TO THE EDITOR:
Mycosis fungoides (MF) is the most common subtype of cutaneous T-cell lymphoma that initially presents in the skin but can progress to involve blood and extracutaneous sites with significant mortality in advanced stages.1 Even in early stages and in the absence of blood involvement, changes in the blood T-cell receptor (TCR) repertoire have been observed in patients with MF.2-5
Indeed, the detection of a monoclonal TCR gene rearrangement by polymerase chain reaction (PCR) in the blood of patients with early-stage MF has been linked to worse overall survival,6 lower response rates,7 and a shorter time to systemic treatment (TTST).8 The impact of dominant blood T-cell clones in early MF becomes more complex if the relationship of the detectable clones in the blood and skin is interrogated. A prior study, using PCR, demonstrated that identical, but not discordant, dominant clones in blood and skin of patients with MF increased the likelihood of disease progression.9
Although prior work has explored the significance of dominant blood T-cell clones in the prognosis of MF,1,9-12 PCR-based assays do not allow for a full characterization of TCR sequences and cannot adequately compare T-cell repertoires in the skin and blood. Here, we used high-throughput TCR sequencing to interrogate skin and blood T-cell repertoires in a large cohort of patients with early-stage MF over the course of their disease. We aimed to determine the relationship of blood and skin T-cell repertories and reassess the impact of dominant blood T-cell clones on selected outcome end points in early-stage MF.
Sixty patients with a confirmed histopathological diagnosis of MF without blood involvement were enrolled at Jefferson’s Cutaneous Lymphoma Clinic (supplemental Tables 1-4). Each patient was staged according to the criteria by Olsen et al,13 and 50 of 60 patients (83%) had early-stage MF (IA-IIA; supplemental Tables 1-3). We used high-throughput TCR sequencing to interrogate the TCRβ complementarity determining region 3 (CDR3) sequences in blood and lesional skin biopsies of enrolled patients (Adaptive Biotechnologies). ImmunoSEQ Analyzer provided T-cell repertoire overlap metric (Morisita index) and diversity measure (Simpson clonality score).3,14-16 TTST was calculated as the time from initial diagnosis to the initiation of the first systemic therapy.
Using ClonoSEQ criteria to establish dominancy,17 we found that 17 of 60 patients (28%) had a dominant T-cell clone in blood. Of these 17 patients, 3 (18%) had an identical dominant T-cell clone in both skin and blood, and 14 (82%) had a discordant dominant clone identified in the skin and blood (supplemental Table 1).
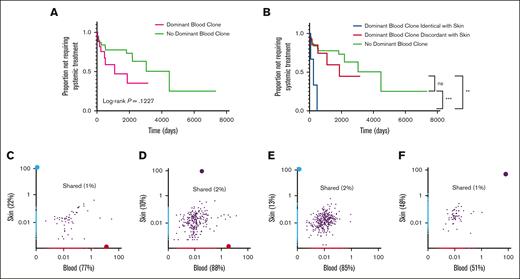
We compared the TTST in patients with a dominant blood clone, regardless of whether they were identical to or discordant with the skin clone, with TTST in patients without a dominant blood clone (supplemental Table 5). We did not find a significant difference in the TTST between these 2 groups (P = .1227; Figure 1A). When we examined the TTST based on the presence or absence of the same dominant T-cell clones in the blood and skin, we found that patients with discordant dominant T-cell clones in the blood and skin as well as those with no dominant blood clones had a longer TTST than those with identical dominant T-cell clones (P = .0057 and P < .0003, respectively; Figure 1B).
Kaplan-Meier analyses of TTST in skin-limited MF and representative plots of the frequency of productive TCRβ CDR3 rearrangements in the blood and skin. Kaplan-Meier analyses of TTST in patients with skin-limited MF (A) with and without a dominant T-cell clone in blood, (B) with an identical dominant T-cell clone in the blood and skin, a discordant dominant T-cell clone in blood and skin, and no dominant blood clones. (C-F) Representative plots of patients’ data depicting the frequency of all TCRβ CDR3 productive sequences either unique to the skin (blue) or blood (red) or shared between blood and skin (purple) in patients with skin-limited MF (C-D) with discordant dominant T-cell clones in the blood and skin, (E) with a dominant clone in the skin but no dominant clone in the blood, and (F) with an identical dominant T-cell clone in the blood and skin. The total numbers of CDR3 sequences for each plot of panel C: 890 (22%), skin; 3121 (77%), blood; and 50 (1%) shared; panel D: 1104 (10%), skin; 9474 (88%), blood; and 227 (2%), shared; panel E: 1515 (13%), skin; 9957 (85%), blood; and 307 (2%) shared; and panel F: 2183 (48%), skin; 2301 (51%), blood; 52 (1%), shared. ∗∗P = .0057; ∗∗∗P < .0003. NS, not significant, P = .5274.
Kaplan-Meier analyses of TTST in skin-limited MF and representative plots of the frequency of productive TCRβ CDR3 rearrangements in the blood and skin. Kaplan-Meier analyses of TTST in patients with skin-limited MF (A) with and without a dominant T-cell clone in blood, (B) with an identical dominant T-cell clone in the blood and skin, a discordant dominant T-cell clone in blood and skin, and no dominant blood clones. (C-F) Representative plots of patients’ data depicting the frequency of all TCRβ CDR3 productive sequences either unique to the skin (blue) or blood (red) or shared between blood and skin (purple) in patients with skin-limited MF (C-D) with discordant dominant T-cell clones in the blood and skin, (E) with a dominant clone in the skin but no dominant clone in the blood, and (F) with an identical dominant T-cell clone in the blood and skin. The total numbers of CDR3 sequences for each plot of panel C: 890 (22%), skin; 3121 (77%), blood; and 50 (1%) shared; panel D: 1104 (10%), skin; 9474 (88%), blood; and 227 (2%), shared; panel E: 1515 (13%), skin; 9957 (85%), blood; and 307 (2%) shared; and panel F: 2183 (48%), skin; 2301 (51%), blood; 52 (1%), shared. ∗∗P = .0057; ∗∗∗P < .0003. NS, not significant, P = .5274.
In order to examine the relationship of blood and skin T-cell clones in MF, we then compared all unique sequences with productive TCRβ CDR3 rearrangements among blood and skin compartments. Representative plots of the frequency of productive T-cell rearrangements among individual patients are shown in Figure 1C-F. We found that, overall, a minority of T-cell clones were shared between the blood and skin, whereas the majority of T-cell clones were present only in the blood or skin compartments (Figure 1C-F; supplemental Figure 1). We identified 4 major patterns of the distribution of dominant clones in our cohort. The first pattern showed a dominant T-cell clone in the skin that was discordant with the dominant clone in the blood (Figure 1C). Second, the dominant clone in the skin was found in the blood but did not reach the level of dominancy in the blood (Figure 1D). Third, the dominant clone in the skin was not present in the blood (Figure 1E). Lastly, dominant clones were identical in both the blood and skin (Figure 1F).
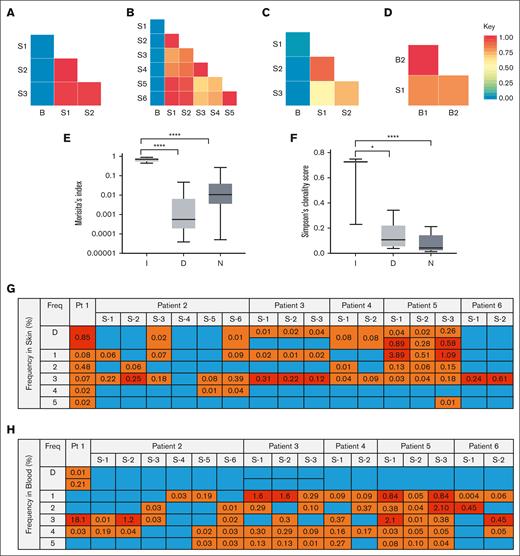
Next, we quantified the degree of T-cell overlap between the blood and skin. We found that when dominant T-cell clones in the blood and skin of those with nonleukemic MF were different, the degree of overall T-cell repertoire overlap between the blood and skin was low, correlating with a lower Morisita score (Figure 2A-C; supplemental Figure 2). In contrast, when the dominant clones in the blood and skin were the same, there was a higher degree of T-cell repertoire overlap (Figure 2D; supplemental Figure 2). Interestingly, the degree of T-cell overlap in the blood and skin appeared to not significantly change over a period of months to years or among skin biopsies obtained from different anatomical locations (Figure 2A-D).
T-cell repertoire overlap between the blood and skin, diversity of the blood T-cell repertoire, and distribution of most frequent T-cell sequences in the blood and skin. (A-D) The degree of clonal overlap between indicated samples measured by Morisita index is demonstrated by a graded color range according to the key, in which blue represents a score of 0 (no overlap) and red represents a score of 1 (maximum overlap) in representative patients. Samples isolated from skin are labeled S, and blood samples are labeled B. (A) Patient with a discordant dominant T-cell clone in the blood and skin. B, year 0; S1, year 1; and S2/S3, year 0. (B) Patient with a discordant dominant T-cell clone in the blood and skin over time. B, year 4; S6, year 0; S5, year 4; S3/S4, year 3 month 1; and S1/S2, year 3 month 10. (C) Patient with a dominant clone in the skin but no dominant clone in the blood. B, month 1; S3, month 2; and S1/S2, month 0. (D) Patient with an identical dominant T-cell clone in the blood and skin. B1, year 0; B2, year 1; and S1, year 0. (E) Average Morisita index for blood and skin overlap and (F) average Simpson clonality score in the blood for patients in indicated groups I, identical dominant clones in the blood and skin; D, discordant dominant clones in the blood and skin; and N, no dominant clone in blood. ∗∗∗∗P < .0001; ∗P = .0130. (G) The frequency of D (dominant) and top 5 most frequent individual blood clones in the skin in patients with discordant dominant blood and skin clones. Numbers represent the frequency (%) of the indicated blood clone in the skin. Freq: the order of frequency of the clones in blood. Blue boxes represent sequences not found in skin, orange boxes represent sequences that were found in skin, and red boxes represent sequences found at high frequency (top 5) in the skin. S-1, S-2, etc represent different biopsy sites. (H) The frequency of D (dominant) and top 5 most frequent individual skin clones in the blood in patients with discordant dominant blood and skin clones. Numbers represent the frequency (%) of the indicated skin clone in blood. Freq: the order of frequency of the clones in skin. Blue boxes represent sequences not found in blood, orange boxes represent sequences that were found in blood, and red boxes represent sequences found at high frequency (top 5) in the blood.
T-cell repertoire overlap between the blood and skin, diversity of the blood T-cell repertoire, and distribution of most frequent T-cell sequences in the blood and skin. (A-D) The degree of clonal overlap between indicated samples measured by Morisita index is demonstrated by a graded color range according to the key, in which blue represents a score of 0 (no overlap) and red represents a score of 1 (maximum overlap) in representative patients. Samples isolated from skin are labeled S, and blood samples are labeled B. (A) Patient with a discordant dominant T-cell clone in the blood and skin. B, year 0; S1, year 1; and S2/S3, year 0. (B) Patient with a discordant dominant T-cell clone in the blood and skin over time. B, year 4; S6, year 0; S5, year 4; S3/S4, year 3 month 1; and S1/S2, year 3 month 10. (C) Patient with a dominant clone in the skin but no dominant clone in the blood. B, month 1; S3, month 2; and S1/S2, month 0. (D) Patient with an identical dominant T-cell clone in the blood and skin. B1, year 0; B2, year 1; and S1, year 0. (E) Average Morisita index for blood and skin overlap and (F) average Simpson clonality score in the blood for patients in indicated groups I, identical dominant clones in the blood and skin; D, discordant dominant clones in the blood and skin; and N, no dominant clone in blood. ∗∗∗∗P < .0001; ∗P = .0130. (G) The frequency of D (dominant) and top 5 most frequent individual blood clones in the skin in patients with discordant dominant blood and skin clones. Numbers represent the frequency (%) of the indicated blood clone in the skin. Freq: the order of frequency of the clones in blood. Blue boxes represent sequences not found in skin, orange boxes represent sequences that were found in skin, and red boxes represent sequences found at high frequency (top 5) in the skin. S-1, S-2, etc represent different biopsy sites. (H) The frequency of D (dominant) and top 5 most frequent individual skin clones in the blood in patients with discordant dominant blood and skin clones. Numbers represent the frequency (%) of the indicated skin clone in blood. Freq: the order of frequency of the clones in skin. Blue boxes represent sequences not found in blood, orange boxes represent sequences that were found in blood, and red boxes represent sequences found at high frequency (top 5) in the blood.
Combining all patients from the representative patterns described earlier, those with discordant dominant T-cell clones and those with no dominant blood clones had lower degrees of overall T-cell repertoire overlap between the blood and skin when compared with those with identical dominant blood and skin T-cell clones (P < .0001; Figure 2E). Because changes in the blood T-cell repertoire diversity are detected in patients with MF regardless of the stage, we then assessed the blood T-cell repertoire diversity based on the relationship of dominant T-cell clones in the blood and skin. Patients with discordant dominant T-cell clones in the blood and skin and patients with no dominant blood clones had higher blood T-cell repertoire diversity than those with identical dominant clones in the blood and skin (P = .013 and P < .0001, respectively; Figure 2F).
To detect the overlap of skin and blood T-cell clones at a higher resolution, we narrowed our analysis to the dominant and top 5 frequent T-cell sequences for all patients with discordant clones. We found that the most dominant and high-frequency blood clones were detected in the skin, albeit at much lower frequencies (Figure 2G; supplemental Figure 3A). The same pattern persisted when detecting high-frequency skin clones in the blood, except for the dominant, presumably malignant skin clone, which mostly did not appear to be present in the blood, even at very low frequencies (Figure 2H; supplemental Figure 3B). Few clones shared their top 5 high-frequency states in both the blood and skin compartments (Figure 2G-H, red squares).
Overall, we examined the complex relationship of blood and skin T-cell repertoires and their relevance to prognosis in skin-limited MF. We found that the mere presence of a dominant blood T-cell clone in MF may not be sufficient to predict prognosis, rather the clonal association between blood and skin dominant clones is the key determining factor.
Dominant blood T-cell clones or T-cell expansions of undetermined significance12 can be detected in 8.7% of healthy individuals aged >65 years, benign inflammatory skin disorders, or after exposure to cytomegalovirus.18,19 Fourteen of 60 patients with early-stage MF (23%) in our cohort had a dominant blood clone discordant with that of the skin, an occurrence higher than expected for the general population and in agreement with prior MF studies.9,10,12,18,20-22 Notably, patients with discordant clones in our cohort did not have cytomegalovirus-specific CDR3 sequences in the blood compartment (supplemental Table 6). The dominant blood clone in the discordant group may represent a distinct population of antitumor T cells, such as CD8+ effector T cells, as previously observed in MF,17,23 or large granular lymphocytes.24
The most striking finding of our study was that the T-cell repertoire overlap in the blood and skin of those with nonleukemic MF appeared to be low over time and across different sites. In addition, the dominant, presumably malignant skin clone, did not appear to be detected in the blood even at low frequencies in a subset of patients. These findings suggest that not all patients with MF have reservoirs of malignant T-cell clones readily available in peripheral circulation to seed new lesions in skin. Additionally, these data support the notion that skin-resident T cells, including malignant clones, are distinct from blood T cells and may not continuously recirculate in the blood.22,25 Lastly, we showed that blood T-cell repertoire diversity was influenced by the relationship of the dominant clones in blood and skin. Interestingly, despite having a dominant blood clone in the discordant cohort, the blood T-cell repertoire diversity was comparable with that of those without dominant clones in the blood.
Our data highlight the importance of establishing clonal associations between dominant T-cell clones of the skin and blood in MF. We propose that patients with early-stage MF with dominant clones in the blood be segregated into 2 cohorts with distinct prognoses and T-cell repertoires based on the identity of their peripheral T-cell clones. Future multicenter prospective studies are needed to evaluate the relevance of these findings to management of patients with MF.
This retrospective review of patient data was approved by the Thomas Jefferson University Institutional Review Board (#21E.724).
Acknowledgments: The authors thank Joshua Banks from the biostatistician division at Thomas Jefferson University for his help with statistics.
N.N. is the recipient of National Cancer Institute grant R03CA2528180.
Contribution: D.J. collected, analyzed, and interpreted data, performed statistical analysis, and wrote the manuscript; S.B., L.B., R.Z., and L.G. collected data; A.M., I.K., and P.P. analyzed and interpreted data; and N.N. designed the research and helped write the manuscript.
Conflict-of-interest disclosure: N.N is an advisory board member for Helsinn and Kyowa Kirin. I.K. is a full-time employee and holder of equity in Adaptive Biotechnologies. The remaining authors declare no competing financial interests.
Correspondence: Neda Nikbakht, Department of Dermatology and Cutaneous Biology, Thomas Jefferson University, 233 S 10th St, Room 409, Philadelphia, PA 19107; e-mail: neda.nikbakht@jefferson.edu.
References
Author notes
Sequencing data can be found in immuneACCESS (https://clients.adaptivebiotech.com/pub/joffe-2023-ba doi 10.21417/DJ2023BA).
Data of the remaining patients are available in the following workbook at https://docs.google.com/spreadsheets/d/146dQutkemMCA9sZ1Nob95MzV-k_UlDBc/edit?usp=drive_link&ouid=109122257459764123209&rtpof=true&sd=true.
Other data are available on request from the corresponding author, Neda Nikbakht (neda.nikbakht@jefferson.edu).
The full-text version of this article contains a data supplement.