Key Points
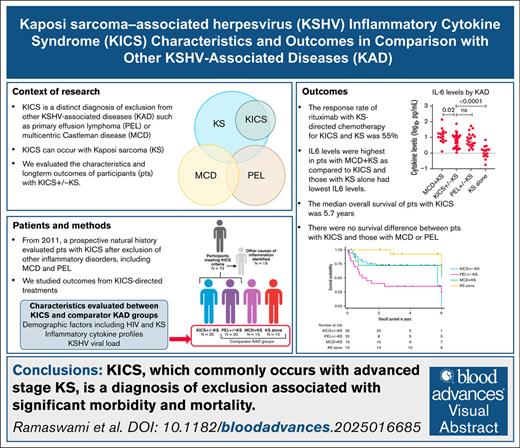
KICS is a distinct condition that is commonly associated with KS and requires exclusion of other KAD, such as MCD and PEL.
Rituximab with KS treatment may be beneficial in selected patients with KICS, but this diagnosis is still associated with high mortality.
Visual Abstract
Kaposi sarcoma–associated herpesvirus (KSHV) inflammatory cytokine syndrome (KICS) is characterized by KSHV infection and severe inflammatory symptoms and signs (elevated cytokines, C-reactive protein, and KSHV viremia), without evidence of other causes. The primary objective of this study was to evaluate KICS natural history after exclusion of other inflammatory disorders, including multicentric Castleman disease (MCD) and, per a revised working definition, primary effusion lymphoma (PEL). Participants with KICS were treated for concurrent Kaposi sarcoma (KS) and/or received KICS-directed treatment with targeted therapies used in other KSHV-associated disorders (KAD), such as rituximab, tocilizumab, or high-dose valganciclovir/azidothymidine. KICS response was evaluated using the Clinical Benefit Response Criteria (KICS-CBR). Baseline inflammatory cytokines were compared between those with KICS and participants with other KAD. Seventy-three participants were enrolled from 2011 to 2022. Following exclusion of other inflammatory processes, 35 (34 with HIV) had KICS, and all but 1 had concurrent stage T1 KS. Among participants with KICS, 11 (43%) received rituximab (with liposomal doxorubicin [8] or paclitaxel [3] for concurrent KS) and 55% (95% confidence interval [CI], 23%-83%) had a response per KICS-CBR. Interleukin-10 (IL-10), IL-6, and IL-1β levels were higher among those with KICS than those with KS alone. The median overall survival among participants with KICS was 5.7 years (95% CI, 5.7 years to not reached). KICS is a diagnosis of exclusion from PEL and MCD. Nearly all patients with KICS have advanced KS, and the syndrome is associated with significant morbidity and mortality. Rituximab may benefit a subset of participants with KICS. This trial was registered at www.clinicaltrials.gov as #NCT01419561.
Introduction
In 1994, Kaposi sarcoma–associated herpesvirus (KSHV), also called human herpesvirus 8, was discovered and identified as the cause of Kaposi sarcoma (KS).1 KSHV was also subsequently recognized as the causative agent of primary effusion lymphoma (PEL) and a subtype of multicentric Castleman disease (MCD).2,3 These KSHV-associated diseases (KAD) are rare, but most common among people with HIV (PWH), although they can arise in other immunosuppressed individuals (eg, transplant recipients).4 KAD can present alone or concurrently, resulting in varying clinical presentations. Patients with MCD and/or PEL exhibit inflammatory symptoms associated with increased levels of interleukin-6 (IL-6), IL-10, and other inflammatory cytokines.5-7 KS, MCD, and PEL have established pathologic diagnostic criteria.
In 2010, our group described a clinical syndrome in 6 patients in which KSHV infection was associated with severe inflammatory signs and symptoms without a pathologic diagnosis of MCD.8 This syndrome was named KSHV-associated inflammatory cytokine syndrome (KICS). These 6 PWH (4 with concurrent KS) presented with inflammatory symptoms including fevers, night sweats, cytopenias, cytokine dysregulation, elevated KSHV viral load (VL), and increased CRP (C-reactive protein), and in some cases with lymphadenopathy or effusions, suggestive of MCD. Each underwent biopsy procedures (including lymph nodes and bone marrow) that did not demonstrate MCD. None developed MCD on follow-up, and autopsy findings from 1 patient did not identify MCD or PEL. On the basis of these observations, we developed an initial working definition of KICS.9
In 2011, we initiated a formal prospective clinical protocol for KICS with nested interventions to study its manifestations, natural history, association with other inflammatory disorders, and treatment. The study was structured to initially enroll individuals with evidence of KSHV infection and inflammatory symptoms. Participants were then carefully evaluated for other causes of inflammation, such as MCD or concurrent opportunistic infections, and to assess the KSHV VL. Those who met the criteria for KICS were prospectively followed up and treated with the protocol. Given that patients with MCD have been shown to benefit from treatment with rituximab,7 high-dose valganciclovir and azidothymidine,10 or tocilizumab,11 we included these agents as potential treatments. During the conduct of this trial, we recognized that essentially all patients with PEL met the criteria for KICS12; patients with PEL continued to be enrolled and followed under the protocol but were considered as a separate cohort.
An analysis of the first 10 participants in this study was reported in 2016.13 In that report, participants with KICS were compared with 2 control cohorts: participants with KSHV and HIV coinfection and participants with HIV infection alone.13 Participants with KICS had more severe symptoms, laboratory abnormalities, and higher IL-6 and IL-10 serum levels compared with the control groups, demonstrating KICS as a well-defined entity distinct from inflammation associated with uncontrolled HIV. The median survival of participants with KICS in this first report was 13.6 months.
Herein we present data on the clinical characteristics and outcomes of 73 participants with suspected KICS enrolled in this prospective study. We focus on the 35 participants who met the definition of KICS following exclusion of other inflammatory causes (including PEL) and were prospectively followed up. We report on response to therapy, survival, and the clinical and immunologic profiles of participants with KICS compared with participants with PEL who were enrolled in this study and with separate cohorts of individuals with MCD and KS or KS alone previously studied by our group.
Methods
KICS study population and study design
Protocol 11-C-0220 (NCT01419561) was initiated in the HIV and AIDS Malignancy Branch of the National Cancer Institute in 2011. The first 10 participants were previously reported13 but are included in this analysis. Adults aged >18 years of any HIV status were eligible if they had at least 2 manifestations possibly related to KICS (Table 1), elevated CRP, evidence of KSHV infection, and no other evident causes of the inflammation. Participants with MCD were excluded, whereas participants with KS and/or PEL could be included. The study allowed for an initial period of evaluation to exclude other inflammatory causes, and assess additional criteria, such as KSHV viremia in either blood or effusions, that were required for a KICS diagnosis. Infection of tissues by KSHV was evaluated using immunohistochemical staining for latency-associated nuclear antigen-1 (anti-ORF73 rat monoclonal antibody; Advanced Biotechnologies, Eldersburg, MD). KS was confirmed by tissue biopsy. Participants with signs or symptoms suspicious for MCD underwent lymph node biopsy, and MCD was evaluated using the World Health Organization classification.14,15 In cases wherein PEL was suspected, the diagnosis was pathologically confirmed by review of cytology, flow cytometry, and/or clonality evaluation of effusions, or biopsy of an extracavitary lesion.5 Participants with PEL at entry or emergence of MCD or PEL were removed from the KICS cohort; participants with PEL continued to be followed in the study as a separate comparator group. In 2023, our group reported a novel approach to MCD diagnosis using multiparametric flow cytometry analyses of effusions from participants.16 These lambda-restricted plasmablasts (LRPs) were positive for CD38, expressed variable CD19, IRF4, Vs38c, and CD319, and were predominantly negative for CD138 and CD20. Participants who initially met the criteria for KICS but had LRPs identified were removed from the KICS cohort because the presence of LRPs was indicative of MCD.
Comparisons with other KAD
Serum inflammatory cytokines, clinical characteristics (including laboratory values), assessment of hemophagocytic lymphohistiocytosis (HLH) using the H-score (https://saintantoine.aphp.fr/score/), and survival outcomes were compared between those with KICS and the KAD comparator group: participants with PEL who were enrolled in the KICS protocol; patients with MCD and KS (MCD + KS) assessed during a flare; and patients with KS alone, who did not meet criteria for KICS. The latter 2 comparator groups were participants in other longitudinal protocols conducted over similar time periods within our group (NCT00006518, NCT00092222). Because all participants with KICS and concurrent KS had advanced (stage T1) KS, for the comparator groups (15 with MCD + KS and 15 with KS alone) we selected participants with stage T1 KS from previously published cohorts from our group.7,17,18 Participants with PEL were not required to have KS. All protocols were approved by the National Cancer Institute and National Institutes of Health Institutional Review Board. All enrolled participants provided written informed consent in accordance with the Declaration of Helsinki.
Treatment
Because there are no standard treatments for KICS, the protocol provided guidelines to select treatment regimens but allowed for a certain amount of flexibility (supplemental Table 1). Response assessment was completed per the Clinical Benefit Response Criteria (KICS-CBR), which our group developed on the basis of baseline systemic symptoms and laboratory findings, and which permitted longitudinal evaluation (supplemental Table 2).7,11 For participants with other concomitant KAD, treatment with standard agents or regimens for KS and/or PEL was permissible. Standard KS-directed therapy19 (where appropriate) was provided before, during, and/or after KICS-directed therapy, as required. Individuals with PEL were treated with standard treatments for HIV-associated non-Hodgkin lymphoma.20 Participants received antiretroviral therapy (ART) and opportunistic infection prophylaxis in accordance with existing guidelines.21
Cytokines and KSHV viral load
Blood for assessment of cytokines and KSHV VL of participants with KICS and PEL was collected at the time of entry when participants had inflammatory symptoms, at the time of flare for the MCD + KS cohort, and at the time of active disease requiring therapy for the KS-alone cohort. The KSHV VL per million peripheral blood mononuclear cells (PBMCs) or effusions was measured in DNA extracted by polymerase chain reaction using K6 primers.22 Measurement of serum levels of inflammatory cytokines (interferon gamma [IFN-γ], IL-10, IL-12, IL-1β, IL-6, IL-18, and tumor necrosis factor-α [TNF-α]) was performed using the V-PLEX Pro-inflammatory 10-plex assay (Meso Scale Discovery, Gaithersburg, MD) and compared between participants with KICS and the other KAD comparator groups at the time of active disease.
Statistical considerations
Differences in baseline characteristics, cytokines, and KSHV and HIV VL among KAD groups were evaluated using the Wilcoxon rank sum test for continuous variables and the Fisher exact test or Mehta’s modification of the Fisher exact test, as appropriate, for categorical variables. Paired comparisons within participants were made using the Wilcoxon signed rank test. Survival was evaluated using the Kaplan-Meier method, and 2-sided log-rank tests were used to determine the statistical significance of differences between curves. Overall survival (OS) was evaluated from study entry for participants with KICS, PEL, and MCD under protocols NCT00006518, NCT00092222, and NCT00099073 until death from any cause or censoring on 1 October 2024. All P values are 2-tailed and reported without any adjustment for multiple comparisons. A P value <.005 was considered to indicate statistical significance and a P value between .005 and .05 a strong trend.
Results
Participant characteristics
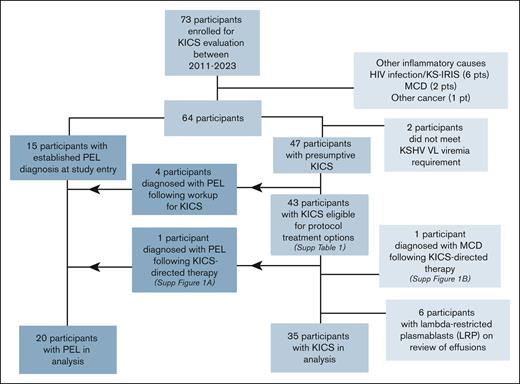
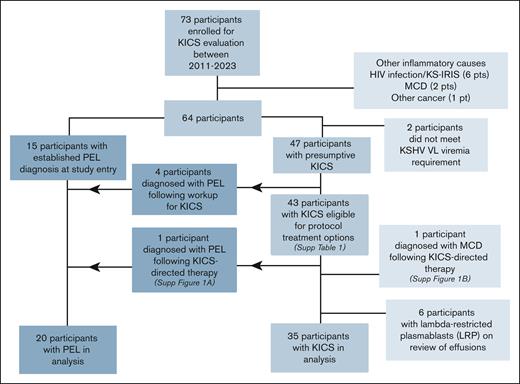
A total of 73 participants initially suspected of having KICS were enrolled. Their median age was 40.6 years; 92% were men and 50% were Black (Table 2). Following evaluation for suspected KICS, 9 participants were found to have other causes of inflammation, including 6 who met the criteria for KS-immune reconstitution inflammatory syndrome (KS-IRIS),23 2 who were diagnosed with MCD following lymph node evaluation, and 1 with a paraganglioma (Figure 1). Two participants did not have KSHV viremia. Seven additional participants were removed from the KICS cohort following an MCD diagnosis; 6 of these participants had LRPs identified in pleural effusions, and 1 participant had MCD following initial evaluation and treatment for KICS (supplemental Figure 1B). Therefore, 35 participants were evaluated in the KICS cohort. Twenty participants had histologic confirmation of PEL, including 15 who had an established diagnosis of PEL at enrollment, 4 who had PEL diagnosed after additional investigations (serial evaluation of existing or new-onset effusions), and 1 who originally met the KICS criteria but later developed PEL (supplemental Figure 1A). All participants with PEL otherwise met the criteria for KICS and were analyzed separately as a comparator PEL cohort.
CONSORT diagram for the study of participants enrolled in the KICS study.
All but 1 of the 35 participants with KICS had concurrent KS, and 15 (75%) with PEL had concurrent KS (Table 2). All cases of concurrent KS among those with KICS or PEL were stage T1, with visceral involvement noted in 83% of participants with KICS. All but 1 participant with KICS had concurrent HIV infection; this individual was diagnosed with an underlying immunodeficiency (CARD11 mutation) following his death. At the time of enrollment, 91% of those with KICS were receiving ART, with a median CD4+ T-cell count of 81 cells per μL and median HIV VL of 75 copies per mL. Among participants with KICS, the median KSHV VL was 882 copies per 106 cells in PBMCs and 12 942 copies per mL in whole blood.
When comparing participants with KICS with comparators with other KAD (20 with PEL, 15 with MCD + KS, and 15 with KS alone), notable differences were observed in the ECOG (Eastern Cooperative Oncology Group) performance status. Among the participants with KICS, 14% had poor ECOG performance status (≥3) as opposed to 55% of participants with PEL and 60% of those with MCD + KS (P = .002 for both comparisons). Among participants with KS alone, the median CD4 T-cell count was 406 cells per μL as opposed to 81 cells per μL among participants with KICS (P = .0001). Although all participants had stage T1 KS, visceral KS involvement was more common among participants with KICS than those with KS alone (Table 2). There were no differences in HIV and KS characteristics (in those with KS) among participants with KICS, MCD + KS, and PEL.
Laboratory, cytokine, and KSHV viral load differences between KICS and other KSHV-associated disorders
Participants with KICS had similar clinical laboratory values compared with those with PEL, except for a trend toward higher albumin levels among those with KICS (median albumin, 3.1 vs 2.6 g/dL; P = .03) (Table 3). Despite a trend toward higher ferritin and triglycerides among participants with PEL compared with those with KICS (median ferritin, 786.5 vs 399.5 μg/L; P = .01; and median triglycerides, 190 vs 137 mg/dL; P = .02), the H-score was not different between these groups (KICS, 62 vs PEL, 81; P = .1). Higher H-score (108), thrombocytopenia, and elevated C-reactive protein levels, as well as a trend toward higher ferritin and hypoalbuminemia, were noted among participants with MCD + KS compared with those with KICS. Participants with KICS had anemia, hypoalbuminemia, and higher CRP levels compared with participants with KS alone. A trend toward higher H-scores was observed among participants with KICS compared with those with KS alone (P = .01).
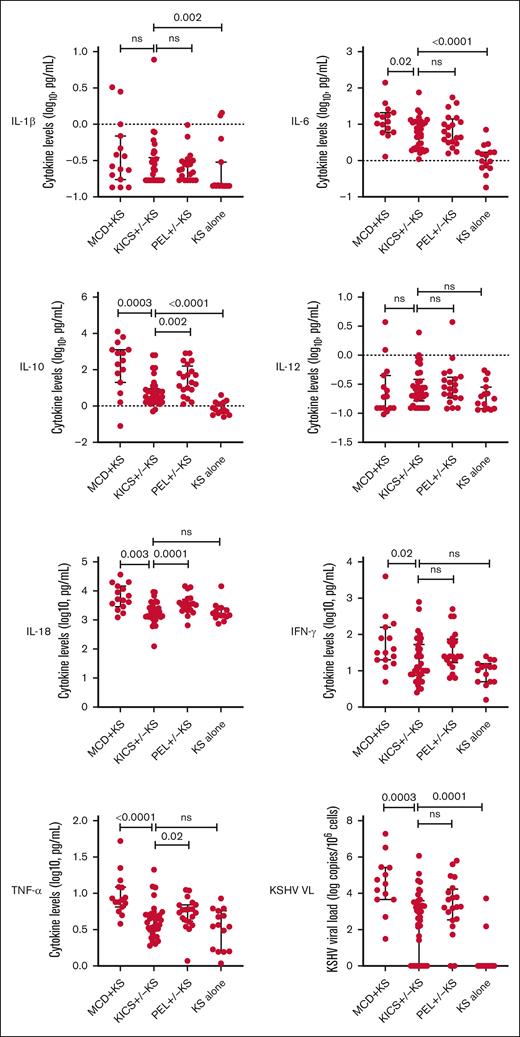
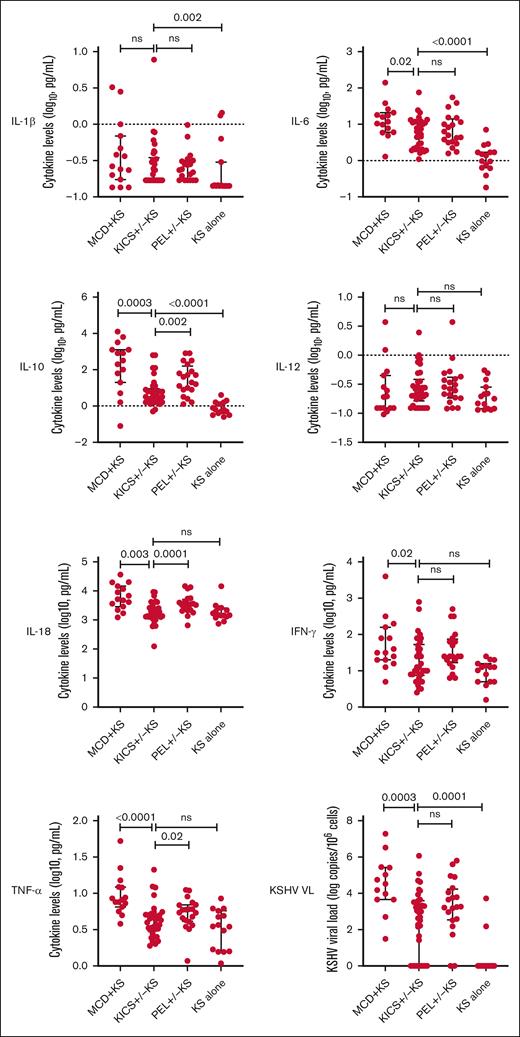
Distinct differences were observed between participants with KICS and those with other KAD. Those with MCD + KS had substantially higher levels of IL-10, IL-18, and TNF-α, and a trend toward higher IL-6 levels compared with those with KICS (Table 3; Figure 2). Levels of IL-10 and IL-18 were also higher among those diagnosed with PEL compared with participants with KICS. Participants with KICS had higher IL-1β, IL-6, and IL-10 levels compared with comparators with stage T1 KS alone. There was no difference in KSHV viremia in PBMCs between participants with PEL and those with KICS, whereas the median KSHV VL was higher among those with MCD + KS than participants with KICS (33 091 vs 882 copies per 106 cells; P = .003). Conversely, participants with KS alone had an undetectable median KSHV VL compared with participants with KICS (0 vs 882 copies per 106 cells; P = .0001).
Cytokine differences among participants at study entry during active diagnoses of KICS with KS and PEL with or without KS from the KICS protocol, and KAD comparators from HAMB protocols at periods of active disease (KS alone and MCD with KS). HAMB, HIV and AIDS Malignancy Branch; ns, not significant.
Cytokine differences among participants at study entry during active diagnoses of KICS with KS and PEL with or without KS from the KICS protocol, and KAD comparators from HAMB protocols at periods of active disease (KS alone and MCD with KS). HAMB, HIV and AIDS Malignancy Branch; ns, not significant.
Treatment and KICS response
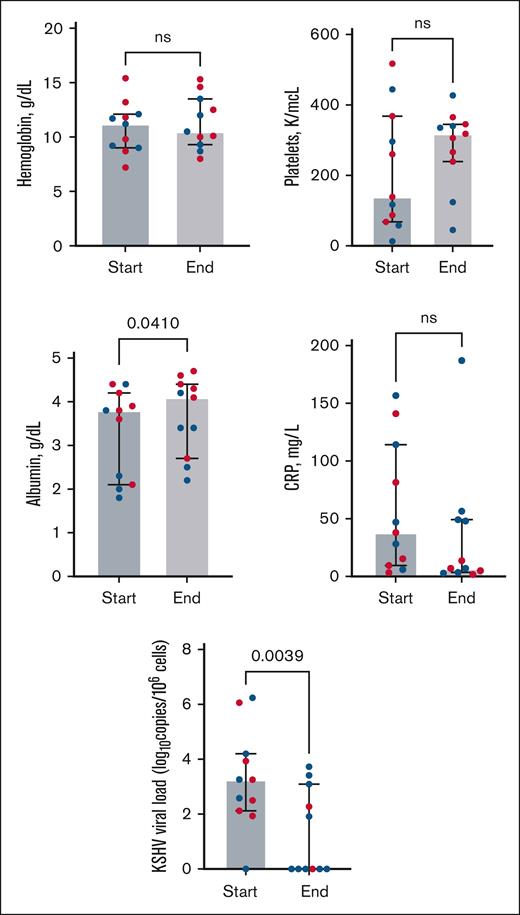
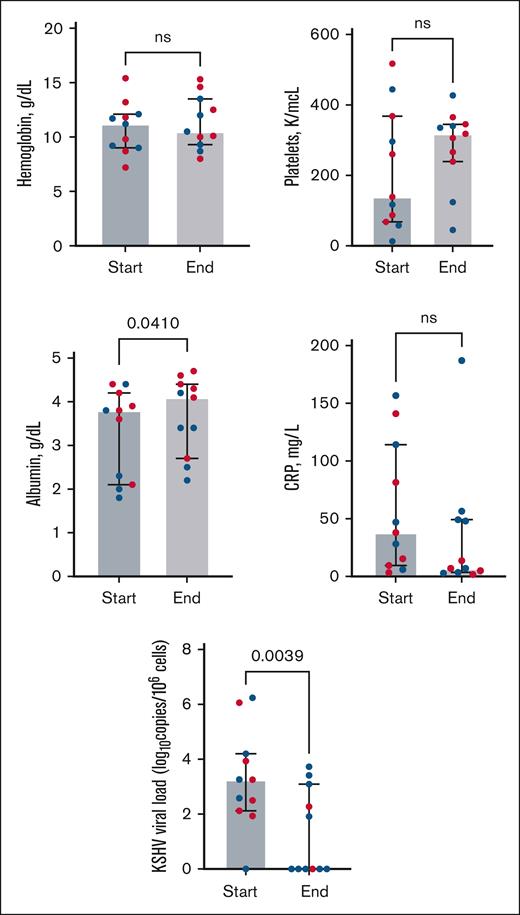
Among 35 participants with KICS, 13 received KICS-directed therapy (supplemental Figure 2) within a median of 18 days of enrollment (interquartile range, 0-63 days). Eleven participants (all with KS) who had evidence of inflammatory symptoms and/or multiorgan dysfunction with elevated KSHV VL received rituximab combined with KS-directed therapy with liposomal doxorubicin (8 participants) or paclitaxel (3 participants). All participants who received rituximab had received KS-directed chemotherapy in the 12 weeks preceding the initiation of rituximab. Seven of 11 participants were hospitalized, and 3 of these participants required intensive care unit support due to KICS and KS symptoms. Participants treated with rituximab combined with KS-directed therapy had a trend toward increased albumin levels and a significant decrease in the KSHV VL from the start to the end of rituximab treatment (Figure 3; supplemental Table 3). Per the KICS-CBR, 6 partial responses were observed during treatment among 11 participants (55%; 95% confidence interval [CI], 23%-83%). Long-term complete responses were observed in 6 participants, with no further recurrence of KICS symptoms during continued treatment for persistent KS symptoms. Participants with KICS and KS who were treated with rituximab continued KS-directed chemotherapy for a median of 4.5 months.
Laboratory parameters from Clinical Benefit Response Criteria (CBR) and KSHV VL values among 11 participants with KICS treated with rituximab in combination with KS-directed therapies (liposomal doxorubicin or paclitaxel). All participants received a median of 3 months of prior KS-directed chemotherapy (range, 6-12 cycles of treatment) before rituximab. “Start” represents treatment initiation of rituximab and KS-directed chemotherapy, and “end” denotes laboratory values following rituximab and KS-directed treatment. Red dots represent participants who met the criteria for partial response as best response (supplemental Table 2) per the CBR. Wilcoxon signed rank tests highlight differences from baseline to the end of treatment in laboratory parameters among all participants who received rituximab-based therapy (see supplemental Table 3 for values).
Laboratory parameters from Clinical Benefit Response Criteria (CBR) and KSHV VL values among 11 participants with KICS treated with rituximab in combination with KS-directed therapies (liposomal doxorubicin or paclitaxel). All participants received a median of 3 months of prior KS-directed chemotherapy (range, 6-12 cycles of treatment) before rituximab. “Start” represents treatment initiation of rituximab and KS-directed chemotherapy, and “end” denotes laboratory values following rituximab and KS-directed treatment. Red dots represent participants who met the criteria for partial response as best response (supplemental Table 2) per the CBR. Wilcoxon signed rank tests highlight differences from baseline to the end of treatment in laboratory parameters among all participants who received rituximab-based therapy (see supplemental Table 3 for values).
Other systemic KICS-directed therapies were used less often. One participant with HIV, tuberculosis-associated immune reconstitution inflammatory syndrome, and KICS without KS (with elevated pleural effusion KSHV VL of 24 000 copies per mL) received tocilizumab (anti–IL-6 receptor antibody). A complete response in the KICS-CBR was observed after 7 cycles without recurrence of KICS symptoms. A second participant with KICS was treated with valganciclovir/azidothymidine but was lost to follow-up, and his response could not be determined.
The other 22 participants with KICS (56%) received KS-directed therapy alone, either as part of standard treatment or clinical trials. Participants who were administered KICS-directed systemic treatments with KS chemotherapy had higher circulatory PBMC KSHV VL levels at entry compared with those who did not receive such treatment (3917 vs 281 copies per 106 cells; P = .002) (supplemental Table 4). Otherwise, there were no differences in demographic characteristics or cytokine levels at entry between these groups.
Survival outcomes
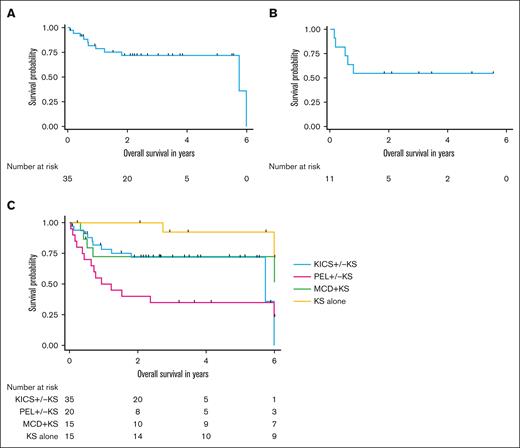
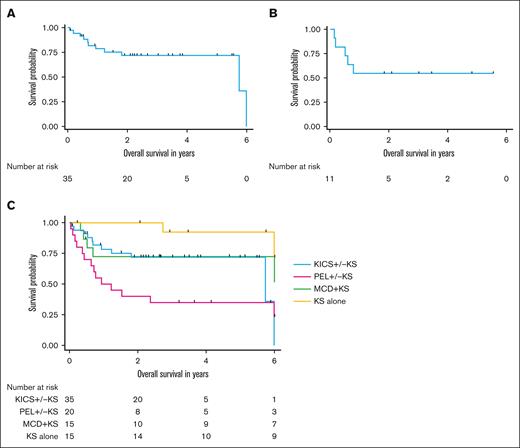
The median follow-up duration for participants with KICS, PEL and KAD comparator groups was 4.7 years. Over the course of the KICS study, 11 participants with KICS and 14 with PEL died. Among the 35 participants with KICS, the median OS was 5.7 years (95% CI, 5.7 years to not estimable) (Figure 4A). Among the 11 participants with KICS who received rituximab with KS-directed therapy, the median OS was not reached, and the 1-year OS was 54.5% (95% CI, 22.9%-78%) (Figure 4B).
Survival outcomes among participants with KICS and participants with other KAD. (A) OS among all 43 participants with KICS. (B) OS among 11 participants with KICS and KS who received rituximab with KS-directed therapy. (C) OS of participants by KAD diagnosis, censored at 6 years for all participants.
Survival outcomes among participants with KICS and participants with other KAD. (A) OS among all 43 participants with KICS. (B) OS among 11 participants with KICS and KS who received rituximab with KS-directed therapy. (C) OS of participants by KAD diagnosis, censored at 6 years for all participants.
Participants with KICS had survival outcomes similar to those of participants with PEL (5-year OS of KICS, 71.9% [95% CI, 52.9%-84.3%] vs PEL, 35% [95% CI, 15.6%-55.2%]; P = .05) (Figure 4C). There was also no survival difference between participants with KICS and those with MCD + KS (5-year OS, 72.4% [95% CI, 42.2%-88.6%]; P = .39). However, participants with KICS had a strong trend toward worse survival compared with those with KS alone (5-year OS, 92.3% [95% CI, 56.6%-98.8%]; P = .006).
Among the 11 participants who died with KICS, 4 died of complications related to progressive KS that led to respiratory complications or liver failure (supplemental Table 5). Two of these 4 participants were treated with rituximab for KICS and received chemotherapy treatment for KS that was ongoing in the weeks preceding their death. Three participants died at outside facilities of unknown causes but had been admitted for progressive KS in the preceding 3 months, suggesting that KS contributed to their death. Three participants with KICS had worsening KSHV-associated inflammation leading to hemodynamic instability and multiorgan dysfunction. Mortality among participants with PEL was due to complications related to progressive lymphoma.
Discussion
In this analysis of 73 participants initially evaluated for KICS in a prospective natural history study, we describe 35 participants who met pre-established criteria for KICS following exclusion of other inflammatory causes and compare them with cohorts of other KAD with established histologic diagnoses: MCD with KS, PEL, and KS alone. KICS is distinct from other KADs; for example, participants with MCD had more significant KSHV viremia, higher H-scores, and elevated IL-10, IL-18, and TNF-α levels compared with those with KICS. This study also explored KICS-directed therapies in 11 participants, including rituximab administered with systemic KS-directed chemotherapies, resulting in a partial response in 6 participants.
Since our initial descriptions of KICS, oncologists and HIV specialists have recognized this condition among patients with KS at other centers,24-26 including in pediatric patients.25 A diagnosis of HIV-associated KS still carries a very high mortality in certain areas worldwide27; a recent study from Kenya and Uganda demonstrated that the 6-month mortality of PWH with KS was 34%, with factors such as low hemoglobin contributing to death.28 Patients with HIV-associated KS and inflammatory symptoms are often treated presumptively for TB; however, recent evidence from South Africa suggests that a proportion of these cases have KICS,29 contributing to their poor outcomes.28,30 Therefore, improved means of diagnosing and treating KICS may have a substantial impact, especially in these regions.
KICS, which is a diagnosis of exclusion after ruling out MCD, PEL, and other infectious or inflammatory diagnoses, presents a diagnostic challenge.31 Patients with KS who are started on ART may develop a flare of their KS with inflammatory symptoms associated with increasing CD4 T-cell counts. This condition is known as KS-IRIS,32 which has similarities to KICS and is also a diagnosis of exclusion. However, KS-IRIS is defined by the occurrence or worsening of KS soon after ART initiation, and is associated with increasing CD4 T-cell counts, which can help differentiate it from KICS. We excluded 6 patients with possible KS-IRIS; participants with KICS had low CD4+ T-cell counts, and had been receiving ART treatment for nearly 1 year, which is outside the range of KS-IRIS.32-34 In this study, 5 participants who initially met the KICS criteria were later diagnosed with PEL. In these cases, worsening of KS, in the presence of recurrent pleural effusions that were repeatedly sampled, led to the identification of PEL. It has been suggested in previous reports that KICS has overlap with conditions associated with HLH.25,26 However, H-scores of participants with KICS were lower than the established HLH criteria within the literature.35,36 The H-scores of participants with KICS were similar to those of participants with PEL, but much lower compared with those of participants with a diagnosis of MCD. Therefore, in the setting of secondary HLH, our analyses support consideration of MCD rather than KICS.31
The pathogenesis of KICS and specifically the origins of the elevated circulatory cytokine levels are unknown. Participants with KICS had higher levels of IL-6, IL-10, and IL-1β than patients with advanced KS alone. However, participants with KICS had substantially lower levels of IL-10 and lower IL-18 and TNF-α levels than those with MCD + KS, suggesting different origins of the cytokine excess. Among participants with MCD, KSHV-infected plasmablasts in lymph nodes are an evident source of viral IL-6 (vIL-6) production, and there is evidence from a murine model that human IL-6 production induced by vIL-6 is a major factor in its pathogenesis.37 The high prevalence of advanced T1 stage KS may be a potential source of vIL-6 or other factors in KICS. Although vIL-6 is rarely detected by standard immunohistochemical staining of KS lesions, a recent study from our group found vIL-6 messenger RNA expression in KS biopsy specimens.38 Another source of cytokine excess in KICS may be inflammasome activation; a recent analysis demonstrated caspase-1/4/5 activity and fluorochrome-labeled inhibitors of caspases (FLICA)-positive ASC speck formation in circulating monocytes indicative of inflammasome activation in participants with KICS.39
Another consideration is whether KICS is a distinct KAD or an early manifestation of MCD. Among 43 participants who initially met the criteria for KICS, only 1 developed MCD confirmed on lymph node biopsy during the time of the study after KICS-directed treatment. An additional 6 participants underwent flow cytometry of effusions, which demonstrated LRPs suggestive of MCD. KSHV-infected plasmablasts have been identified in the circulation during MCD flares.40 Our group reported LRPs within pleural effusions among participants with KICS and also those with biopsy-confirmed MCD who had effusions,16 raising the possibility that a subset of participants with KICS may have clinical characteristics that overlap with MCD without lymph node involvement.
Partly on the basis of rituximab’s efficacy in treating MCD and overlapping clinical features,7 we hypothesized that rituximab would have benefit in the treatment of KICS. Eleven participants received rituximab with KS-directed chemotherapy with a response rate of 55%. This is lower than the response rate observed among participants treated with rituximab for MCD.7,41-43 Participants receiving rituximab had less consistent increases in their hemoglobulin, platelet, or CRP levels following treatment than previously reported for patients treated with rituximab for MCD.7 The decreases in KSHV VL noted following rituximab treatment suggest an impact on KSHV-infected CD20+ cells in the circulation in patients with KICS. In limited-resource settings, ART, corticosteroids, and KS-directed chemotherapy have been used to treat KICS with KS in the absence of rituximab.25,44
The median survival of 5.7 years in this KICS cohort exceeds that of the previously reported series of 10 participants, which included some patients with PEL; however, survival in this analysis was similar to that observed among patients with MCD and PEL treated during the same period. Death among participants with KICS was related to multiorgan dysfunction or progressive KS (despite ongoing KS-directed therapy), which underscores the severity of this disease and need for novel therapies. We hypothesize that the cytokine excess in KICS may potentiate and worsen KS. Understanding the disease mechanisms of KS and KICS will be crucial to the development of improved therapies.
To our knowledge, this is the largest cohort of patients with KICS reported to date and the first study to compare the inflammatory profiles among various KSHV-associated disorders. A limitation of this study is that it included adult patients only, with a wide range of KICS severity. Although this study is prospective with a priori definitions of KICS and observation of the patients within a defined clinical trial, the analyses are partially retrospective given the longitudinal observation and additional diagnostic investigations (ie, sampling of effusions) that identified PEL or LRPs in some participants. Other limitations include referral bias given that our center is a tertiary referral center for patients with KAD, and there was lack of randomization of KICS-directed treatments, which was influenced by clinical presentation and physician assessment. Furthermore, because the participants were evaluated in high-resource settings, the mortality outcomes may be better than those in limited-resource areas. However, this large cohort of comprehensively evaluated participants in a specialized setting provides insight into KICS and the challenges associated with the management of this rare and serious condition.
In summary, KICS is a distinct diagnosis of exclusion among PWH and other immunodeficiencies and is observed among individuals with advanced KS. A subset of participants with KICS may respond to rituximab with KS-directed therapies, but the condition still has a high morbidity and mortality. Further study is required to determine the pathogenesis of KICS, its relationship with HIV and other KAD, and the optimal therapy for this disorder.
Acknowledgments
The authors sincerely thank the individuals who volunteered for this study and their families for providing their time, energy, and effort to contribute to scientific and clinical advancement for these rare conditions. The authors also thank the nurses, physicians, the participant care and clinical research support staff at the National Cancer Institute and the National Institutes of Health Clinical Center, and the physicians who referred participants.
This work was supported, in part, by the Intramural Research Program of the National Cancer Institute, National Institutes of Health (NIH), and in part with federal funds from the National Cancer Institute, NIH, under contract (75N91019D00024, HHSN261200800001E, ZIA BC 010888). The contributions of the NIH author(s) were made as part of their official duties as NIH federal employees, are in compliance with agency policy requirements, and are considered works of the US government. However, the findings and conclusions presented in this paper are those of the author(s) and do not necessarily reflect theviews of the NIH or the US Department of Health and Human Services.
Authorship
Contribution: R.Y., T.S.U., M.N.P., E.T., and R.R. developed the protocol and study design; R.R., V.A.M., D.W., and A.R. conducted data analysis; E.S.J., H.-W.W., M.J.R., A.C.F., and S.P. were involved in evaluation of pathology to diagnose primary effusion lymphoma, multicentric Castleman disease, and Kaposi sarcoma; K.L., M.N.P., A.W., I.E., T.S.U., R.R., and R.Y. contributed to care of the study participants; and all authors reviewed the study and were involved in writing the manuscript, which was led by R.R. and R.Y.
Conflict-of-interest disclosure: R.Y., R.R., and K.L. report receiving research support from Celgene (now Bristol Myers Squibb), CTI BioPharma (a Sobi A.B. Company), PDS Biotech, and Janssen Pharmaceuticals through cooperative research and development agreement (CRADAs) with the National Cancer Institute (NCI); drugs for clinical trials from Merck, EMD Serono, and Eli Lilly; and preclinical material from Lentigen Technology through CRADAs or material transfer agreements with the NCI. T.S.U. reports receiving other commercial research support from Roche through a clinical trial agreement with Fred Hutchinson Cancer Center; and is now employed by Regeneron Pharmaceuticals. T.S.U., R.Y., and D.W. are coinventors on US Patent 10001483 entitled “Methods for the treatment of Kaposi’s sarcoma or KSHV-induced lymphoma using immunomodulatory compounds, and uses of biomarkers.” R.Y. is also a coinventor on patents on a peptide vaccine for HIV and on the treatment of Kaposi sarcoma with IL-12, and an immediate family member of R.Y. is a coinventor on patents related to internalization of target receptors, KSHV viral IL-6, and the use of calreticulin and calreticulin fragments to inhibit angiogenesis. All rights, title, and interest to these patents have been or should by law be assigned to the US Department of Health and Human Services; the government conveys a portion of the royalties it receives to its employee inventors under the Federal Technology Transfer Act of 1986 (P.L. 99-502). The remaining authors declare no competing financial interests.
Correspondence: Ramya Ramaswami, HIV and AIDS Malignancy Branch, Center for Cancer Research, National Cancer Institute, 10 Center Dr, Room 6N106, Bethesda, MD 20892; email: ramya.ramaswami@nih.gov.
References
Author notes
Presented, in part, at the Conference on Retroviruses and Opportunistic Infections, Seattle, WA, 19 to 22 February 2023.
Data are available on request from the corresponding author, Ramya Ramaswami (ramya.ramaswami@nih.gov).
The full-text version of this article contains a data supplement.