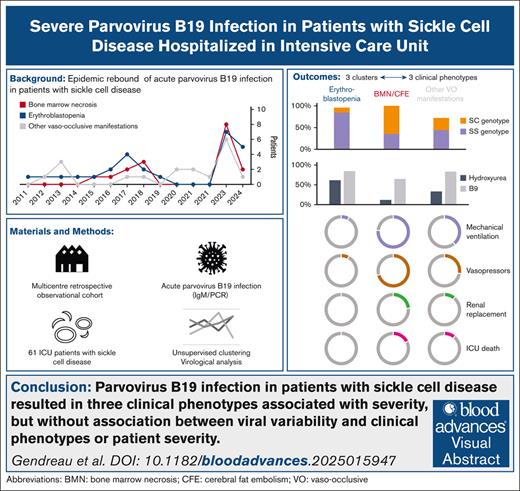
Key Points
Adults with SCD and parvovirus B19 infection showed 3 phenotypes: erythroblastopenia, BMN, and vaso-occlusive events.
Patients with BMN had longer hospital stay but no differences in parvovirus B19 genotype or diversity across groups.
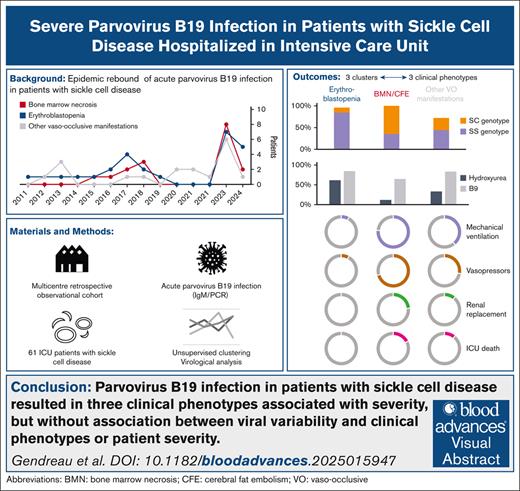
Visual Abstract
Parvovirus B19 infection can lead to severe complications in patients with chronic hemolysis. The aim of this study was to describe severe parvovirus B19 infections in adult patients with sickle cell disease (SCD). In this multicenter, retrospective, observational cohort study, adult patients with SCD admitted to intensive care units (ICUs) between 2011 and 2024 with acute parvovirus B19 infection were included. Unsupervised analysis was performed including clinical and biological characteristics to identify clusters of patients with different outcomes. Clinical phenotypes were defined based on patient clustering. Parvovirus B19 genomes from ICU (n = 15) and non-ICU control patients (n = 15) admitted to the hospital during the same period were sequenced and compared. Sixty-one patients (52% female; median age, 29 years [interquartile range, 24-38]) from 8 ICUs in France were included. Three clusters of patients were identified. From these clusters, 3 groups of patients with distinct clinical phenotype were identified: erythroblastopenia (n = 26), bone marrow necrosis (BMN) and fat cerebral embolism syndrome (CFE; n = 17), and other vaso-occlusive manifestations (n = 18). Length of stay in the ICU and hospital was longer in patients with BMN/CFE. There was no difference in parvovirus B19 genotype or NS1 or VP1/2 amino acid diversity between the groups. Similar results were observed between patients who were admitted to the ICU and those who were not. ICU patients with SCD and acute parvovirus B19 infection presented 3 clinical phenotypes associated with different initial severity and outcome but with similar parvovirus B19 clades and amino acid diversity.
Introduction
Parvovirus B19 is an airborne virus that is highly tropic to human erythroid progenitor cells and typically causes brief epidemics or can occasionally be transmitted by blood transfusion.1 Infections are more common in children,2 and although most cases are asymptomatic, primary infection with parvovirus B19 can lead to severe complications in patients who are immunocompromised or those undergoing significant bone marrow regeneration. Indeed, its tropism for erythroid progenitor cells can lead to erythroblastopenia in individuals with chronic hemolysis.3
Sickle cell disease (SCD) is a congenital hemoglobinopathy characterized by recurrent vaso-occlusive and hemolytic events.4 Chronic hemolysis, caused by the fragility of red blood cells carrying hemoglobin S, is associated with significant bone marrow regeneration, which stabilizes the constitutional anemia observed in these patients. However, parvovirus B19 infection, by blocking the regeneration of erythroid progenitors, leads to acute erythroblastopenia, which has been described in this population.5 Parvovirus B19 infection may also be associated with macrophage activation syndrome,6 bone marrow necrosis (BMN),7 or hyperhemolysis,8 although the clinical phenotype and outcome of severe adult forms are poorly documented, particularly in patients with SCD. Parvovirus B19 viremia has been shown to be associated with greater severity of infection.9 Persistent viremia is more common in immunocompromised patients2 and has been associated with severe anemia and transfusion support. Intravenous immunoglobulins may be useful to reduce the duration of reticulocytopenia and transfusion requirements10,11 but the evidence for this intervention is weak, particularly in patients with SCD.
The presentation of parvovirus B19 infection in patients with SCD ranges from isolated erythroblastopenia5 to a full-blown multiorgan failure syndrome, manifesting as BMN.7 However, the distribution of these manifestations in the intensive care setting, as well as their potential association with specific clinical phenotypes, has not yet been described. We conducted a retrospective multicenter study of adult patients admitted to intensive care units (ICUs) with confirmed parvovirus B19 infection. The primary objective of the study was to identify clinical phenotypes and outcomes. The secondary objective of the study was to assess the association between clinical severity and phenotypes and genomic variability of parvovirus B19.
Methods
Patients
This was a multicenter retrospective observational cohort of adult patients with SCD admitted to ICUs in France, between 1 January 2011 and 4 April 2024. Patients were identified from medical records and/or virological results from the participating centers’ laboratories. They were eligible if they were admitted to the ICU of 8 participating centers (see detailed list in supplemental Table 1, involving centers of the French National Sickle Cell Referral Centre and the Groupe de Recherche Respiratoire en Réanimation Onco-Hématologique) for an acute complication of SCD (initial diagnosis of SCD was made by hemoglobin electrophoresis interpreted by a senior physician) and had a confirmed acute parvovirus B19 infection, defined by a positive polymerase chain reaction or positive immunoglobulin M directed against parvovirus B19. Our study was approved by the institutional review board of the French Intensive Care Society (no. CE SRLF 23-083). Data were analyzed using R 4.1.0 (R Foundation for Statistical Computing, Vienna, Austria). Some patients were included in a previous analysis on cerebral fat embolism.12
Data collection
Baseline characteristics were collected from medical records and included: type of hemoglobinopathy, steady-state hemoglobin and platelet counts, and history and long-term treatment of SCD. Admission severity and organ failures were assessed using the simplified acute physiology score 2 (SAPS2) and sequential organ failure assessment score.13,14 Vital parameters and clinical presentation at the time of ICU admission were recorded, as were laboratory findings (on admission, and worst values). ICU and hospital length of stay, supportive care, transfusion therapy, and hospital death were also recorded.
Unsupervised clustering was first performed to identify the characteristics that best defined the population. Each cluster of patients with similar characteristics was secondarily evaluated by the investigators (S.G., N.d.P., and L.-M.C.) to determine whether a specific parvovirus-associated clinical phenotype could correlate with these clusters. The clinical phenotypes identified were defined as follows: (1) transient erythroblastopenia, defined as severe isolated anemia, with reticulocytopenia (<150 × 109/L); (2) BMN, defined as either medullary necrosis on bone marrow examination,7 or cerebral fat embolism (CFE) syndrome on magnetic resonance imaging12; and (3) other vaso-occlusive manifestations, including vaso-occlusive crisis (VOC) or acute chest syndrome (ACS).
Virological analysis
We conducted an in-depth analysis of the genome diversity of parvovirus B19 to identify whether differences in viral genes could explain the variability of clinical presentations (ie, clinical severity and phenotypes).
Full-length parvovirus B19 genomes were sequenced by means of next-generation sequencing.15 Samples were obtained from patients admitted in the ICU of Henri Mondor hospital (n = 15, 12 from whole blood and 2 from bone marrow) and from control patients (n = 15) diagnosed with nonsevere parvovirus B19 infection during the study period. Briefly, samples were subjected to a mechanical (glass beads), enzymatic (proteinase K), and chemical (chaotropic lysis buffer) treatment. Total nucleic acid extractions were then performed on a QIAsymphony, using QIAsymphony DSP DNA midi kit (Qiagen, Hilden, Germany). Mixed DNA/RNA libraries were constructed using the viral surveillance panel version 2 kit, and were sequenced on a NovaSeq 5000 sequencer (Illumina). The raw data were demultiplexed using BCLConvert on a Dragen server, and Fastq files were then analyzed on the BaseSpace cloud (Illumina), using Dragen Microbial Enrichment Plus software, to obtain B19 consensus sequences. These sequences were aligned using MAFFT version 7.525 (bootstrap, n = 1000) and phylogenetic trees were constructed using IqTree version 2.1.4 (bootstrap, n = 10 000; substitution model: TN + F + I + G4 [whole genome] and: HKY + F + G4 [nonstructural protein (NS)1/VP1]) and annotated with iTol.
Phylogenetic trees are diagrams representing the evolutionary relationships between different parvovirus viral sequences based on their genetic similarities and differences. Contextual references of genotype 1b, 2, and 3 were only available for the VP1 gene; therefore, 1 tree was constructed with NS1-VP1 sequences to assign a genotype to each clinical ICU presentation, and non-ICU sequences (Figure 3); and 1 tree with near full-length sequences (supplemental Figure 3) was constructed to check the sequence clustering, between ICU and non-ICU, and the 3 previously defined clinical phenotypes.
To explore the diversity of amino acids in NS1 and VP1/2 viral proteins, Shannon entropy was calculated and compared between ICU and non-ICU protein sequences, using Los Alamos HIV Shannon entropy online software (https://www.hiv.lanl.gov/content/sequence/ENTROPY/entropy.html). Statistical confidence of differential entropy was evaluated by a randomization strategy (n = 1000, with replacements). Only full-length sequences without gaps were retained (ICU, n = 14; non-ICU, n = 9).
Statistics
After removal of variables with >30% of missing values, an exploratory unsupervised clustering analysis was achieved using both quantitative and qualitative variables in the data set with factor analysis of mixed data (FAMD; see the supplemental Methods).16 FAMD explored data with both continuous and categorical variables using a principal components method to identify both differences and similarities between individuals as well as relationships between variables. Variables with the highest contribution were presented to identify clusters of patients with similar characteristics. A self-organized map (SOM) was then built to identify patients with similar features using the Kohonen self-organized map methodology17 using the R package Numero18 (see the supplemental Methods). Missing data were removed from SOM analysis.
Each cluster of patients with similar characteristics was subsequently reviewed by the investigators (S.G., N.d.P., and L.-M.C.) and the clusters identified were matched to previously described clinical phenotypes. Continuous data were expressed as medians [25th and 75th percentiles] and compared between groups of clinical phenotypes using the Kruskal-Wallis test for independent samples. Categorical variables, expressed as percentages, were evaluated using χ2 or Fisher exact tests, as appropriate.
Results
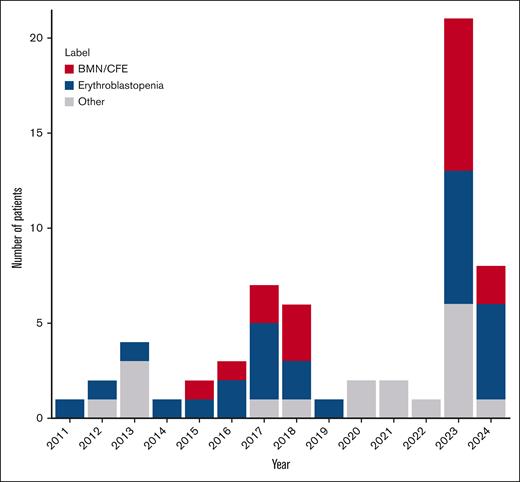
Between 2011 and 2024, 61 patients (48% male; median age, 29 [interquartile range, 24-38] years) from 8 ICUs in France were included (Figure 1). There was a significant increase in the annual number of cases included in 2023 and 2024 compared with those included between 2011 and 2022.
Time course of ICU admission of patients with SCD and acute parvovirus B19 infection (n = 61). Note that the inclusion period ended in May 2024.
Time course of ICU admission of patients with SCD and acute parvovirus B19 infection (n = 61). Note that the inclusion period ended in May 2024.
Identification of clusters of patients with SCD with parvovirus B19 infection
FAMD analysis and SOMs were used to identify clusters of patients. In the FAMD analysis, the proportions of variance retained by the first 3 dimensions were 16%, 9%, and 7%, respectively. The 10 variables with the highest contribution to the first 2 dimensions are shown in supplemental Table 2.
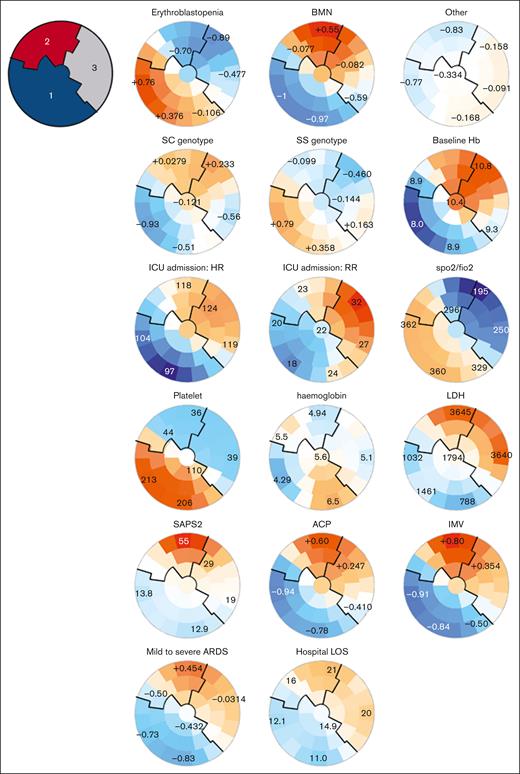
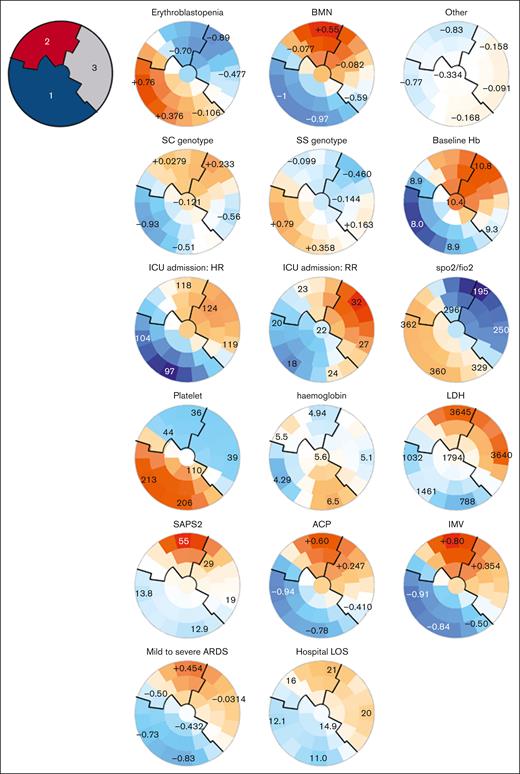
Three clusters of patients were identified according to these clinical characteristics (Figure 2; supplemental Table 3; supplemental Figures 1 and 2): on self-organizing maps, patients with similar characteristics were located by the SOM algorithm on the maps, defining 3 clusters. Cluster 1 consisted of patients with SS genotype, lower baseline hemoglobin levels, a history of alloimmunization or delayed hemolytic transfusion reaction (DHTR), chest and extrathoracic pain on admission to the ICU, low heart rate, respiratory rate, and a normal platelet count with low lactate dehydrogenase (LDH) level (Figure 2; supplemental Table 3); cluster 2 included older patients with multiorgan failure, a high SAPS2 score, and high LDH and creatinine levels. They had more frequent neurological manifestations, acute cor pulmonale, mild to severe acute respiratory distress syndrome, and required more frequently mechanical ventilation, inhaled nitric oxide, and catecholamines. They had longer mechanical ventilation duration, and ICU length of stay. Cluster 3 included patients with highest baseline hemoglobin, less hydroxyurea (HU) and folate treatments, acute chest syndromes with more severe thoracic pain, lower ratio of peripheral arterial oxygen saturation to the inspired fraction of oxygen (SpO2/FiO2) but less need for organ support than cluster 2, and a lower SAPS2 score.
SOMs of unsupervised analysis of the clinical and biological characteristics of 61 patients with SCD and acute parvovirus B19 infection. Unsupervised analysis of the clinical and biological characteristics of the 61 patients with SCD and parvovirus B19 infection. Patients with similar characteristics are located by the SOM algorithm closely on the maps, within one of the small groupings (“districts”). Each individual map shows the mean values or proportions per district for each characteristic: blue indicates the lowest average values, red the highest, with numbers shown for a selection of representative districts in each SOM. For instance, patients with erythroblastopenia were more frequently located in the lower left districts and had SS genotype, lower baseline hemoglobin, higher platelet counts, and less requirement for vasopressors and mechanical ventilation. 1, cluster 1; 2, cluster 2; 3, cluster 3; ACP, acute cor pulmonale; ARDS, acute respiratory distress syndrome; FiO2, fraction of inspired O2; Hb, hemoglobin; HR, heart rate; IMV, invasive mechanical ventilation; LOS, length of stay; RR, respiratory rate; SpO2, oxygen peripheral saturation.
SOMs of unsupervised analysis of the clinical and biological characteristics of 61 patients with SCD and acute parvovirus B19 infection. Unsupervised analysis of the clinical and biological characteristics of the 61 patients with SCD and parvovirus B19 infection. Patients with similar characteristics are located by the SOM algorithm closely on the maps, within one of the small groupings (“districts”). Each individual map shows the mean values or proportions per district for each characteristic: blue indicates the lowest average values, red the highest, with numbers shown for a selection of representative districts in each SOM. For instance, patients with erythroblastopenia were more frequently located in the lower left districts and had SS genotype, lower baseline hemoglobin, higher platelet counts, and less requirement for vasopressors and mechanical ventilation. 1, cluster 1; 2, cluster 2; 3, cluster 3; ACP, acute cor pulmonale; ARDS, acute respiratory distress syndrome; FiO2, fraction of inspired O2; Hb, hemoglobin; HR, heart rate; IMV, invasive mechanical ventilation; LOS, length of stay; RR, respiratory rate; SpO2, oxygen peripheral saturation.
These clusters and the visual distribution of patients within the SOMs corroborated the 3 clinical phenotypes previously found in patients with SCD with a diagnosis of parvovirus B19 infection (Figure 2; supplemental Figure 2; supplemental Table 3), including: erythroblastopenia without crisis (phenotype 1, corresponding to patients from cluster 1); BMN and fat embolism syndrome (phenotype 2, corresponding to patients from cluster 2); and patients with other vaso-occlusive manifestations (phenotype 3, corresponding to patients from cluster 3).
Clinical presentation and outcomes of patients according to their clinical phenotype on admission to the ICU
Of 61 patients, 26 (43%) had transient erythroblastopenia (phenotype 1), 17 (28%) had a clinical presentation consistent with BMN or CFE (phenotype 2), and 18 (30%) had other vaso-occlusive manifestations (phenotype 3). There were significant differences regarding the demographic and clinical characteristics of these 3 phenotypes on admission to the ICU (Table 1; supplemental Table 5).
Patients with erythroblastopenia (phenotype 1) included most SS genotype (85%), with lower baseline hemoglobin count, more frequent past history of ACS, and underlying HU treatment (Table 1). Of note, >50% of these patients had a previous history of alloimmunization or DHTR, and half had been admitted to the ICU for treatment of acute anemia. Fewer patients (68%) received a transfusion than in the other groups, in line with previous findings. They had a higher parvovirus B19 serum viral load (median, 8.87 [IQR 7-9.4] log copies/mL) and lower hemoglobin and reticulocyte counts than others (Table 2). No patient died in this group.
Patients with BMN/CFE (phenotype 2) had a more frequent SC genotype (n = 11, 65%) and almost never had an ACS episode before the current episode (Table 1). Most of these patients were also free of underlying SCD-specific treatment other than folate supplementation. They had the most severe presentation on ICU admission, as indicated by the highest SAPS2 and sequential organ failure assessment score; the lowest SpO2:FiO2 ratio, reflecting impaired oxygenation; and altered neurological status (median Glasgow coma scale: 10 [IQR 9-15]; Table 2). They had lower platelet counts and higher LDH levels than their counterparts. Notably, 77% of them required invasive mechanical ventilation support, 65% had mild to severe acute respiratory distress syndrome, and 71% had shock associated with acute cor pulmonale (Table 3). BMN was confirmed by bone marrow examination in 80% of cases (n = 12/15) and CFE by magnetic resonance imaging in 92% of cases (n = 12/13). All patients in this group received erythrocyte transfusion.
Most patients with phenotype 3 (ie, those with vaso-occlusive crisis and no erythroblastopenia/no evidence of BMN/CFE) had previous episodes of VOC and ACS (Table 1) and had been admitted to the ICU for a vaso-occlusive event (either VOC or ACS). They most commonly (89%) presented with VOC on admission, and 67% of them also had ACS (Table 2). The pain score was the highest, and 94% of patients had thrombopenia (lowest platelet counts: 32 × 109/L [IQR 24.0-39.5]). One third of patients required invasive mechanical ventilation or catecholamines (Table 3).
Therapeutic interventions and outcomes
Specific therapeutic interventions included intravenous immunoglobulins, given in 25% of patients (n = 15), and erythropoietin in 32% (n = 19/60; Table 3), with no significant differences between clinical phenotypes. No deaths were reported in patients with erythroblastopenia, 3 (18%) in patients with BMN/CFE, and 2 (11%) in patients with other vaso-occlusive manifestations (P = .1). ICU and hospital length of stay was longer in patients with BMN/CFE, consistent with their more severe presentation of disease.
Virological findings
Near full-length viral genome sequences were obtained for 14 ICU and 9 non-ICU samples. Full-length sequence could not be obtained for 3 ICU and 6 non-ICU samples because of low or intermediate viral loads (see supplemental Table 4). VP1 sequences could be obtained for 2 additional non-ICU samples.
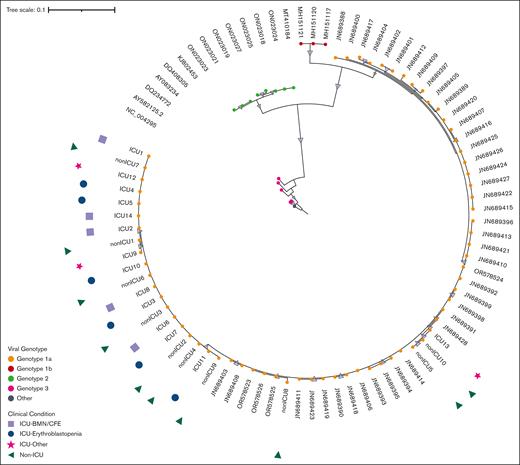
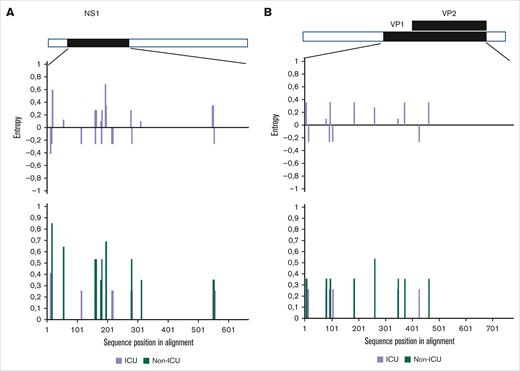
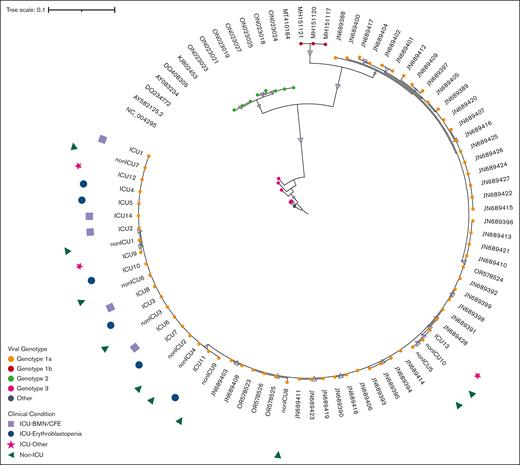
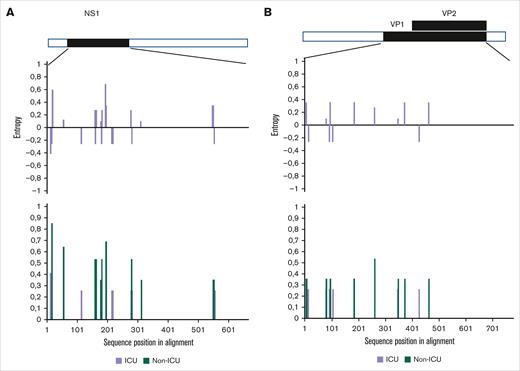
Phylogenetic analysis of the NS1 and VP1 genes showed that all sequences belong to the parvovirus B19 genotype 1a (Figure 3), which is the main circulating genotype in Europe. Parvovirus genome analysis showed no difference between ICU and non-ICU patients, nor between phenotypes: ICU patients, whatever their clinical phenotype, and non-ICU patients did not form separate clusters on both NS1-VP1 and near full-length genome phylogenetic analyses (Figure 3; supplemental Figure 3). The tree constructed with the near-full tree sequences (supplemental Figure 3) exhibited the same topology as the tree including partial NS1-VP1 sequences (Figure 3), suggesting no potential association between genetic variations of NS1-VP1 and disease severity, nor recombination events outside the partial NS1-VP1 sequence. Entropy analyses of the 3 viral proteins (NS1, and VP1/2) showed very little variability in the protein sequences of ICU and non-ICU related samples, also suggesting no potential link between protein variations of NS1 and VP1/2, and disease severity. No amino acid residue in any of the viral proteins was a signature of the ICU-related viral sequences (Figure 4).
Phylogenetic tree of NS1-VP1 sequence in patients with parvovirus B19 infection and different clinical conditions. This figure shows NS1-VP1 genome phylogenetic analysis including sequences from contextual references (eg, JN689428), and sequences from ICU and non-ICU patients (eg, ICU13 or non-ICU10). All patients from this cohort presented with genotype 1a (genotype 1a, 1b, 2, and 3 sequences are colored in orange, red, green, and pink, respectively). Samples from ICU and non-ICU patients did not form separate clusters, and the 3 phenotypes of ICU patients (ie, erythroblastopenia in blue; BMN/CFE in lilac, and other vaso-occlusive manifestations in pink) were equally distributed within the ICU-related viral sequences.
Phylogenetic tree of NS1-VP1 sequence in patients with parvovirus B19 infection and different clinical conditions. This figure shows NS1-VP1 genome phylogenetic analysis including sequences from contextual references (eg, JN689428), and sequences from ICU and non-ICU patients (eg, ICU13 or non-ICU10). All patients from this cohort presented with genotype 1a (genotype 1a, 1b, 2, and 3 sequences are colored in orange, red, green, and pink, respectively). Samples from ICU and non-ICU patients did not form separate clusters, and the 3 phenotypes of ICU patients (ie, erythroblastopenia in blue; BMN/CFE in lilac, and other vaso-occlusive manifestations in pink) were equally distributed within the ICU-related viral sequences.
Viral amino acidic variations in NS1 and VP1/2 viral proteins from patients admitted or not to the ICU. Shannon entropy calculated for (A) NS1 and (B) VP1/2 viral proteins (lower panels) and between ICU and non-ICU sequences (upper panels). The upper entropy graph shows differences between ICU and non-ICU subsets (purple: not significant). The lower entropy graph shows single viral amino acid variations within the ICU-related viral sequences (purple) and non-ICU–related viral sequences (green). Low entropy differences between protein sequences from ICU and non-ICU subsets suggest conserved proteins and no viral evolution.
Viral amino acidic variations in NS1 and VP1/2 viral proteins from patients admitted or not to the ICU. Shannon entropy calculated for (A) NS1 and (B) VP1/2 viral proteins (lower panels) and between ICU and non-ICU sequences (upper panels). The upper entropy graph shows differences between ICU and non-ICU subsets (purple: not significant). The lower entropy graph shows single viral amino acid variations within the ICU-related viral sequences (purple) and non-ICU–related viral sequences (green). Low entropy differences between protein sequences from ICU and non-ICU subsets suggest conserved proteins and no viral evolution.
Discussion
We report here the largest cohort of severe parvovirus B19 infection in ICU patients with SCD and identified 3 phenotypes associated with different outcomes: transient erythroblastopenia, BMN/fat embolism syndrome, and other vaso-occlusive manifestations. These 3 clinical phenotypes were associated with different initial severity and outcomes but with similar parvovirus B19 clades and amino acid diversity.
Patients with transient erythroblastopenia, a previously described manifestation of parvovirus B19,5 were referred to the ICU if they had either profound anemia and/or a transfusion contraindication (either alloimmunization or history of DHTR). Specific DHTR prevention and management may indeed require close monitoring in these circumstances20 and this organization may explain the presentation of phenotype 1. Patients with BMN/CFE syndrome had a severe presentation, leading to multiple organ failure. Previous studies have found similar results; a 2014 study by Tsitsikas et al showed an apparent association between fat embolism syndrome and parvovirus B19 infection7 and non-SS genotypes.21 The severe thrombocytopenia observed in this group was consistent with the overall severity of patients, as described previously. The last group consisted mainly of patients with vaso-occlusive events (either ACS or VOC), possibly triggered by parvovirus B19 infection.
The identification and characterization of these 3 clinical phenotypes allows early adjustment of patient management, because there is no specific treatment for parvovirus B19 infection. Patients with erythroblastopenia are particularly challenging in terms of transfusion compatibility. Their management in cases of previous DHTR or alloimmunization relies on transfusion-sparing strategies (such as erythropoietin and intravenous iron), or pretransfusion preparation (including compatibility testing, provision of the most compatible red blood cell units, or administration of rituximab22,23). In this population, at risk of death from acute anemia, immunomodulation with intravenous immunoglobulin may be considered to improve viral clearance and promote hematopoiesis recovery.11 Patients with BMN, the most severe phenotype, required prompt and intensive transfusion therapy24 as recommended for multiorgan failure in patients with SCD. In these cases, the primary concern is no longer bone marrow regeneration but rather organ support because of fat embolism and vaso-occlusive damages. Finally, patients presenting with severe VOC or ACS should receive standard care, following the established management guidelines for these complications as recommended in guidelines.25,26
The prevalence of parvovirus B19 infection changed during the study period. The decrease observed between 2020 and 2022 could be the consequence of the COVID-19 pandemic and the implementation of mandatory preventive measures in France in 2020 and 2021. Parvovirus B19 is indeed an airborne virus and social distancing as well as mask wearing could have been effective in preventing its transmission. This hypothesis is supported by data from blood donation screening for parvovirus B1927 confirming a low circulation during the first COVID-19 pandemic waves and a rebound in 2023 with an atypical seasonal pattern. Indeed, there was the same nonseasonal epidemic in parvovirus B19 cases reported in our study from 2023 onward. One hypothesis for this subsequent higher parvovirus B19 circulation after 2023 could be a subsequent reduction in herd immunity, although this is not supported by robust data. Another hypothesis for this epidemic could involve mutational changes in the virus; enhancing viral fitness; increasing transmissibility; and, eventually, immune escape. However, the phylogenetic analysis showed that ICU and non-ICU patients were infected by the same genotype (1a) and that amino acid variability was not significantly different in the former than in the latter group, corroborating previous works reporting the limited viral diversity of parvovirus B19, genotype 1a.28 Virological analyses also suggested that clinical phenotypes were not related to viral diversity. A change in seasonal outbreak was also described for respiratory syncytial virus (RSV) and influenza after the COVID-19 pandemic,29,30 associated with a reduced genomic diversity. Phylogenetic analyses showed major collapse of RSV and influenza lineages during years 2020 and 2021 compared with those circulating before the COVID-19 pandemic.
Possible explanations for these phenotypic differences include host-related factors. It has previously been reported that SC genotype may be associated with more ICU admission during parvovirus infection.31 BMN has previously been associated with SC genotype,7 a finding consistent with our study. Several hypotheses could be proposed to explain this association, including higher baseline hemoglobin level, leading to increased blood viscosity, or a lack of severe previous complications from SCD, because only 35% of patients in this group in our study had previously experienced ACS, possibly accounting for less compromised immunological features (including splenic function) and delayed diagnosis.7,21 Although the exact pathophysiology of BMN remains unclear, our findings suggest that both genotype and patient history contribute to the risk of developing this phenotype. Regarding erythroblastopenia, the severity of this phenotype primarily stems from the difficulty in transfusing patients with a history of DHTR or alloimmunization. The least severe phenotype, erythroblastopenia, was more frequently observed in patients on long-term HU therapy. This is consistent with the higher proportion of patients with the SS genotype in this group. The potential protective effect of HU against parvovirus B19 complications has been explored in several studies. In vitro findings have shown that HU inhibits parvovirus B19 replication in erythroid progenitor cells, providing experimental evidence of its antiviral activity at clinically relevant concentrations measured in patients with SCD receiving HU therapy.32 This protective effect on erythroid progenitors could potentially extend to other cell populations susceptible to infection, and contribute, at least in part, to phenotypic differences in disease severity. In vivo, a retrospective study of parvovirus B19–associated aplastic crises in children found that HU treatment was associated with fewer transfusions and a higher hemoglobin nadir during transient aplastic crisis. These findings suggest a protective role for HU, that may extend to endothelial invasion and prevention of the most severe clinical phenotypes.33
Numerous microorganisms are involved in the infectious etiology of SCD complications. Bacterial infections, including pulmonary infections34 and bacteremia,35 are known to trigger VOC and ACS, as are viral infections such as influenza36 and COVID-19.37,38 In addition, primary Epstein-Barr virus infections have been reported to cause organ failure and splenic sequestration.39,40 During bone marrow infection, parvovirus B19 typically induces apoptosis of infected erythroid progenitors, leading to aplastic crisis. However, endothelial dysfunction can be induced by parvovirus B19 infection of nonerythroid tissues, including endothelial cells,41,42 under certain conditions. Other localizations of parvovirus, including tubular and vascular involvement within renal tissue,43 have indeed been suggested previously. Endothelial viral invasion may increase vascular adhesion and vaso-occlusion, particularly in patients with higher baseline hemoglobin levels. The increased rate of pulmonary thrombosis observed in this group, although not statistically significant in our study, supports this hypothesis. Bone marrow endothelial dysfunction and vaso-occlusive mechanisms could lead to necrosis. The mechanisms underlying the differential targeting of erythroid progenitors vs endothelial cells remain unclear. However, our study provides insight into the absence of a specific viral protein or genetic etiology driving these phenotypic differences. Viral loads were similar across all 3 phenotypic groups, ruling out a direct inoculum effect on presentation. One hypothesis is that variability in the host’s constitutive or adaptive immune response, possibly influenced by an unidentified genetic component, may play a role. In addition, the preservation of spleen function in non-SS genotypes may also contribute to these differences. From an immunological perspective, 88% of patients were seropositive for anti-parvovirus B19 immunoglobulin G, suggesting previous exposure to the virus. This is consistent with published data in adults, in cases in which subclinical parvovirus B19 infection has been reported in children with sickle cell anemia.44
Our study has some limitations. First, although multicentric, most patients were admitted in 1 reference hospital (Henri Mondor, Assistance Publique Hôpitaux de Paris, Creteil, France). There are also several biases inherently related to the retrospective design of the study. We could not exclude a higher severity of patients, sometimes referred for specialized care, which precludes generalization to the general SCD population. However, our intention was to focus on severe parvovirus B19 infection in patients with SCD, and this primary objective was achieved in this population. Secondly, because ICU admission was an inclusion criterion, patients with erythroblastopenia were characterized by a particular severity and/or past history of transfusion contraindication or DHTR among this group. Moreover, this cohort was established in expert SCD centers, with a potential recruitment bias, because asymptomatic or mildly symptomatic patients may not have undergone parvovirus testing. However, this cohort primarily aimed to describe the most severe phenotypes requiring intensive care, and this inclusion/memory bias seemed to mildly affect this objective. There is some overlap between the BMN/CFE phenotype and the patients with most severe vaso-occlusive manifestations on SOM analysis. These common features may be because of some degree of BMN in these patients, which may not have been detected by imaging or biological assessment. The decision to prioritize clinical definitions over unsupervised clustering was made to ensure the most clinically relevant approach, given the therapeutic implications. Because of the retrospective nature of this study, some data were missing, which may have influenced the results. Data imputation was applied for the FAMD analysis, whereas patients with missing data were excluded from SOMs and clustering analyses. However, the identified clinical patterns remained consistent across analyses, suggesting that the missing data likely had minimal impact on the overall findings. In addition, the use of immunoglobulins was rare, and the size and diversity of the population, as well as the low mortality rate, precluded any analysis to conclude on a beneficial effect of this treatment on mortality. Finally, to our knowledge, no previous studies have specifically compared statistical analyses in patients with SCD or applied clustering methods to parvovirus B19 infections. Although unsupervised clustering has been used in the context of other viral infections, such as in patients who were critically ill with COVID-1945 or those with RSV46 infection using self-organizing maps or FAMD, these approaches have not yet been explored in the specific clinical setting we investigated.
However, to our knowledge, this is the largest study to describe severe parvovirus B19 infection in ICU patients with SCD, and the identification of clinical subtypes associated with severity and outcomes may help guide their management.
Conclusion
In patients with SCD, parvovirus B19 infection resulted in 3 clinical phenotypes associated with severity: erythroblastopenia, BMN /fat embolism, and other manifestations associated with vaso-occlusive events. Parvovirus B19 genotype 1a was involved in all cases, and we found no association between viral variability and clinical phenotypes or patient severity. Clinicians should be aware of the risk of multiorgan failure in patients with BMN, especially given the epidemic rebound observed since 2023. Each phenotype requires a specific and prompt therapeutic approach, integrating either transfusion-sparing strategies or transfusion support with standard patient care. Further investigation into the cause of this increased viral circulation is urgently needed to consider preventive or curative measures for parvovirus B19 infection, which has potentially severe consequences in patients with SCD.
Acknowledgments
The authors thank all study investigators, the nurses and physicians who took care of the patients, the laboratory staff who took care of virological samples, and the patients and their families for agreeing to participate in the study.
Authorship
Contribution: S.G. and L.-M.C. conducted the study; S.G., L.-M.C., and N.d.P. designed the research study, analyzed the clinical data, and wrote the initial version of the article; S.G., L.-M.C., A.B., N.V., A.H., M.A., S.L.J., L.A., S.B., G.B., P.C., S.H., A. Lafarge, and A.P. contributed to data acquisition; P.C., A. Ly, and S.F. analyzed the virological data; S.G. is the guarantor; and all authors revised and approved the article, and the corresponding author attests that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted.
Conflict-of-interest disclosure: S.G. reports support from Pharma Dom for attending a meeting, outside the submitted work. S.F. has served as a speaker for GlaxoSmithKline, AstraZeneca, MSD, Pfizer, Cepheid, and Moderna. N.d.P. has served as an advisor or speaker for Moderna and AstraZeneca. The remaining authors declare no competing financial interests.
Correspondence: Ségolène Gendreau, Service de Réanimation Médicale, Hôpital Henri Mondor, 1 Rue Gustave Eiffel, 94000 Créteil, France; email: segolene.gendreau@aphp.fr.
References
Author notes
S.G. and L.-M.C. contributed equally to this study.
Full-length parvovirus B19 genomes have been deposited in GenBank (accession no. PV031965.1 to PV031988.1).
Source data are provided with this article. Because of the sensitive nature of the data, all the clinical data sets generated and/or analyzed during this study are available on reasonable request from the corresponding author, Ségolène Gendreau (segolene.gendreau@aphp.fr) and would require new patient information before data sharing.
The full-text version of this article contains a data supplement.