TO THE EDITOR:
Infections are the most common complication of treatment with novel immunotherapies and the second most common cause of death in multiple myeloma after the disease itself.1 Information on the incidence of infections in patients with relapsed/refractory myeloma treated with bispecific antibodies (BsAbs) is important for adjusting therapy dose, frequency, and timing, as well as selecting and intensifying preventive measures, especially because these therapies are associated with a disproportionately high risk of infection.2 However, infection frequencies reported in individual studies vary widely, leading to potentially biased comparisons of infection rates between studies due to the large differences in observation time. This appears to be particularly relevant given the cumulative incidence of infections during treatment with BsAbs. In 1 study, the number of patients with infections increased from 41% at 3 months to 64% at 12 months, and this pattern was the same for bacterial, viral, and fungal infections.3
Therefore, we sought to review the existing evidence on the incidence of infections in BsAb-treated patients and propose a harmonized reporting model for infections due to inconsistent description of infection burdens.4 We considered all publications and abstracts that included ≥50 patients, published by 31 December 2023,5-14 and excluded studies combining BsAbs with CD38 antibodies or other myeloma drugs, because these have been shown to increase the risk of infection, potentially distorting the true effect of BsAbs on infection risk. Of note, premedication with dexamethasone in immunosuppressive doses (16 or 20 mg) before the administration of the currently approved BsAbs (teclistamab, talquetamab, and elranatamab) is recommended, which may increase the infection risk.
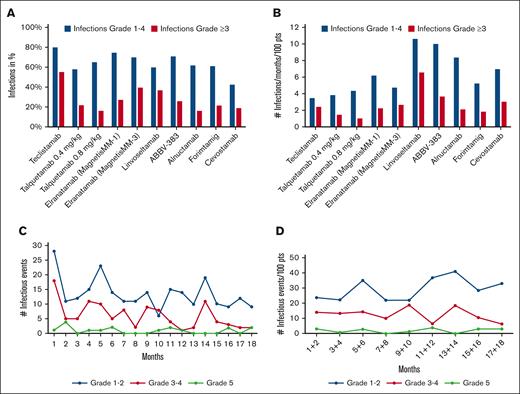
Among the 9 studies identified (the MonumenTAL-1 study results include 2 dose levels), infection rates varied widely, ranging from 42.5% for cevostamab to 80% for teclistamab (Table 1). These findings partly reflect the different duration of follow-up, given the cumulative pattern of infection incidence.3 In addition, the risk of infection is lower in BsAbs targeting GPRC5D (G protein-coupled receptor class C group 5 member) or FcRH5 (Fc receptor homolog 5) because neutropenia, B-cell depletion, and hypogammaglobulinemia are less severe with the later BsAbs.15,16 To correct the reported data (Figure 1A) for the unequal observation periods, we adjusted the data on the incidence of infections using the following formula: percentage of infections per 100 patients per month. Using this equation, the risk of infections varied between 3.5 and 10.7 per 100 patients per month (Figure 1B). To assess whether the incidence of infections varies at different time points during BsAbs treatment, we aimed to obtain data on the temporal incidence of infections. We used the WebPlotDigitizer17 to plot the data reported by Nooka et al for the MajesTEC-1 study,5 focusing on infection numbers over the first 18 months of teclistamab therapy, generating an image showing the monthly infections rate. In contrast to common trial practice, in which an adverse event (AE) is only counted once,18 albeit by documenting its highest grade, we counted every infection observed during the entire follow-up period. The resulting graph showed a higher incidence of infections within the first month of therapy, both for grade 1/2 and grade 3/4 infections (Figure 1C). Besides minor peaks around months 5 and 14, a general tendency for a decrease in the infection rates became evident. Importantly, Figure 1C does not account for the decreasing number of patients still on study. Therefore, we corrected the data for the number of patients in each time period, revealing a fairly even distribution of infections, including both those with moderate and higher grade. A comparison of the infection rates between the first 8 and the subsequent 8 months of therapy showed no statistically significant difference (Wilcoxon signed-rank test, P = .114; Figure 1D).
Graphical representation of incidence of infections. (A) Incidence of infections and of grade ≥3 infections in percent reported in studies with BsABs. (B) The incidence of infections normalized to a 1-month observation period per 100 patients. (C) The incidence of each infectious event per month in the MajesTEC-1 trial. (D) The incidence of each infectious event every 2-months period normalized to 100 patients in the MajesTEC-1 trial (data digitized from Nooka et al4).
Graphical representation of incidence of infections. (A) Incidence of infections and of grade ≥3 infections in percent reported in studies with BsABs. (B) The incidence of infections normalized to a 1-month observation period per 100 patients. (C) The incidence of each infectious event per month in the MajesTEC-1 trial. (D) The incidence of each infectious event every 2-months period normalized to 100 patients in the MajesTEC-1 trial (data digitized from Nooka et al4).
The observed pattern is noteworthy because of 2 reasons. First, contrary to clinicians expectations, the MajesTEC-1 study data do not show a decrease in infection rates per patient with ongoing therapy; Secondly, the relatively constant incidence of infections suggests that most of the infection risk is due to immunosuppression by the BsAb therapy rather than due to poorly controlled myeloma,19 because most of these patients must have achieved a myeloma response to justify continued treatment. Another important feature of this analysis is the much higher incidence of infections by counting each infectious episode. The usual study practice of reporting AEs resulted in 123 infections in the cohort of 156 patients in the MajesTEC-1 trial, but when each individual infection was appreciated, 436 infections were documented. This highlights the pitfalls of the present AE reporting policy, resulting in significant underreporting of complications, a problem that becomes even more relevant with increasing study durations and potential complications emerging at multiple time points.20 When analyzing all observed infections in 2-month intervals, we found a consistent incidence over the entire 18-month follow-up period. Therefore, it seems appropriate to change the reporting of infectious complications in BsAb-treated patients from counting only the highest grade of infection per patient to counting the number of events per patient per month during the entire treatment period. This differs from reporting infections in patients treated with chimeric antigen receptor T cells (CAR-T cells) because these patients are subject to a time-dependent risk of infection, with the majority of infections occurring during the first 100 days.21 Recording infections in these patients should account for those facts with dichotomizing infection risk within 3 months after chimeric antigen receptor T-cell infusion and beyond. Such a strategy could be a useful step toward harmonization, as previously called for.4 In our opinion, it also seems reasonable in this treatment area to count each observed infection as a single event. In some cases, this can be difficult, but it is of clinical interest whether a viral infection of the upper respiratory tract subsequently leads to bacterial pneumonia.
Nevertheless, some caveats should be kept in mind when reporting the incidence of infections. In our study, the incidence rates of infections were based on the results of the MajeTEC-1 study with long follow-up. Although there is little reason to assume that the infection patterns vary among different BsAbs, this fact may be interpreted as a limitation of our findings. Furthermore, diagnosing infections can be complex at times when distinguishing between infectious and noninfectious causes of febrile episodes such as cytokine release syndrome or drug fever. In addition, the incidence of infections may have been influenced by COVID-19 infections in trials conducted at the height of the COVID-19 pandemic. In addition, our diagnostic tools for identifying the infection-causing pathogens remain suboptimal, although ongoing efforts aim to enhance sensitivity and faster identification through modern laboratory techniques such as mass spectrometry, polymerase chain reaction (PCR), multiplex polymerase chain reaction, next generation sequencing (NGS), and whole genome sequencing.22
In summary, our proposal to report all infections observed in an individual patient per observation time in months after starting treatment with BsAbs would eliminate the usual biases due to different follow-up periods and counting only 1 event per each patient, a limitation inherent in the current practice of AE reporting in clinical trials. Applying the proposed model could provide much clearer information about an individual patient’s risk of treatment-related infectious AE. For easier documentation and evaluation, we have developed a Myeloma Infection Score calculator, which is available at https://www.myelomainfectionscorecalculator.org, and provides a quick overview of monthly documented infections and a graphical representation of the progress (supplemental Figure 1). Suggestions for the period after the end of treatment with BsAbs should rely on further research, experience, and robust data, although continuing the recommended practice seems sensible.
Acknowledgment: The authors thank Julie Krainer for the development of the electronic version of the Myeloma Infection Score Calculator.
The authors also thank the Austrian Forum against Cancer for financial support.
Contribution: H.L. and I.S. designed the project; I.S. digitized the data of the MajesTec-1 study and created the figures; N.C.M., E.T., N.R., P.M., A.N., H.L., and I.S. discussed and reviewed the data and the manuscript; and all authors approved the final version of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Heinz Ludwig, Wilhelminen Cancer Research Institute, c/o Department of Medicine I, Clinic Ottakring, Montleartstr 37, Vienna 1160, Austria; email: heinz.ludwig@extern.gesundheitsverbund.at.
References
Author notes
Data are available on request from the corresponding author, Heinz Ludwig (heinz.ludwig@extern.gesundheitsverbund.at).
The full-text version of this article contains a data supplement.