Constellation Pharmaceuticals Inc, a MorphoSys company, Incyte, Kronos, Kura, and Protagonist. R.K.R. received consulting fees from AbbVie, Blueprint Medicines, BMS/Celgene, Constellation Pharmaceuticals Inc, a MorphoSys company, CTI BioPharma, Disc Medicine, Galecto, Incyte, Jazz Pharmaceuticals, Novartis, PharmaEssentia, Promedior, Sierra Oncology, Stemline Therapeutics, and Sunimoto Dainippon, and research funding from Constellation Pharmaceuticals Inc, a MorphoSys company, Incyte, Stemline Therapeutics, and Zentalis. M.T. received research funding from Arcus and BMS, and has participated in advisory boards for Arcus, BMS, GSK, Imago BioSciences, Kyowa Kirin, Novartis, SDP (Sumitomo), and Sierra Oncology. J.-J.K. received payment honoraria from Novartis and participates on a data safety or advisory board for AbbVie, AOP Orphan, BMS, Incyte, and Novartis. A.M.V. received payment honoraria from AbbVie, BMS, Incyte, Novartis, and Roche, and participates on a data safety monitoring board or advisory board for AbbVie, Blueprint Medicines, BMS, Incyte, and Novartis. S.V. received research funding from Constellation Pharmaceuticals Inc, a MorphoSys company, and participates on advisory boards for Constellation Pharmaceuticals Inc, a MorphoSys company. G.C. is employed by Constellation Pharmaceuticals, Inc, a MorphoSys company and is a current holder of stock options in MorphoSys AG, a privately held company. D.D. is employed by MorphoSys AG. C.H. received consulting fees from AOP, Galecto, Keros, and Roche; research funding from Celgene, Constellation Pharmaceuticals, a MorphoSys company, and Novartis; acts in an advisory role and receives speaker funding from AbbVie, Celgene, CTI Biopharma, Constellation Pharmaceuticals Inc, a MorphoSys company, Janssen, and Novartis; received support for attending meetings from Novartis; serves on advisory boards for AbbVie, AOP Pharma, CTI BioPharma, Galecto, Geron, Promedior, and Roche; and participates in a leadership role at the European Hematology Association and MPN Voice.
The current affiliation for S.V. is Kartos Therapeutics, Redwood, CA.
Correspondence: Vikas Gupta, Princess Margaret Hospital, Medical Oncology and Hematology, 610 University Ave, Toronto, ON M5G2M9, Canada; e-mail: vikas.gupta@uhn.ca.
References
Author notes
Data from the MANIFEST study are available on request, starting 12 months from acceptance of the manuscript and until 36 months thereafter, only for noncommercial use and on a case-by-case basis from MorphoSys (delphine.elmehdi@morphosys.com); approval may be subject to a data access agreement.
The full-text version of this article contains a data supplement.