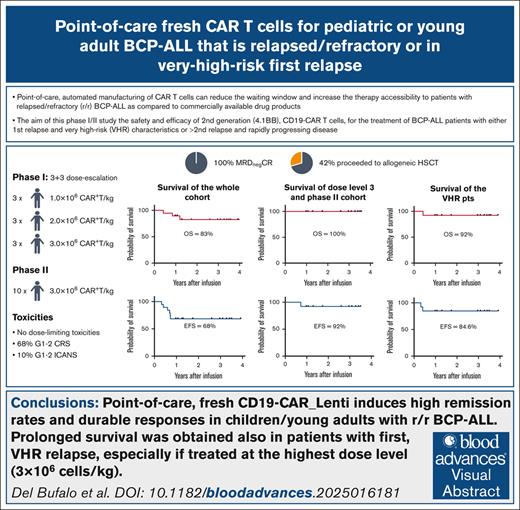
Key Points
Point-of-care CD19-CAR_Lenti induces high remission rates and durable responses in children/young adults with relapsed/refractory BCP-ALL.
Prolonged survival was obtained also in patients with first, VHR relapse, especially if treated at the highest dose level (3×106 cells/kg).
Visual Abstract
The point-of-care, automated manufacturing of chimeric antigen receptor (CAR) T cells can reduce the waiting window and increase the therapy accessibility to patients with B-cell precursor acute lymphoblastic leukemia (BCP-ALL) as compared with commercially available drug products (DPs). We conducted a phase 1/2 trial (NCT04787263) on the use of second-generation (4.1BB), CD19-CAR T cells, for the treatment of patients with BCP-ALL with either first relapse and very-high-risk (VHR) characteristics or second/subsequent relapse and rapidly progressing disease. CAR T cells were manufactured with a 12-day process using CliniMACS Prodigy, from a fresh apheresis and infused fresh. Three dose levels were tested in the phase 1: 1.0, 2.0, and 3.0×106 CAR+ T cells/kg. Nineteen patients were enrolled, 13 (68%) in first VHR relapse. The designed dose was always successfully produced. No dose-limiting toxicities were observed in the phase 1. Grade 1 to 2 cytokine release syndrome was observed in 13 (68%) patients. Grade 1 to 2 immune effector cell–associated neurotoxicity syndrome occurred in 2 patients and resolved spontaneously. All patients achieved bone marrow complete remission (CR) with negative minimal residual disease. Eight patients (42%) received hematopoietic stem cell transplantation (HSCT) consolidation. Overall, CR was maintained in 13 of 19 patients (68%), 7 of whom having received HSCT. Importantly, all but 1 patient treated with 3.0×106 CAR+ T cells/kg maintained CR. The 3-year event-free survival and overall survival of the whole cohort are 68% and 83%, respectively. Our data suggest that the point-of-care manufacturing of autologous, anti-CD19 CAR T cells can successfully treat patients with BCP-ALL including those with first relapse and VHR characteristics.
Introduction
The development of chimeric antigen receptor (CAR) T cells has tremendously advanced the landscape of therapeutic options for patients with B-cell neoplasms. To date, 7 products are commercially available for the treatment of B-cell acute lymphoblastic leukemia (B-ALL), B-cell non-Hodgkin lymphomas, and multiple myeloma. All these commercial products are prepared using a centralized process, based on the shipment of the apheresis from the treating center to the central good manufacturing practice facility, in which the drug product (DP) is manufactured and then shipped back to the center. However, this model carries some major limitations, namely: (1) it is time consuming; (2) the manufacturing availability cannot be timely and flexibly adapted to the clinical need of each single patient; and (3) it is associated with significant costs and, therefore, accessibility is limited to those countries that can afford these products.1 As demonstration of the difficulties of adapting a centralized model to patient clinical need, in the phase 2 global clinical trial that tested tisagenlecleucel in pediatric/young adult patients with relapsed/refractory (R/R) B-ALL, a remarkable proportion of patients could not receive the DP due to disease progression.2
Alternatives to the approved products manufactured centrally have been investigated in the last years. Point-of-care manufacturing of CAR T-cell products developed academically represents a promising option, allowing for a more manageable scheduling of the manufacturing and reduction of costs. In addition, point-of-care manufacturing allows DPs to be produced and infused fresh, without any step of cryopreservation, further reducing the vein-to-vein time.3
Such a reduction of the waiting window is particularly important for patients with more aggressive diseases. In the pediatric population, very-high-risk (VHR) first relapse of B-ALL represents an example and, indeed, it remains an unmet medical need, with a 10-year event-free survival (EFS) of 15% to 20%.4 The VHR subset includes patients experiencing very early relapse (ie, within 18 months from diagnosis) or whose disease displays unfavorable, recurrent cytogenetic/molecular alterations (ie, TP53 alterations; hypodiploidy; and KMT2A::AFF1, TCF3::PBX1, and TCF3::HLF translocations) and continue to face a grim prognosis.5-8 The early introduction of immunotherapy strategies, at first relapse, may improve the chances of cure of these children. The use of a CAR T-cell approach that can be delivered timely and potentially provide a deep eradication of the disease with long-term control represents a promising strategy.
We report the results of our phase 1/2 clinical trial on the use of CD19-directed CAR T cells generated with an automated process, from fresh apheresis, and reinfused as fresh DP (CD19-CAR_Lenti) for pediatric/young adult patients with R/R B-ALL, including first VHR relapses.
Methods
Clinical trial design
At the Bambino Gesù Children’s Hospital, Rome, Italy, we conducted a phase 1/2 clinical trial (ClinicalTrials.gov identifier: NCT04787263) to test CD19-CAR_Lenti in patients with R/R B-ALL. The phase 1 portion of the study was conducted according to a conventional 3+3 dose-escalation design and 3 cohorts (namely: 1.0 × 106, 2.0 × 106, 3.0 × 106 CAR+ T cells per kg recipient total body weight) were considered. Both the National Competent Authority and the institutional review board approved the study. Patients up to the age of 25 years with either (1) first relapse with either VHR characteristics or refractoriness to the second-line treatment; or (2) second or subsequent relapse and rapidly progressing disease preventing access to the commercially available DPs were enrolled.
The institutional review board (Bambino Gesù Children's Hospital Ethical Committee) approved the clinical trial.
CD19-CAR_Lenti cells manufacturing and infusion
The construct, a second-generation (containing 4.1BB as costimulatory molecule), CD19-directed CAR, is vehiculated through a lentiviral vector and has been previously described.9 The vector pLTG1563 (aCD19 CAR) is manufactured by Miltenyi Biotec in Teterow, Germany. CD19-CAR_Lenti was produced and released as fresh product, with a 12-day-long process using CliniMACS Prodigy and including an initial selection of CD4+/CD8+ cells, and a fixed vein-to-vein time of 14 days.
All patients received a lymphodepleting regimen including fludarabine (30 mg/m2 on days −5, −4, and −3) and cyclophosphamide (500 mg/m2 on days −4 and −3), and CD19-CAR_Lenti cells were subsequently administered as single infusion.
Immunomonitoring of patients who received an infusion of CD19-CAR_Lenti cells
The details of patient’s immunomonitoring are provided in the supplemental Materials and methods.
Toxicity grading and response evaluation
Toxicity was graded using the Common Terminology Criteria for Adverse Events (version 5.0), the Lee criteria for cytokine release syndrome (CRS), and the American Society for Transplantation and Cellular Therapy (ASTCT) consensus grading for immune effector cell–associated neurotoxicity syndrome (ICANS), as recommended in the protocol.10,11 CRS and ICANS have also been reported according to the more recent European Society for Blood and Marrow Transplantation (EBMT), Joint Accreditation Committee of International Society for Cellular Therapy and EBMT (JACIE), and European Haematology Association (EHA) guidelines.12 Neutropenia/immune effector cell–associated hematotoxicity (ICAHT) was classified according to the EHA/EBMT consensus.13 Information on the response evaluation is available in the supplemental Materials. Data cutoff for analysis was 15 April 2025.
Statistics
The clinical trial was designed according to a conventional 3+3 dose finding design for the phase 2. The phase 2 had a Fleming 2-stage design.14 Further details on the statistical design of the phase 2, the general statistics, and the definitions of overall survival (OS) and EFS are provided as supplemental Materials. Stringent EFS was calculated adding, to the conventional EFS, also the following events: measurable residual disease (MRD) emergence after reaching complete remission (CR) with or without hematological recovery, and receipt of further antileukemia therapy, including allogeneic hematopoietic stem cell transplantation (HSCT; if performed for early loss of B-cell aplasia [BCA]). Statistical significance of curve comparison was evaluated with the log-rank (Mantel-Cox) test.
CRS prediction rule
The details on the methodology applied for the CRS prediction are included in the supplemental Materials.
Results
Patient characteristics and bridging therapies
Between March 2021 and April 2024, 19 patients were enrolled in the study, 9 in the phase 1 portion and 10 additional children/young adults in the phase 2. Characteristics of the patient population are summarized in Table 1. Thirteen patients (68%) were enrolled for a first relapse of their disease; 11 of 13 (85%) with VHR characteristics (including 1 patient with TP53 mutation), and 2 of 13 (15%) with refractoriness to the second-line treatment and unfavorable cytogenetics (TCF3::PBX1 and KMT2A-rearrangements). The remaining 6 children (32%) were enrolled for a second or subsequent relapse and rapidly progressing disease, preventing access to the commercially available DPs; all 6 patients had relapsed after allogeneic HSCT.
In terms of previous therapies, beside chemotherapy, 12 of 19 patients (63%) had previously received blinatumomab (10/12 obtaining CR after the first cycle, 8/10 with molecular MRD negativity, 1 of 12 after the second cycle, and 1 of 12 showing an increase of MRD levels after treatment) and 6 of 19 (32%) had previously received inotuzumab ozogamicin. Most patients (16/19) did not receive a bridging therapy, considering the short interval between lymphoapheresis and start of lymphodepletion (9 days, with a fixed vein-to-vein time for CAR T-cell infusion of 14 days). Only 3 patients received low-dose systemic chemotherapy (namely, cyclophosphamide 300 mg/m2 and etoposide 300 mg/m2 on day −13; vincristine 1.5 mg/m2 and methotrexate 1 g/m2 on day −13; or a single dose of vincristine 1.5 mg/m2 on day −13).
We did not record any dropout from the study for progressive disease or infection during CAR T-cell manufacturing.
DP characteristics
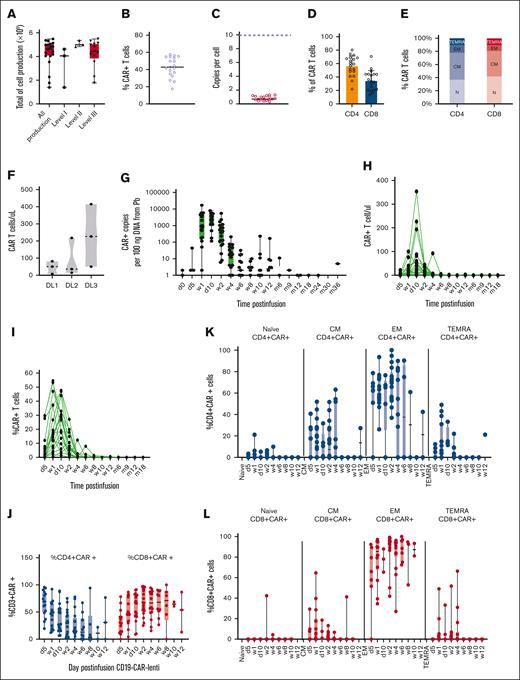
Manufacturing of CD19-CAR_Lenti was successful in all patients and all the target doses were obtained. The median number of cells obtained at the end of manufacturing was 4.24 × 109 (range, 1.39 × 109 to 5.48 × 109), and no significant difference was observed among the total yield of manufactured cells in the DPs of the patients enrolled across the 3 different dose levels (DLs; Figure 1A). Median CAR expression was 42.91% (range, 17.5%-57.4%) and median vector copy number per cell was 0.7 (range, 0.3-1.3; Figure 1B-C). The median CD4:CD8 ratio in the DPs was 1.7 (range, 0.3-5.9), with most of the DPs showing a predominance of CD4+ cells (Figure 1D); most CD4+ and CD8+ CAR+ T cells were represented by naïve and central memory (CM) cells, with a minor proportion of effector memory (EM) and effector memory cells reexpressing CD45RA (TEMRA; Figure 1E).
CD19-CAR_Lenti expansion after infusion
According to the design of the clinical trial, 3 patients received 1.0 × 106 CAR+ T cells per kg recipient total body weight (DL1), 3 children 2.0 × 106 (DL2) and the remaining 13, 3.0 × 106 transduced cells per kg (DL3). We observed a trend toward a higher maximum peak of expanded CAR T cells after infusion in the children enrolled in the DL3 as compared with those who were treated at the DL1 and DL2, although nonsignificant, with a median peak of 50.7 CAR+ cells per μL in DL1, 36.1 in DL2 and 226.8 in DL3 (Figure 1F). In terms of kinetics of expansion, both by quantitative polymerase chain reaction (qPCR) and flow cytometry, the peak of expanded CD19-CAR_Lenti in the peripheral blood (PB) was observed between 1 and 2 weeks after infusion (Figure 1G-I), with no difference across all 3 DLs (supplemental Figure 1).
Long-term persistence of CD19-CAR_Lenti was evaluated in all patients who did not receive consolidation with allogeneic HSCT. The gene-modified T cells could be detected by qPCR up to 36 months after infusion (Figure 1G) and to 6 months by flow cytometry (Figure 1H-I). Upon expansion in the PB, an inversion of the CD4:CD8 ratio was observed, with a predominant expansion of the CD8+ T cells over the CD4+ T cells (Figure 1J).
The expanded CD4+ and CD8+ CAR T cells exhibited a significant decrease in the proportion of naïve T cells, in comparison with the DPs before infusion (Figure 1K-L). By contrast, there was an increase in the proportion of CM T cells, along with a higher frequency of EM and TEMRA cells (Figure 1K-L). In the CD8+ CAR T-cell population, we observed a substantial expansion of EM cells (Figure 1L). Flow cytometry analysis identified CD3+ CAR+ T-cell infiltration in the bone marrow (BM) 2 weeks after infusion, with a median peak of 8.1% ± 6.1% (range, 0.4%-20.3%; Figure 1M). In addition, qPCR detected the persistence of CAR DNA copies for up to 2 years after CAR DP infusion also in the BM (Figure 1N). Finally, when comparing the CD4+:CD8+ ratio of CD19-CAR_Lenti infiltrating the BM with preinfusion DP profiles, an inversion is observed, with a prevalence of CD8+ CAR+ cells (median, 66.9% ± 26.30% [range, 11.1%-97.9%]), over CD4+CAR+ cells (median, 28.8% ± 25.3% [range, 1.6%-88.9%]) from week 2 onward (Figure 1O). Long-term persisting CAR+ T cells in the BM (week 12) showed no significant differential distribution between the CD4 and CD8 compartments.
Activity of CD19-CAR_Lenti
Functional sustained activity of CAR T cells was documented >6 months after infusion in 6 of 11 patients who did not receive HSCT through the persistence of BCA (Figure 2A). In particular, 4 children with an adequate follow-up remain with BCA ≥12 months after infusion (up to 40 months in 1 patient) and 1 recovered B-cell counts 24 months after infusion. Of the remaining 5 children, 1 lost BCA 6 months after infusion and 4 patients experienced an early loss of BCA, 3 months after infusion (Figure 2A). Notably, 6 of 7 patients with persisting BCA received the higher dose of 3.0 × 106 CAR+ T cells per kg.
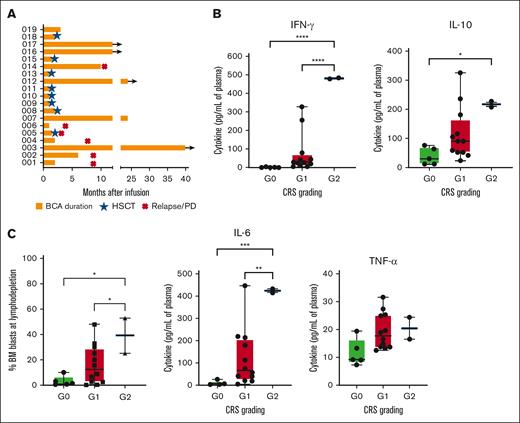
Evaluation of the BCA and cytokine profile according to CRS grading. (A) The kinetics of circulating CD19+ cells was monitored over time in the PB in all the treated patients by flow cytometry. BCA was defined according to the criteria established in the ELIANA phase 2 trial, namely as <3% B lymphocytes in total PB leukocytes (or absolute count of <50/μL) or <1% of CD19+ B cells in the BM.15 (B) The peak levels of the 4 most relevant cytokines (IFN-γ, IL-10, IL-6, and TNF-α) were measured in the sera of each treated patient and correlated with the severity of the CRS. (C) The percentage of blasts in the BM at lymphodepletion, evaluated by flow cytometry, was correlated with the severity of the CRS experienced by patients after infusion of CD19-CAR_Lenti cells. IFN-γ, interferon gamma; PD, progressive disease; TNF-α, tumor necrosis factor α.
Evaluation of the BCA and cytokine profile according to CRS grading. (A) The kinetics of circulating CD19+ cells was monitored over time in the PB in all the treated patients by flow cytometry. BCA was defined according to the criteria established in the ELIANA phase 2 trial, namely as <3% B lymphocytes in total PB leukocytes (or absolute count of <50/μL) or <1% of CD19+ B cells in the BM.15 (B) The peak levels of the 4 most relevant cytokines (IFN-γ, IL-10, IL-6, and TNF-α) were measured in the sera of each treated patient and correlated with the severity of the CRS. (C) The percentage of blasts in the BM at lymphodepletion, evaluated by flow cytometry, was correlated with the severity of the CRS experienced by patients after infusion of CD19-CAR_Lenti cells. IFN-γ, interferon gamma; PD, progressive disease; TNF-α, tumor necrosis factor α.
Toxicity
None of the patients developed severe (grade ≥3) CRS, according to the Lee criteria adopted in the clinical trial11; 11 children had grade 1 CRS, and 2 had grade 2 CRS (Table 2). When evaluated according to the new EBMT/JACIE/EHA criteria, 2 children had grade 3 CRS, because of the use of low-dose inotropic agents, 1 had grade 2 CRS, and 10 had grade 1 CRS.12 A comparable grading of CRS was observed when the ASTCT criteria were adopted.11 CRS had a median onset on day 5 after infusion (range, 0-10) and a median duration of 2 days (range, 1-6). CRS resolved spontaneously in all patients; tocilizumab was administered only to 1 child with constitutional trisomy 21, who developed grade 2 CRS, because of the additional risks related to the underlying constitutional syndrome. Severity of CRS correlated significantly with the levels of interferon gamma, interleukin-10 (IL-10), and IL-6 measured in patient sera (Figure 2B) and with the disease burden in the BM before lymphodepletion (Figure 2C).
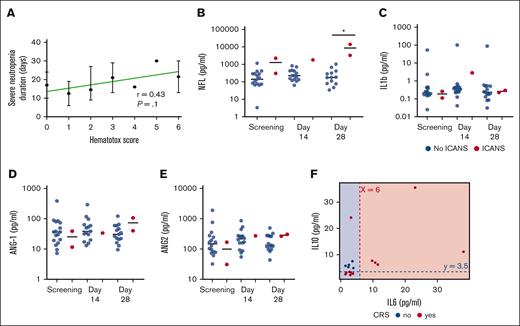
Hematological toxicity developed in all patients (Table 2). Early ICAHT developed in all children and reached maximum grade 4 in 1 and grade 3 in 10 children. In 11 of 19 patients, cytopenia involving ≥1 line was present before infusion of CD19-CAR_Lenti. Late ICAHT (ie, occurring >30 days after CAR T-cell infusion) was recorded in 16 patients, reaching maximum grade 4 in 2 patients and grade 3 in 9; the median duration of grade 3 to 4 late ICAHT was 7 days (range, 1-113) and the only 3 patients with late ICAHT lasting >14 days were all children with constitutional trisomy 21. None of the patients developed clinically relevant infections during neutropenia. In terms of risk factors associated with postinfusion ICAHT, the most relevant were associated to the disease status, to previous therapies, and to baseline marrow status. In particular, 5 patients had previous HSCT; 7 had >2 previous lines of therapy; 10 had >5% BM infiltration before infusion; and 3 had preexisting, clinically relevant cytopenia, before lymphodepletion.13 None of the children had active infections before/during/immediately after infusion of CD19-CAR_Lenti. In terms of CAR-HEMATOTOX score, 10 of 19 (52%) patients had a score ≥2 before treatment and developed a trend, not statistically significant, toward a longer duration of severe neutropenia, with a median of 15 days (range, 9-30), as compared with 13 days (range, 6-24) of patients with a CAR-HEMATOTOX score of 0 to 1 (Figure 3A).
Evaluation of biomarkers of toxicity. (A) Correlation between the CAR-HEMATOTOX score before infusion and the duration of severe neutropenia; (B-E) Measurement of the levels of NF-L, IL-1b, and ANG-1 and ANG-2 in the CSF of patients overtime, after infusion of CD19-CAR_Lenti cells. (F) The scatterplot illustrates how the rule identified by the JRip model classifies CRS categories (yes/no CRS) using samples from patients before the infusion of CAR T cells (day 0) in our data set. Samples for cytokine evaluation were unavailable for 2 patients. The red dashed line indicates the IL-6 cutoff at 6 pg/mL, and the blue dashed line indicates the IL-10 cutoff at 3.5 pg/mL. The background colors represent the predicted classification areas for patients with CRS negativity (blue) and CRS positivity (red), respectively; with a high Kappa value of 0.866, the model indicates substantial agreement beyond chance. The P value for exceeding the no-information rate (71%) is .02, suggesting that the model performs significantly better than a random classifier. The balanced accuracy is 95%, further highlighting its good performance. ANG-1/2, agiopoietin-1/2.
Evaluation of biomarkers of toxicity. (A) Correlation between the CAR-HEMATOTOX score before infusion and the duration of severe neutropenia; (B-E) Measurement of the levels of NF-L, IL-1b, and ANG-1 and ANG-2 in the CSF of patients overtime, after infusion of CD19-CAR_Lenti cells. (F) The scatterplot illustrates how the rule identified by the JRip model classifies CRS categories (yes/no CRS) using samples from patients before the infusion of CAR T cells (day 0) in our data set. Samples for cytokine evaluation were unavailable for 2 patients. The red dashed line indicates the IL-6 cutoff at 6 pg/mL, and the blue dashed line indicates the IL-10 cutoff at 3.5 pg/mL. The background colors represent the predicted classification areas for patients with CRS negativity (blue) and CRS positivity (red), respectively; with a high Kappa value of 0.866, the model indicates substantial agreement beyond chance. The P value for exceeding the no-information rate (71%) is .02, suggesting that the model performs significantly better than a random classifier. The balanced accuracy is 95%, further highlighting its good performance. ANG-1/2, agiopoietin-1/2.
One patient each developed grade 1 and grade 2 ICANS (according to both ASTCT and EBMT/JACIE/EHA criteria of classifications), both occurring 7 days after infusion and resolving spontaneously within 48 hours. None of the 19 patients required admission to the intensive care unit. In order to find biomarkers possibly correlated to the development of ICANS, we evaluated the levels of neurofilaments light chain (NF-L),16 IL-1b,17 and angiopoietin-1 and -218 in the cerebrospinal fluid (CSF) of patients. We observed a significant increase in NF-L in the CSF of the 2 patients who developed ICANS, whereas the other biomarkers evaluated remained comparable between patients who did or did not develop ICANS (Figure 3B-E).
We also sought to identify a pattern of cytokines plasma levels before the infusion of CD19-CAR_Lenti (day 0) that could predict the CRS occurrence. We observed that the combination of IL-6 of ≤6 pg/mL and IL-10 of ≥3.5 pg/mL is a relevant classification model that achieved an accuracy of 94%. Sensitivity is 100%, Whereas specificity is at 92%, demonstrating that the model is able to correctly identify patients that will not develop CRS (Figure 3F). The positive predictive value is 83%, whereas the negative predictive value is 100%.
Clinical responses
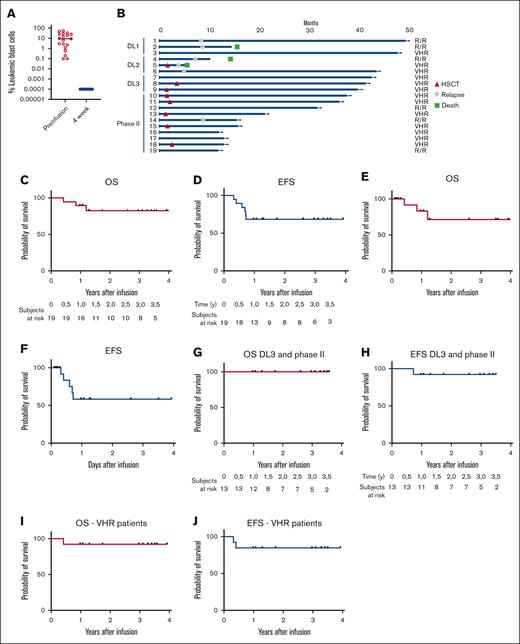
All patients showed CR with molecular MRD negativity in the BM (Figure 4A). One patient treated at DL2 with combined BM and bone disease, while achieving CR in the BM, obtained a partial response in the bone in which the disease subsequently progressed; the patient ultimately died of leukemia progression. All other patients with EM disease achieved CR also in the EM compartment. At time of analysis, 13 of 19 patients (68%) are alive and disease free, with a median follow-up of 3.0 years (range, 1-4; Figure 4B); 7 (50%) of whom were given allogeneic HSCT as consolidation of the remission, whereas the remaining 6 did not receive any additional treatment (Figure 4B). The reasons for consolidating these patients with an allograft were related to the fact that: (1) they showed very-poor-risk features; (2) they had never received a previous allograft; (3) several reports indicate that in children and young adults HSCT may be the most effective approach for preventing further relapse19; and (4) 5 patients had characteristics of high disease burden, as defined by Schultz et al.20 Six children experienced a relapse of the disease, 1 of them after consolidation with HSCT, at a median of 7.7 months (range, 3.8-8.7) after infusion. All relapses were CD19+, were heralded by loss of BCA within 6 months after infusion and 5 of 6 occurred at DL1 or DL2 (Figure 2A); in addition, 4 of 6 patients relapsed with EM disease, at the same sites present before treatment, all but 1 with concomitant BM disease. In 3 of 4 of the EM relapses, the disease could be biopsied and leukemia cells showed persistent CD19 positivity. All but 1 patient treated at DL3 displayed longer duration of BCA and remain in remission (Figure 2A). In addition, we observed a significant correlation between the levels of ferritin before lymphodepletion and the occurrence of relapse (supplemental Figure 2A).
Patient outcome and survival. (A) Evaluation of the leukemic blast infiltration in the BM before and 4 weeks after infusion of CD19-CAR_Lenti T cells, by flow cytometry. (B) Swimmer plot of all patients treated in the clinical trial. The survival of the patients was evaluated, in terms of OS and EFS for the whole cohort of patients, either without (C-D) or with censoring for HSCT (E-F), for the patients treated at the DL of 3 × 106 CAR+ cells per kg (G-H) and for the cohort of patients treated for a VHR first relapse (I,J).
Patient outcome and survival. (A) Evaluation of the leukemic blast infiltration in the BM before and 4 weeks after infusion of CD19-CAR_Lenti T cells, by flow cytometry. (B) Swimmer plot of all patients treated in the clinical trial. The survival of the patients was evaluated, in terms of OS and EFS for the whole cohort of patients, either without (C-D) or with censoring for HSCT (E-F), for the patients treated at the DL of 3 × 106 CAR+ cells per kg (G-H) and for the cohort of patients treated for a VHR first relapse (I,J).
In terms of durability of response, the 3-year OS and EFS of the whole cohort were 83% and 68%, respectively (Figure 4C-D), 71% and 58%, respectively, when patients were censored at time of HSCT (Figure 4E-F) whereas for the cohort of patients treated at DL3, survivals increased to 100% and 92%, respectively (Figure 4G-H). Importantly, when only patients with VHR relapses are considered, we recorded a 3-year OS and EFS of 92% and 84.6%, respectively (Figure 4I,J). When a more stringent definition of event was adopted, the 3-year EFS was 58% for the whole cohort, 77% for the patients treated at DL3 and 64% for patients with VHR relapses. We also evaluated the impact of the CAR-HEMATOTOX score on the survival and did not observe a statistically significant difference of survivals between the cohort with a low score (3-year OS and EFS: 100% and 73%, respectively) and that with a high score (3-year OS and EFS, 67% and 47%, respectively), although a trend toward improved outcome was recorded (supplemental Figure 2B-C). Neither previous exposure to blinatumomab nor to inotuzumab correlated with the probability of EFS and OS (data not shown).
Discussion
We describe the feasibility, safety, and efficacy of the use of CD19-CAR_Lenti, produced from fresh lymphocytes and infused as fresh DPs after a point-of-care manufacturing, in children and young adults with first relapse of B-cell precursor ALL (BCP-ALL) and VHR characteristics or with R/R disease. Manufacturing was successful in all patients, and CD19-CAR_Lenti cells were infused into all enrolled children, including patients with rapidly progressing disease. The toxicity profile of the treatment compared favorably with the safety signals conventionally described in children treated with CAR T cells, and we were able to clearly establish that the recommended dose of CD19-CAR_Lenti is 3 × 106 per kg recipient bodyweight. The efficacy of CD19-CAR_Lenti is promising, with 100% of patients showing BM CR after infusion and a significant proportion of them benefiting from sustained response, especially in the cohort treated at the highest DL.
Few groups have reported the successful treatment of patients with various B-cell malignancies using CAR T cells manufactured with an automated, CliniMACS Prodigy–based process, in a point-of-care model. In particular, a Spanish group pioneered this field with the development of ARI-0001, a point-of-care manufactured, second-generation (4.1BB) CD19 CAR T-cell DP.21 After proving that such system allows the academic development of a CAR T-cell DPs at affordable costs (in the order of €89 000, in a hospital-exemption setting), they confirmed the feasibility and efficacy of the approach across different clinical trials, for different indications, finally obtaining both the reimbursement of the treatment from the Spanish national health insurance system and the PRIME (priority medicines) designation by the EMA.22-24 These results have boosted the enthusiasm of the scientific community and academic developers, proving the possibility of increasing the affordability, and therefore accessibility, of CAR T cells. In 2021, the results of the point-of-care manufacturing at 2 different clinical sites (Moscow, Russia; and Cleveland, OH), sharing the same viral construct and manufacturing process that we have used, confirmed the feasibility of the approach and showed consistent toxicity and efficacy profiles between the 2 institutions, and comparable with those observed with the commercially available products.9
Our experience confirms the results obtained by the other groups. We were able to obtain high numbers of total cells at the end manufacturing in all patients, with a median transduction of 43%, which results in the availability of several doses of CD19-CAR_Lenti for each enrolled patient. These results imply that the expansion window of the manufacturing could be reduced and, therefore, even shorter timing of manufacturing could be envisaged in the future (with a consequent further reduction of the costs of each DP), in line with the results previously reported with ARI-0001.25
The results obtained in our study, and by other groups, on the point-of-care manufacturing model support further investment in developing these approaches, not only for the abovementioned reasons but also to foster the academic research and development of new CAR T-cell products for uncovered indications. This aspect is of particular relevance in pediatric hematology/oncology, because the rarity of most childhood malignancies discourages the development of dedicated CAR T-cell approaches by pharmaceutical companies, leaving several unmet medical needs for these patients. This topic is intensely discussed in the pediatric community of scientists involved in the development of advanced therapy medicinal products and new models of development are emerging, involving virtuous collaboration between academia and the pharmaceutical industry and point-of-care manufacturing.26,27
The DPs obtained with our manufacturing process were characterized by a predominance of naïve and CM CD4+ cells. After infusion, the expansion kinetics of CAR T cells revealed a dose-dependent trend, with patients receiving the highest dose (DL3) exhibiting a higher peak expansion of CAR T cells. Although not statistically significant, this trend may suggest a potential advantage of higher doses in achieving robust CAR T-cell expansion. The long-term persistence of CAR T cells, with detectable gene-modified cells in the PB up to 36 months after infusion, is a promising indicator of sustained treatment efficacy, particularly in patients who did not receive subsequent allogeneic HSCT. Phenotypically, CAR T cells shifted from a predominance of naïve and CM T cells before infusion to an enrichment EM and TEMRA T cells after infusion. This shift suggests an in vivo maturation and differentiation of CAR T cells, likely in response to antigen exposure and proliferation. Notably, CD8+ CAR T cells showed a marked increase in EM compartment, correlating with their cytotoxic functionality. High infiltration rates of CAR T cells in the BM further suggest effective homing to sites of disease. In addition, the inversion of the CD4:CD8 CAR T-cell ratio observed in the BM, with a predominance of CD8+ CAR T cells, indicates a cytotoxic-oriented immune response within this critical site. Persistence of BCA in most patients who did not receive transplantation supports the functional longevity of CAR T cells. Among patients maintaining BCA at last follow-up, a significant proportion received the highest DL, underscoring the potential impact of dose on functional persistence. However, some patients experienced an early loss of BCA, suggesting variability in patient response and the influence of individual factors.
The toxicity profile was generally manageable, with most patients experiencing low-grade CRS that resolved spontaneously. CRS severity was associated with elevated levels of cytokines, including interferon gamma, IL-10, and IL-6. Notably, our model incorporating IL-6 and IL-10 levels before infusion is associated with high accuracy in predicting CRS development, supporting the potential for cytokine profiling as a tool for risk stratification, although the model has been developed in this cohort of patients characterized by the occurrence of low rates and mild CRS. Hematological toxicity was observed in most patients, with severe grades correlating with previous therapy, disease burden, and BM status. Although ICAHT is not validated in the pediatric ALL population and is going to be implemented for these patients, we confirm its validity as a scoring system for the hematological toxicity. ICANS was rare and self-limiting, with elevated NF-L levels in the CSF, potentially indicating an early marker for neurotoxicity.
Children with trisomy 21 exhibited a prolonged duration of cytopenia, with 3 patients showing late ICAHT lasting >14 days, which aligns with their predisposition to hematological abnormalities. None of the patients, however, developed clinically relevant infections. Importantly, the treatment was well tolerated by patients with trisomy 21, despite their comorbidities and the well-known frailty of these children.
In terms of efficacy, the use of CD19-CAR_Lenti is associated with a 100% MRD-negative CR rate in the BM, and all but 1 patient obtained CR also in the EM compartment. These responses are durable in 13 of 19 (68%) patients, 6 of whom without any additional therapy and, in the others, after consolidation with HSCT. Importantly, 4 of 6 relapses involved extramedullary sites of disease, confirming that the EM compartment remains a feature predisposing to high risk of treatment failure.28 In addition, our data suggest that the use of higher doses of CAR T cells is associated not only with higher peaks in PB but, most importantly, with improved outcomes, because these patients maintain prolonged BCA and durable responses. Of 6 relapses recorded in our study cohort, 5 occurred in patients treated at the first 2 DLs.
We observed a significant correlation between prelymphodepletion ferritin levels and relapse risk. This correlation suggests that patients with elevated ferritin before lymphodepletion may have an underlying inflammatory or immunologic profile that could influence their response to CAR T-cell therapy and warrants further investigation. Similarly, a high CAR-HEMATOTOX score correlated with worse survival outcomes, although the difference does not reach significance, differently from what was reported in adults with various hematological malignancies.29-32 A larger cohort of patients is required to evaluate whether the score could be validated also in children with R/R BCP-ALL. Children with BCP-ALL have higher rates of baseline cytopenias and, in a study evaluating the role of the score in children with BCP-ALL, baseline BM disease emerged as an independent predictor of severe prolonged neutropenia, possibly reducing the relevance of the score in this population of patients and highlighting the need of a scoring system tailored for this patient population, as the ALL CAR-HEMATOTOX recently proposed.29,33
To the best of our knowledge, this is the first clinical trial documenting the efficacy of CAR T cells for children in first relapse of the disease and VHR characteristics. In this cohort of patients, we obtained remarkable 3-year OS and EFS of 92% and 84.6%, respectively, suggesting that the approach may substantially benefit this population of patients. A prospective, multicenter trial on the use of CD19-CAR_Lenti in first VHR BCP-ALL is going to be activated by the IntReALL consortium.
Acknowledgments
The authors are grateful to C. Geraci, M. Guercio, and S. Manni for their technical support and assistance during this work.
The experimental work was supported by grants awarded by Accelerator Award (Cancer Research United Kingdom/Associazione Italiana Ricerca per la Ricerca sul Cancro [AIRC]), INCAR project (F.L.), AIRC Special Project 5×1000 (number 9962 [F.L.]), AIRC IG 2018 (identifier 21724 [F.L.]), AIRC IG 2023 (identifier 29057 [B.D.A.]), and MFAG 21979 (C.Q.); Italian Ministry of Health projects Ricerca Corrente and 5×1000 grant (B.D.A., C.Q., and F.D.B.); Ministero dell’Università e della Ricerca (grant PRIN 2017 [F.L.]; grant PRIN 2020 [F.L.]; grant PRIN 2022 [F.L.]; and grant PRIN 2022 [C.Q.]); Italian Ministry of Health projects (GR-2021-12372614 [F.D.B.] and RF-2021-12374120 [C.Q.]); Italian network Alliance Against Cancer (RCR-2022-23682287 Preclinical Models [B.D.A.] and RCR-2023-23684268 chimeric antigen receptor T-cell therapy [F.L.]); European Union Next Generation EU, Mission 4, Component 2 (CUP B93D21010860004), National Center for Gene Therapy and Drugs Based on RNA Technology (F.L.); Hub Life Science Terapia Avanzata (PNC-E3-2022-23683269, CUP E83C22006230001) from the Italian Ministry of Health, “Piano Nazionale Complementare Ecosistema Innovativo della Salute,” cod PNC-E.3. (F.L.); and IMI JU/T2EVOLVE (grant number 945393 [F.L.]).
Authorship
Contribution: F.D.B. participated in the elaboration of the treatment, managed patients clinically, analyzed data, and wrote the manuscript; M.B. and C.R. managed patients clinically and analyzed data; P.M., M.A., D.P., F.G., M.M., and V.P. clinically managed patients and revised the manuscript; M.G.C. supervised the conduct of the clinical trial under Good Clinical Practice rules; L.H. contributed vital reagents and revised the manuscript; V.B., M.S., S.D.C., and L.I. participated in the immunomonitoring of the patients; V.F. developed the predictive model of cytokine release syndrome based on serum cytokine levels; G.L.P. and G.L. took care of the cell collection and manipulation and revised the manuscript; B.D.A. and C.Q. supervised the immunomonitoring, analyzed data, contributed to writing the manuscript, and critically revised the manuscript; F.L. supervised the project and the clinical management of patients, analyzed data, and critically revised the manuscript; and all authors had access to primary clinical trial data.
Conflict-of-interest disclosure: L.H. is an employee of Miltenyi Biomedicine. The remaining authors declare no competing financial interests.
Correspondence: Franco Locatelli, Department of Hematology/Oncology, Cell and Gene Therapy, IRCCS, Bambino Gesù Children’s Hospital, Piazza S Onofrio, 4-00165, Rome, Italy; email: franco.locatelli@opbg.net.
References
Author notes
Data sets and/or deidentified individual participant data that underlie the reported results are available upon reasonable request from the corresponding author, Franco Locatelli (franco.locatelli@opbg.net) and in compliance with the general data protection regulation.
The full-text version of this article contains a data supplement.