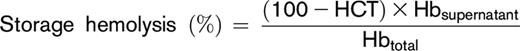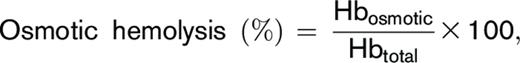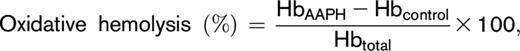Key Points
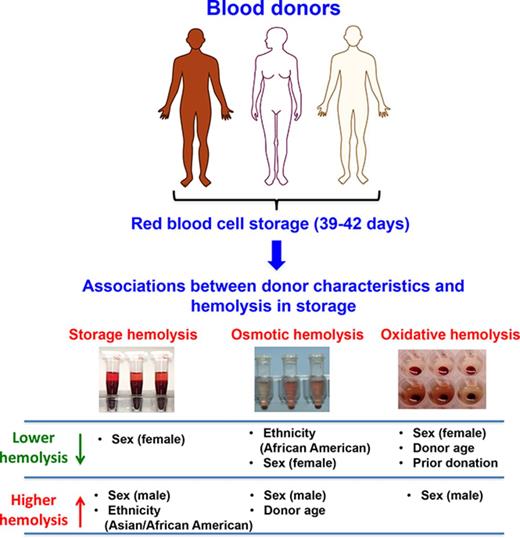
Genetic and biological variability in blood donors may impact RBC predisposition to hemolysis during cold storage and after transfusion.
Male sex, Asian or African American racial background, and older age (>45 years) are significant modifiers of hemolysis.
Abstract
Genetic polymorphisms in blood donors may contribute to donor-specific differences in the survival of red blood cells (RBCs) during cold storage and after transfusion. Genetic variability is anticipated to be high in donors with racial admixture from malaria endemic regions such as Africa and Asia. The purpose of this study was to test the hypothesis that donor genetic background, reflected by sex and self-reported ethnicity, significantly modulates RBC phenotypes in storage. High throughput hemolysis assays were developed and used to evaluate stored RBC samples from 11 115 African American, Asian, white, and Hispanic blood donors from 4 geographically diverse regions in the United States. Leukocyte-reduced RBC concentrate-derived samples were stored for 39 to 42 days (1-6°C) and then evaluated for storage, osmotic, and oxidative hemolysis. Male sex was strongly associated with increased susceptibility to all 3 hemolysis measures (P < .0001). African American background was associated with resistance to osmotic hemolysis compared with other racial groups (adjusted P < .0001). Donor race/ethnicity was also associated with extreme (>1%) levels of storage hemolysis exceeding US Food and Drug Administration regulations for transfusion (hemolysis >1% was observed in 3.51% of Asian and 2.47% of African American donors vs 1.67% of white donors). These findings highlight the impact of donor genetic traits on measures of RBC hemolysis during routine cold storage, and they support current plans for genome-wide association studies, which may help identify hereditable variants with substantive effects on RBC storage stability and possibly posttransfusion outcomes.
Introduction
Blood donors represent a genetically diverse population with inherited differences in red blood cell (RBC) characteristics that may modulate predisposition to hemolysis and RBC recovery in response to various stress conditions, including cold storage of RBC units.1 A growing number of studies have discussed the potential impact of donor characteristics on RBC storage lesions2 and posttransfusion outcomes,3-7 which led to the scrutiny of genetic and biological factors in blood donors that may contribute to variations in the quality of RBC units. Although the clinical consequences of such differences have not been established in humans, studies in mice have demonstrated strain-specific susceptibility to RBC injury from cold storage that correlates with posttransfusion RBC recovery and function.8,9
Genetic or biological factors that modulate RBC responses to stress and favor hemolysis may impact the efficacy of RBC transfusions and increase the risk of transfusion complications via enhanced destruction of stored RBCs in the patient circulation. For example, intravascular hemolysis releases cell-free hemoglobin, heme, arginase, adenosine 5′-diphosphate, and other RBC metabolites that may induce hypertension and endothelial dysfunction by several mechanisms such as hemoglobin-mediated nitric oxide scavenging,10-12 oxidative stress,13 and inflammation.14 Similarly, extravascular hemolysis has been proposed to modulate posttransfusion outcomes by increasing non-transferrin–bound free iron levels, which promote bacterial growth and enhance inflammatory responses in the transfusion recipient.15
Current understanding of genetic and biological variables that may affect RBC storage quality is limited to known RBC disorders, in which genetic enrichment for mutations related to hemolytic diseases, such as sickle cell disease,7 thalassemia, or glucose-6-phosphate dehydrogenase deficiency, may modulate RBC rheology or antioxidant capacity and may compromise RBC recovery during storage and after transfusion.16,17 Functionally relevant RBC polymorphisms are anticipated to be high in donors with racial origin or admixture from malaria-endemic regions, such as equatorial Africa, Asia, and South and Central America.18 In these donors, certain conserved RBC mutations that historically conferred resistance against malaria may impact RBC functional characteristics and be disadvantageous with regard to cold storage. For example, prolonged cold storage of blood from donors with sickle cell trait reduces cellular viability and posttransfusion recovery in murine models.7
In addition to racial background, donor sex has been shown to affect storage hemolysis and RBC storage outcomes. Several studies have indicated that RBCs from male humans and mice exhibit increased susceptibility to stress-induced hemolysis after cold storage.3,6,19,20 Recent studies implicated testosterone effects on RBC production as a mechanism for this sex effect.6 In addition, a recent observational study has associated female sex and a young donor age with increased risk of posttransfusion mortality.5
In an effort to further characterize donor differences in predisposition to storage-induced RBC hemolysis parameters, the National Heart, Lung, and Blood Institute Recipient Epidemiology Donor Evaluation Study III (REDS-III) program launched the Red Blood Cell-Omics (RBC-Omics) study. One aim of the RBC-Omics study is to define genetic and metabolomic bases for donor-specific differences in RBC storage stability. In this first analysis of the RBC-Omics cohort, the associations between donor characteristics and hemolysis in stored RBCs are identified and quantified. We report evaluations of sex, age, and racial/ethnic differences in hemolytic responses to routine blood bank cold storage, including spontaneous end-of-storage hemolysis and responses of stored RBCs to osmotic and oxidative stress. Our findings further emphasize the potential impact of genetic variants, reflected by population sex and self-reported racial/ethnic background, on RBC characteristics during storage. We anticipate that future genetic and metabolomic studies in this cohort will identify novel variants that may impact transfusion safety and efficacy.
Materials and methods
Human subjects
RBC-Omics was conducted under regulations applicable to all human subject research supported by federal agencies. The Data Coordinating Center (RTI International, Rockville, MD) of REDS-III was responsible for the overall compliance of human subjects to regulatory protocols, including institutional review board approval from each participating blood center, from the REDS-III Central Laboratory (Blood Systems Research Institute, San Francisco, CA) and the Data Coordinating Center.
Donor recruitment and data linkages
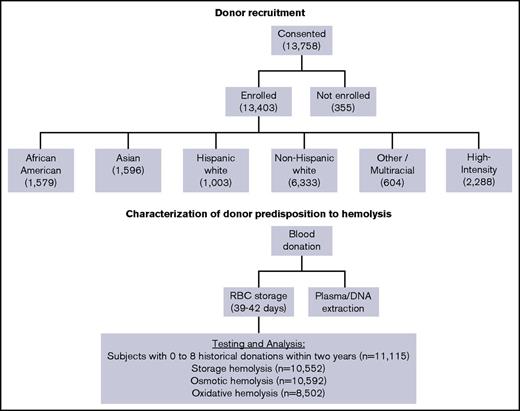
Donor selection and recruitment for RBC-Omics was performed at 4 large blood centers: the American Red Cross (Farmington, CT), the Institute for Transfusion Medicine (Pittsburgh, PA), Blood Center of Wisconsin (Milwaukee, WI), and Blood Centers of the Pacific (San Francisco, CA). Overall, 97% (13 403) of the whole blood donations provided by 13 758 participant donors age 18 years or older who provided informed consent were fully evaluable for storage hemolysis parameters (Figure 1). Donors were categorized into self-reported racial/ethnic groups: non-Hispanic white, Hispanic white, non-Hispanic African American, and non-Hispanic Asian. Donors with multiple races, Hawaiian Americans, Native Americans, and other donors were grouped as “Other.” In addition, we recruited a group of 2288 high-intensity donors, who met the specific criteria of 10 or more successful blood donations in the prior 24 months without a low hemoglobin deferral. These donors were excluded from the current analyses to limit the effects of frequent donation on RBC properties (such as iron status), allowing for a focused analysis of hereditable traits such as donor sex and race/ethnicity.
Flowchart of the RBC-Omics study cohort and donor testing for hemolysis.
Demographic data, including race and ethnicity, were collected directly from enrollment interviews and recorded in the RBC-Omics Study Management System database. Additional demographic data, including weight, height, and date of birth, as well as donation history were derived from the blood centers’ routine donor/donation databases and linked through donor identification, donation date, and donation identification number. Donor age at time of the enrollment donation was derived by calculating the difference between enrollment date and donor date of birth. A biological specimen inventory was used to track biospecimens.
Blood collection and components
Whole blood units were processed according to each blood centers’ standard operating procedures. Each whole blood component was filtered to generate a leukocyte-reduced packed RBC (LR-pRBC) unit in additive solution-1 or 3. A representative portion (10-15 mL) of RBCs from each LR-pRBC unit was then sterile transferred into a customized transfer bag (Haemonetics, Braintree, MA), which was made specifically for this study by using the same materials as the parent RBC storage bag. Pilot studies have demonstrated strong correlations in storage outcomes between types of bags (data not shown). The LR-pRBC parent units were released for distribution for transfusion to patients, whereas the transfer bags were sent to RBC-Omics testing laboratories (University of Pittsburgh, Pittsburgh, PA, and Blood Systems Research Institute, San Francisco, CA).
Evaluation of hemolytic propensity in stored RBCs
After storage under routine blood bank conditions (1-6°C) for 39 to 42 days, the contents of each transfer bag was transferred into a 15-mL conical tube from which 2 aliquots (1 mL each) were processed for the hemolytic assays. One aliquot was used for the quantification of spontaneous storage hemolysis and the other for the stress-induced hemolysis assays. Percent end-of-storage hemolysis was determined according to the following equation:
Sample hematocrit (HCT) was determined by collecting blood samples into capillary tubes, which were centrifuged in a micro-HCT centrifuge (LW Scientific, Lawrenceville, GA). Hbsupernatant refers to the levels of free hemoglobin obtained after centrifugation (1500g, 10 minutes, 18°C) measured in the supernatant. Hbtotal refers to the total amount of sample hemoglobin before centrifugation. In the entire study, hemoglobin concentrations (micromolar) were determined by Drabkin’s method.21
For the evaluation of stress-induced hemolysis, stored RBCs were washed (1500g, 10 minutes, 18°C) 3 times with phosphate-buffered saline (PBS) to remove plasma and additive solution, and immediately subjected to osmotic or oxidative stress assays.
RBC osmotic hemolysis.
Osmotic hemolysis was determined by a modified pink test assay previously used for diagnosis of genetic mutations that affect RBC osmotic fragility (eg, sickle cell disease, thalassemia, and spherocytosis).22,23 Washed RBCs were incubated under static conditions (4 hours at 22°C) in pink test buffer (a hypotonic Bis-Tris buffer containing 25 mmol/L sodium chloride, 70 mmol/L 2,2-Bis(hydroxymethyl)-2,2′,2″nitrilotriethanol (Bis-Tris) buffer, and 135 mmol/L glycerol; pH 6.6) at a final concentration of 1.6% ± 0.2% after which samples were centrifuged (1500g, 10 minutes, 18°C), and percent osmotic hemolysis was determined:
for which Hbosmotic corresponds to supernatant cell-free hemoglobin of pink test–treated RBCs, and Hbtotal refers to the total amount of hemoglobin in each sample.
RBC oxidative hemolysis.
Testing for donor differences in RBC susceptibility to oxidative hemolysis was performed by incubating RBCs in the presence of 2,2′-azobis-2-methyl-propanimidamide dihydrochloride (AAPH; 150 mmoL). Thermal (37°C) decomposition of AAPH generates peroxyl radicals leading to lipid peroxidation–mediated hemolysis.24 Washed RBCs were suspended with PBS to a final concentration of 3.5% ± 0.5%. Aliquots (0.21 mL) were transferred into microplates to which 0.09 mL of AAPH (0.5 M) or PBS was added. The plates were incubated (37°C) under static conditions for 1.5 hours, after which the plates were centrifuged (1500g, 10 minutes, 18°C), and AAPH-induced oxidative hemolysis was determined by using the following formula:
where HbAAPH corresponds to supernatant cell-free hemoglobin of AAPH-treated RBCs, Hbcontrol corresponds to supernatant cell-free hemoglobin from untreated RBCs, and Hbtotal refers to the total amount of hemoglobin in each sample.
Statistical analyses
Data adjustment for blood center–specific differences in RBC production and hemolysis measurements.
To account for center-specific differences in RBC production procedures, per site data adjustment was separated from the multivariable analysis. This decision was made because of known technical (nonbiological) differences in the procedures used to manufacture blood components between the blood centers (eg, blood bags and leukoreduction filter types and timing), and small differences in methods used to generate the hemolysis data between the 2 testing laboratories (eg, differences in instrumentation). The hemolysis measurements in self-reported white donors were adjusted per site so results centered to the same mean (ie, the overall mean of the white samples across the sites). Per site adjustment from the whites were applied to the samples from non-white participants to adjust for the hub-specific effects without adjusting away race- and ethnic-specific effects. Whites were selected as a reference population because they were the largest group of donors across all sites, which provided a robust distribution of the 3 hemolytic measures for the reference group.
Association between donor age and hemolysis.
Evaluation of the association between donor age and each hemolysis measurement was performed by regression analysis using smoothing splines on donor ages. R package ggplot2 (version 2.1.0) and gam (generalized additive model; version 1.12), which use a back-fitting algorithm to combine different smoothing methods (smoothing across 80 points) was used. The fitted lines indicated the predicted mean hemolysis for corresponding ages, and the shaded areas highlight the 95% confidence intervals for the standard error of the mean.
Race/ethnicity differences in hemolysis.
Frequency plots for each of the hemolysis measurements were generated for each racial-ethnic category by using the ggplot2 packages (version 2.1.0) in R statistical software for Windows, version 3.3.1.
Correlations among the 3 hemolytic measures.
The associations among the 3 hemolysis measurements were determined by Pearson’s correlation coefficient. Statistical significance was determined by using a Student t test with N−2 degrees of freedom, where N is the total number of donors with hemolysis measures for each comparison. Calculations were performed by using R statistical software for Windows, version 3.3.1. The null hypothesis was rejected when the P value was smaller than .01 to allow a modest correction for multiple tests.
Multivariable analyses.
A fixed effects multivariable linear model analysis was used to assess the effects of sex, race (non-Hispanic white as the reference), donation history, and age on the various hemolysis outputs. We have chosen this model because our data included the complete distributions of donation history being considered (0-8 donations in 2 years), and nearly the full range of ages for adults who donated blood (18-90 years).
Multiple comparison adjustment.
To account for the fact that we performed multiple statistical tests and to keep the overall type I error rate below .05, we determined tests with P values < .001 to be statistically significant.
Results
RBC-Omics donor demographic characteristics
Between December 2013 and December 2015, 13 758 blood donors consented to participate in the REDS-III RBC-Omics study. Of those, 11 115 donors (excluding high-intensity donors) provided sufficient samples and data for inclusion in this analysis. Their sex, age, and racial-ethnic distributions are provided in Table 1. Donors from both sexes were well represented in this study (53.1% females and 46.9% males). The mean age varied among the racial-ethnic groups, with white donors older (P < .0001) than the other groups.
Blood donor demographic (n = 11 115) characteristics from the RBC-Omics study
| Characteristic . | No. of females . | No. of males . | Female age (y) (mean ± SD) . | Male age (y) (mean ± SD) . |
|---|---|---|---|---|
| White | 3380 | 2953 | 46.5 ± 16.0 | 47.5 ± 15.9 |
| Hispanic | 607 | 396 | 33.2 ± 13.1 | 34.2 ± 13.6 |
| African American | 862 | 717 | 40.1 ± 15.8 | 39.2 ± 15.4 |
| Asian | 732 | 864 | 34.9 ± 13.4 | 36.7 ± 12.6 |
| Other | 322 | 282 | 35.3 ± 14.4 | 37.3 ± 14.3 |
| Characteristic . | No. of females . | No. of males . | Female age (y) (mean ± SD) . | Male age (y) (mean ± SD) . |
|---|---|---|---|---|
| White | 3380 | 2953 | 46.5 ± 16.0 | 47.5 ± 15.9 |
| Hispanic | 607 | 396 | 33.2 ± 13.1 | 34.2 ± 13.6 |
| African American | 862 | 717 | 40.1 ± 15.8 | 39.2 ± 15.4 |
| Asian | 732 | 864 | 34.9 ± 13.4 | 36.7 ± 12.6 |
| Other | 322 | 282 | 35.3 ± 14.4 | 37.3 ± 14.3 |
SD, standard deviation.
Sex associations with hemolysis
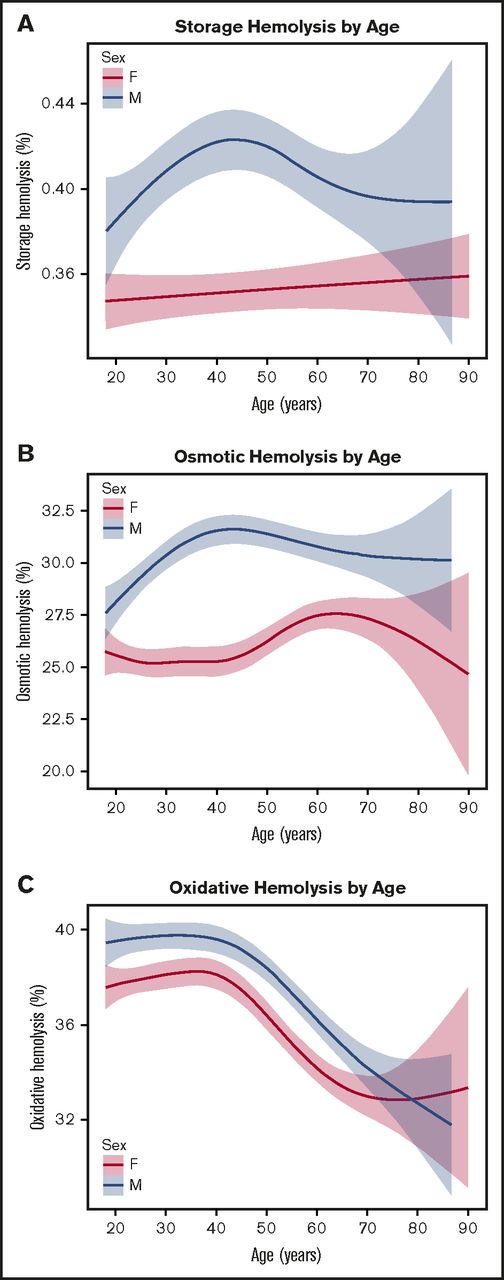
Evaluations of hemolysis in stored RBCs revealed that male sex was associated with increased levels of all 3 hemolysis measures at 39 to 42 days of storage, consistent with reported sex hormone effects on RBC function6 (Figure 2A-C). The sex differences were most notable in storage and osmotic hemolysis (males 0.41% vs females 0.35% for storage hemolysis and males 30.5% vs females 26.0% for osmotic hemolysis; all adjusted P values < .0001) (Table 2).
Distribution of spontaneous storage or stress-induced hemolysis by donor age and sex. RBC concentrates from male or female donors age 18 to 90 years old were stored (1-6°C) for 39 to 42 days in transfer bags and tested for storage or stress-induced hemolysis as described in “Materials and methods.” (A) Percent spontaneous storage hemolysis. (B) Percent osmotic hemolysis (4-hour Pink test). (C) Percent AAPH-induced oxidative hemolysis (incubation conditions: 150 mmol/L, 1.5 hours, 37°C). The fitted line indicates the predicted mean hemolysis for corresponding age, and the shaded areas highlight the 95% confidence intervals for the standard error of the mean.
Distribution of spontaneous storage or stress-induced hemolysis by donor age and sex. RBC concentrates from male or female donors age 18 to 90 years old were stored (1-6°C) for 39 to 42 days in transfer bags and tested for storage or stress-induced hemolysis as described in “Materials and methods.” (A) Percent spontaneous storage hemolysis. (B) Percent osmotic hemolysis (4-hour Pink test). (C) Percent AAPH-induced oxidative hemolysis (incubation conditions: 150 mmol/L, 1.5 hours, 37°C). The fitted line indicates the predicted mean hemolysis for corresponding age, and the shaded areas highlight the 95% confidence intervals for the standard error of the mean.
Sex differences in storage, osmotic, or oxidative hemolysis
| Hemolysis (%) . | Females . | Males . | P (t test) . | No. of females . | No. of males . |
|---|---|---|---|---|---|
| Storage | 0.35 ± 0.32 | 0.41 ± 0.29 | <.0001 | 5585 | 4967 |
| Osmotic | 26.0 ± 12.5 | 30.5 ± 13.7 | <.0001 | 5598 | 4994 |
| Oxidative | 36.6 ± 9.8 | 38.3 ± 9.9 | <.0001 | 4488 | 4014 |
| Hemolysis (%) . | Females . | Males . | P (t test) . | No. of females . | No. of males . |
|---|---|---|---|---|---|
| Storage | 0.35 ± 0.32 | 0.41 ± 0.29 | <.0001 | 5585 | 4967 |
| Osmotic | 26.0 ± 12.5 | 30.5 ± 13.7 | <.0001 | 5598 | 4994 |
| Oxidative | 36.6 ± 9.8 | 38.3 ± 9.9 | <.0001 | 4488 | 4014 |
Values represent mean ± SD percent storage or stress-induced hemolysis in stored (39-42 days) RBCs from female and male donors. Because of multiple testing, differences were considered significant at P < .001.
Effect of age on sex associations with hemolysis
Further evaluation of predisposition to hemolysis based on age revealed age-specific differences across all hemolytic measurements (Figure 2). For storage and osmotic hemolysis, we found sex-specific differences between age and hemolysis. In females, age was associated with a gradual increase in spontaneous storage hemolysis (Figure 2A), whereas in males, the shape of the curve indicated that hemolysis increased from approximately 0.38% to 0.42% between the ages of 18 and 45 years, followed by a gradual decrease in older donors. A similar pattern was observed when stored and washed RBCs from male donors were exposed to osmotic hemolysis (Figure 2B), for which there was an increase from ∼27.5% to 32% between the ages of 18 and 45 years, followed by a decrease to ∼30% in older donors.
Increasing donor age was associated with reduced predisposition to oxidative hemolysis in RBCs from both males and females (Figure 2C). In both sexes, younger age (18-45 years) had a minor effect on predisposition to oxidative hemolysis, whereas age older than 45 years was strongly associated with increased resistance to AAPH exposure. This effect is demonstrated in the difference in average oxidative hemolysis between donors younger than age 21 years (38.3% ± 9.2%) and those older than age 65 years (33.8% ± 9.7%; P < .0001 by variance t test).
Self-reported race/ethnicity associations with hemolysis
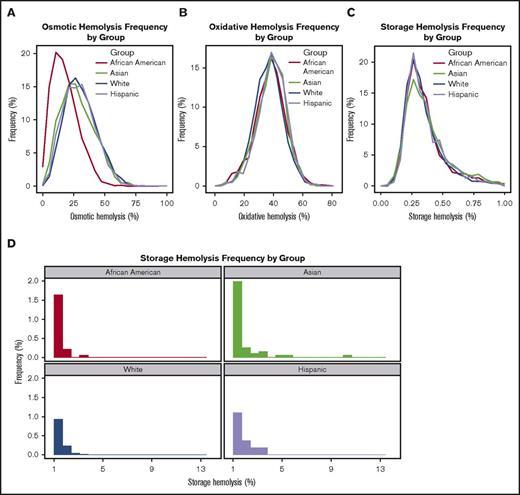
Substantial racial-ethnic differences in predisposition to osmotic hemolysis were observed, consistent with known major effects of thalassemia mutations on reducing osmotic hemolysis. Most notably, RBCs from African American donors exhibited enhanced resistance to osmotic lysis demonstrated by a left shift in the frequency distribution histogram (Figure 3A), for which the mean osmotic hemolysis (17.9% ± 10.6%) was significantly lower in African Americans than in other racial-ethnic groups (ranging from 27.4% ± 13.6% in other donors to 30.6% ± 12.7% in whites; adjusted P < .0001). Smaller differences were observed among the racial groups for mean oxidative hemolysis, which was lowest in white (36.7% ± 9.6%) and highest in Hispanic stored RBCs (39.0% ± 9.6%) (Table 3; Figure 3B).
Frequency distribution of spontaneous storage or stress-induced hemolysis by donor racial background. RBC concentrates from RBC-Omics donors were stored (1-6°C) for 39 to 42 days in transfer bags and tested for storage or stress-induced hemolysis as described in “Materials and methods.” The plots represent the frequency distribution of percentage of storage or stress-induced hemolysis in donors of different racial backgrounds including non-Hispanic white, Hispanic white, non-Hispanic African American, and non-Hispanic Asian. For simplification, hemolysis data from other donors were excluded from this figure and can be viewed in Table 3. (A) Percent osmotic hemolysis (4-hour Pink test). (B) Percent AAPH-induced oxidative hemolysis (incubation conditions: 150 mmol/L, 1.5 hours, 37°C). (C) Percent storage hemolysis at the range of 0% to 1% (acceptable for transfusion) and (D) above 1%.
Frequency distribution of spontaneous storage or stress-induced hemolysis by donor racial background. RBC concentrates from RBC-Omics donors were stored (1-6°C) for 39 to 42 days in transfer bags and tested for storage or stress-induced hemolysis as described in “Materials and methods.” The plots represent the frequency distribution of percentage of storage or stress-induced hemolysis in donors of different racial backgrounds including non-Hispanic white, Hispanic white, non-Hispanic African American, and non-Hispanic Asian. For simplification, hemolysis data from other donors were excluded from this figure and can be viewed in Table 3. (A) Percent osmotic hemolysis (4-hour Pink test). (B) Percent AAPH-induced oxidative hemolysis (incubation conditions: 150 mmol/L, 1.5 hours, 37°C). (C) Percent storage hemolysis at the range of 0% to 1% (acceptable for transfusion) and (D) above 1%.
Effect of donor racial background on osmotic, oxidative, or storage hemolysis
| Hemolysis (%) . | Mean ± SD . | Range . | P . | Percentiles . | No. of donors . | ||||
|---|---|---|---|---|---|---|---|---|---|
| 5 . | 25 . | 50 . | 75 . | 95 . | |||||
| Osmotic | |||||||||
| White | 30.6 ± 12.7 | 1.3-93.4 | 11.4 | 21.3 | 29.4 | 39.0 | 53.3 | 6059 | |
| African American | 17.9 ± 10.6 | 0.0-65.1 | <.0001 | 3.9 | 9.7 | 16.2 | 24.4 | 37.7 | 1497 |
| Asian | 28.1 ± 13.3 | 0.1-74.5 | <.0001 | 8.6 | 17.9 | 26.5 | 37.0 | 51.4 | 1498 |
| Hispanic | 29.3 ± 12.6 | 1.1-73.7 | .005 | 10.5 | 19.9 | 28.7 | 37.8 | 51.0 | 966 |
| Other | 27.4 ± 13.6 | 1.9-69.9 | <.0001 | 7.3 | 16.9 | 26.2 | 36.2 | 51.7 | 572 |
| Oxidative | |||||||||
| White | 36.7 ± 9.6 | 6.0-74.3 | 20.8 | 30.5 | 36.8 | 42.9 | 52.7 | 4663 | |
| African American | 37.7 ± 10.7 | 3.9-77.1 | .0028 | 18.6 | 31.0 | 38.1 | 44.6 | 54.3 | 1387 |
| Asian | 38.4 ± 9.8 | 4.1-67.6 | <.0001 | 21.4 | 31.9 | 39.2 | 45.3 | 54.0 | 1178 |
| Hispanic | 39.0 ± 9.6 | 5.9-70.0 | <.0001 | 22.5 | 33.3 | 39.5 | 45.3 | 53.5 | 837 |
| Other | 38.5 ± 10.3 | 5.6-68.2 | .0005 | 20.3 | 31.8 | 38.9 | 45.7 | 53.9 | 437 |
| Storage | |||||||||
| White | 0.36 ± 0.21 | 0.00-3.63 | 0.17 | 0.24 | 0.31 | 0.41 | 0.70 | 6031 | |
| African American | 0.38 ± 0.23 | 0.09-3.19 | .0028 | 0.17 | 0.25 | 0.32 | 0.42 | 0.80 | 1497 |
| Asian | 0.43 ± 0.44 | 0.05-10.5 | <.0001 | 0.17 | 0.25 | 0.34 | 0.47 | 0.91 | 1491 |
| Hispanic | 0.41 ± 0.58 | 0.06-16.2 | .0189 | 0.18 | 0.25 | 0.32 | 0.45 | 0.75 | 963 |
| Other | 0.36 ± 0.27 | 0.09-3.74 | .9735 | 0.16 | 0.23 | 0.31 | 0.41 | 0.70 | 570 |
| Hemolysis (%) . | Mean ± SD . | Range . | P . | Percentiles . | No. of donors . | ||||
|---|---|---|---|---|---|---|---|---|---|
| 5 . | 25 . | 50 . | 75 . | 95 . | |||||
| Osmotic | |||||||||
| White | 30.6 ± 12.7 | 1.3-93.4 | 11.4 | 21.3 | 29.4 | 39.0 | 53.3 | 6059 | |
| African American | 17.9 ± 10.6 | 0.0-65.1 | <.0001 | 3.9 | 9.7 | 16.2 | 24.4 | 37.7 | 1497 |
| Asian | 28.1 ± 13.3 | 0.1-74.5 | <.0001 | 8.6 | 17.9 | 26.5 | 37.0 | 51.4 | 1498 |
| Hispanic | 29.3 ± 12.6 | 1.1-73.7 | .005 | 10.5 | 19.9 | 28.7 | 37.8 | 51.0 | 966 |
| Other | 27.4 ± 13.6 | 1.9-69.9 | <.0001 | 7.3 | 16.9 | 26.2 | 36.2 | 51.7 | 572 |
| Oxidative | |||||||||
| White | 36.7 ± 9.6 | 6.0-74.3 | 20.8 | 30.5 | 36.8 | 42.9 | 52.7 | 4663 | |
| African American | 37.7 ± 10.7 | 3.9-77.1 | .0028 | 18.6 | 31.0 | 38.1 | 44.6 | 54.3 | 1387 |
| Asian | 38.4 ± 9.8 | 4.1-67.6 | <.0001 | 21.4 | 31.9 | 39.2 | 45.3 | 54.0 | 1178 |
| Hispanic | 39.0 ± 9.6 | 5.9-70.0 | <.0001 | 22.5 | 33.3 | 39.5 | 45.3 | 53.5 | 837 |
| Other | 38.5 ± 10.3 | 5.6-68.2 | .0005 | 20.3 | 31.8 | 38.9 | 45.7 | 53.9 | 437 |
| Storage | |||||||||
| White | 0.36 ± 0.21 | 0.00-3.63 | 0.17 | 0.24 | 0.31 | 0.41 | 0.70 | 6031 | |
| African American | 0.38 ± 0.23 | 0.09-3.19 | .0028 | 0.17 | 0.25 | 0.32 | 0.42 | 0.80 | 1497 |
| Asian | 0.43 ± 0.44 | 0.05-10.5 | <.0001 | 0.17 | 0.25 | 0.34 | 0.47 | 0.91 | 1491 |
| Hispanic | 0.41 ± 0.58 | 0.06-16.2 | .0189 | 0.18 | 0.25 | 0.32 | 0.45 | 0.75 | 963 |
| Other | 0.36 ± 0.27 | 0.09-3.74 | .9735 | 0.16 | 0.23 | 0.31 | 0.41 | 0.70 | 570 |
P values were obtained by t tests, which compared the mean hemolysis value of each minority group with that of white donors. Because of multiple testing, differences were considered significant at P < .001.
RBCs from Asian donors exhibited significantly higher storage hemolysis compared with those from white donors (0.43% ± 0.44% vs 0.36% ± 0.21%, respectively; P < .0001) (Table 3; Figure 3C-D). Further evaluation of racial-ethnic differences in storage hemolysis at levels exceeding US Food and Drug Administration regulations (>1%) suggested that the extreme hemolysis was more frequent in RBCs donated by Asian and African American donors than in RBCs from white donors. As shown in Figure 3D and Table 3, 3.51% of Asian donors and 2.47% of African American donors had more than 1% storage hemolysis compared with 1.67% and 2.09% for white and Hispanic donors, which supports the existence of genetic variants that significantly affect RBC structure and function.
Multivariable analyses of donor demographics associated with hemolysis
Analysis using a multivariable linear model that adjusted for donation history, donor sex, age, and race (Table 4) found that storage hemolysis was significantly associated with donor sex, Asian and Hispanic race, and donor age (all P values < .0001). Donation history did not have a significant independent effect on storage hemolysis (Table 4). This model also found that on average, storage hemolysis in male donors is 0.055% higher than in females, and that the racial-ethnic differences in storage hemolysis were consistent among male and female donors.
Multivariable linear modeling of hemolysis measures
| Hemolysis . | Parameter estimate . | P . |
|---|---|---|
| Storage | ||
| Male sex | 0.055 | <.0001 |
| Race/ethnicity | ||
| African American | 0.024 | .0086 |
| Asian | 0.070 | <.0001 |
| Hispanic | 0.059 | <.0001 |
| Donation history | −0.003 | .065 |
| Age | 0.001 | <.0001 |
| Osmotic | ||
| Male sex | 4.509 | <.0001 |
| Race/ethnicity | ||
| African American | −12.882 | <.0001 |
| Asian | −3.099 | <.0001 |
| Hispanic | −1.071 | .015 |
| Donation history | −0.267 | <.0001 |
| Age | 0.017 | .042 |
| Oxidative | ||
| Male sex | 1.878 | <.0001 |
| Race/ethnicity | ||
| African American | −0.021 | .94 |
| Asian | 0.113 | .73 |
| Hispanic | 0.874 | .020 |
| Donation history | −0.317 | <.0001 |
| Age | −0.080 | <.0001 |
| Hemolysis . | Parameter estimate . | P . |
|---|---|---|
| Storage | ||
| Male sex | 0.055 | <.0001 |
| Race/ethnicity | ||
| African American | 0.024 | .0086 |
| Asian | 0.070 | <.0001 |
| Hispanic | 0.059 | <.0001 |
| Donation history | −0.003 | .065 |
| Age | 0.001 | <.0001 |
| Osmotic | ||
| Male sex | 4.509 | <.0001 |
| Race/ethnicity | ||
| African American | −12.882 | <.0001 |
| Asian | −3.099 | <.0001 |
| Hispanic | −1.071 | .015 |
| Donation history | −0.267 | <.0001 |
| Age | 0.017 | .042 |
| Oxidative | ||
| Male sex | 1.878 | <.0001 |
| Race/ethnicity | ||
| African American | −0.021 | .94 |
| Asian | 0.113 | .73 |
| Hispanic | 0.874 | .020 |
| Donation history | −0.317 | <.0001 |
| Age | −0.080 | <.0001 |
Differences are considered significant at P < .001 after adjustment for race/ethnicity, sex, donor age, and donation history.
Osmotic hemolysis was strongly associated with African American racial background, donor sex, and donation history (all P values < .0001) (Table 4). Donor age did not correlate with osmotic hemolysis in the entire male population. However, younger age (≤45 years) was positively (P = .0002) associated with osmotic hemolysis, whereas a negative (P = .00023) association was observed in donors older than age 45 years. Conversely, donor age in both sexes was strongly and negatively associated with susceptibility to oxidative hemolysis. Other variables that were significantly associated with oxidative hemolysis were donor sex and donation history (all P values < .0001). Donor race/ethnicity was not significantly associated with oxidative hemolysis (Table 4).
Weak correlations between storage and stress hemolysis measurements
The possibility that donor predisposition to spontaneous storage hemolysis correlated with stress-induced hemolysis (osmotic fragility and oxidative stress) was examined (Table 5). In both cases, storage hemolysis demonstrated weak correlation with osmotic hemolysis (Pearson’s r = 0.08) or oxidative hemolysis (r = 0.02). Likewise, weak correlation was observed between the stress hemolysis assays (r = 0.05).
Pearson r test estimates of the correlations among the 3 hemolytic measurements
| Hemolysis . | Pearson’s r . | P . |
|---|---|---|
| Storage vs osmotic | 0.08 | <.0001 |
| Storage vs oxidative | 0.02 | .069 |
| Osmotic vs oxidative | 0.05 | <.0001 |
| Hemolysis . | Pearson’s r . | P . |
|---|---|---|
| Storage vs osmotic | 0.08 | <.0001 |
| Storage vs oxidative | 0.02 | .069 |
| Osmotic vs oxidative | 0.05 | <.0001 |
Discussion
Our study provides new evidence in large ethnically diverse cohorts of blood donors and demonstrates the impact of donor characteristics on RBC predisposition to spontaneous and stress hemolysis after cold storage consistent with routine blood bank practice. The data have identified and quantified broad genetic and biological variables that contribute to donor differences in RBC storage and stress stability. Among the selected variables, male sex, Asian and African American race/ethnicity, and older age demonstrated the strongest associations with spontaneous or stress hemolysis. Although these new observations are limited to in vitro measurements of RBC propensity for hemolysis, they provide new insights into donor characteristics that may impact the efficacy and adverse consequences of transfused RBC components and may provide phenotypes for evaluating genetic polymorphisms associated with altered RBC function in response to varied stressors.
The relationship between in vitro measurements of hemolysis used in this study and RBC posttransfusion recovery is unclear. In preclinical rodent studies of RBCs and transfusion, we found that alterations in osmotic and storage hemolysis do predict lower posttransfusion RBC recovery.6,7 In patients with hereditary spherocytosis, enhanced susceptibility of hereditary spherocytosis RBCs to osmotic fragility was associated with poor RBC in vivo recovery after autologous transfusion of stored RBCs.25 A few observational studies that examined the association between donor characteristics and patient outcomes suggested that female RBCs may increase the risk of posttransfusion mortality,5,26 whereas comparable studies found no associations between donor sex or age and posttransfusion mortality.27,28 Because packed RBC units contain plasma, these studies could evaluate only the clinical effects of total blood component transfusions; hence, donor plasma and/or RBCs may have been responsible for transfusion-related adverse outcomes.
Although sex differences in spontaneous storage hemolysis have been recently reported in a cohort of blood donors from Canada,3,6 there has been no prior large-scale characterization of predisposition to stress-induced hemolysis. The sex differences in osmotic and oxidative hemolysis after storage are likely intrinsic to the RBCs, because these hemolytic assays were performed on washed RBCs in the absence of donor plasma or additive solution. The molecular mechanisms that promote sex differences in hemolysis are not clear, although a recent study has linked testosterone with enhanced susceptibility to hemolysis in storage and after transfusion in male mice.6 In that study, orchiectomy improved the posttransfusion recovery of stored RBCs compared with control male RBCs, whereas testosterone repletion in orchiectomized mice was associated with increased susceptibility to osmotic and oxidative hemolysis. It should be noted that in mice, the magnitude of the sex and strain differences in storage or stress hemolysis are significantly greater than those reported in the human data presented here. Therefore, the differences we documented here should be further evaluated in humans to determine the clinical impact of stored RBCs from donors with distinct differences in predisposition to hemolysis.
Interestingly, we found that aging in both sexes was associated with enhanced resistance to AAPH-induced oxidative hemolysis. The cause for this phenomenon is not clear and requires further investigation. Previous studies have demonstrated increased levels of oxidative stress and compromised antioxidant activity in RBCs from elderly subjects (age 50-75 years) compared with younger subjects (age 20-30 years).29 The mechanisms that confer resistance to AAPH oxidation in the stored RBCs of older blood donors are not likely to be related to sex hormones as suggested by the similar trend observed in both sexes. The recent report that linked transfusion of RBCs from older donors with reduced risk of posttransfusion mortality5 may be explained in part by our observations; however, further characterization of age-related differences in RBC characteristics and clinical consequences after transfusion are required.
The most profound association between race/ethnicity and hemolysis was observed in African American donors and osmotic hemolysis. The unique resistance of African American donors’ RBCs to osmotic fragility may stem from the high prevalence of genetic traits for hemolytic diseases (eg, sickle cell disease and thalassemia) in blood donors of African descent that are known to reduce osmotic hemolysis.30,31 A recent study that evaluated the impact of sickle cell trait on RBC storage stability and posttransfusion recovery demonstrated that sickle trait RBCs exhibited high resistance to osmotic hemolysis, with accelerated degradation during cold storage and reduced posttransfusion recovery in mice.7 Because sickle cell trait affects 8% to 10% of African Americans,32 given the magnitude of our observations, we hypothesize that other genetic factors may also modulate the osmotic hemolysis pattern of stored RBCs from African American donors. This finding also likely relates to the high prevalence of α-thalassemia variants in African Americans,33 because they are associated with enhanced resistance to osmotic fragility.34
Less pronounced associations between donor race/ethnicity and hemolysis were observed in Asian donors who had increased sensitivity to storage and oxidative hemolysis and Hispanic donors who had increased sensitivity to oxidative hemolysis. It may be argued that in the case of spontaneous storage hemolysis, the magnitude of the differences among the groups is relatively small. However, evaluation of extreme hemolyzers (≥1% storage hemolysis) suggests that there may be underlying differences among ethnic groups. On the basis of this finding, we anticipate that whole genome sequencing and association studies will identify hereditable variants with substantive effects on RBC storage and posttransfusion recovery that will be of clinical relevance.
Although we have observed significant (P < .0001) associations between storage and osmotic hemolysis or between osmotic and oxidative hemolysis, Pearson’s r analyses suggested that such associations are weak. This may be explained by differences in genetic and biological variables that are associated with each stress phenotype. On the basis of the results of multivariable analyses, storage hemolysis is predominantly associated with donor sex, and osmotic hemolysis is predominantly associated with donor race/ethnicity, whereas predisposition to oxidative hemolysis is most strongly modulated by donor age. Such differences and the presence of confounding factors that are unique to a subset of the donor population may have weakened the associations between the hemolytic measurements. It should be noted that in addition to genetic factors, our study has revealed that donation history may significantly modulate the hemolytic propensity of stored RBCs. This observation was most relevant to oxidative hemolysis, which was negatively associated with the number of previous donations (up to 8 donations in 2 years before the study). Because the likelihood of intensive donation history increases with age, it is possible that the enhanced resistance to AAPH hemolysis observed in older donors is partially associated with lifetime donation history and other age-related factors contributing to reduced iron availability for erythropoiesis.
In conclusion, our study has identified and quantified the impact of donor sex, age, and race/ethnicity on spontaneous and stress-induced hemolysis in stored RBCs. These finding emphasize the need for linked studies that will evaluate clinical outcomes of RBC transfusions from donors with high vs low hemolysis. An analysis is in progress to determine the outcomes, including hemoglobin increments and clinical findings, after transfusions of ∼20 000 RBC units derived from the RBC-Omics donors reported in this study. The RBC-Omics study data set and biospecimens (DNA and cryopreserved RBC samples) will be invaluable for ongoing studies of genetic and metabolic biomarkers relevant to the RBC storage lesions, to optimizing RBC storage conditions and donation policies, and to broader insights into the etiology and pathogenesis of hemolytic diseases.
Acknowledgments
The authors thank the RBC-Omics research staff at all participating blood centers, the testing laboratories for performing tests and for their contribution to this project, and all blood donors who agreed to participate in this study.
This study was supported by the National Institutes of Health, National Heart, Lung, and Blood Institute (NHLBI) contracts HHSN2682011-00001I, HHSN2682011-00002I, HHSN2682011-00003I, HHSN2682011-00004I, HHSN2682011-00005I, HHSN2682011-00006I, HHSN2682011-00007I, HHSN2682011-00008I, and HHSN2682011-00009I, which supported the Recipient Epidemiology and Donor Evaluation Study III (REDS-III) RBC-Omics study, and by National Institutes of Health, NHLBI grant R01HL098032-04 (M.T.G.), which partially supported research staff and assay development for this study.
Authorship
Contribution: T.K. and M.T.G. contributed to the design and execution of the study, wrote the manuscript, developed the hemolysis assays, supervised Pittsburgh’s testing laboratory, and performed data analyses and interpretation; M.P.B. and S. Kleinman developed the study protocols, supervised donor recruitment and blood center and testing laboratory activities, participated in data analyses and interpretation, and edited and finalized the manuscript; M.C.L., M.S., and S. Keating developed the study’s protocols, including quality assurance of the hemolysis assays, supervised the testing labs at Blood Systems Research Institute, and performed data analysis; D.J.T., and J.E.K. participated in the design and execution of the study, data interpretation, and editorial input for the manuscript; A.E.M. participated in study design, data interpretation, and writing the manuscript; R.G.C. prepared and coordinated key protocol sections of the study, participated in the writing group, and reviewed data and manuscript drafts; G.P.P. and Y.G. designed and performed the statistical analyses of data for the study; S.M.E. assisted with oversight of data coordination from all participating sites; and E.L.M. supervised collection of data and helped editing the manuscript.
Conflict-of-interest disclosure: D.J.T. served as a consultant to Fresenius Kabi. A.E.M. received research funding from Novo Nordisk and has received honoraria from Siemens. The remaining authors declare no competing financial interests.
A complete list of the members of the NHLBI REDS-III Program appears in “Appendix.”
Correspondence: Tamir Kanias, Pittsburgh Heart, Lung, Blood, and Vascular Medicine Institute and Division of Pulmonary, Allergy, and Critical Care Medicine, Department of Medicine, University of Pittsburgh, Starzl Biomedical Science Tower, E1200-22B, 200 Lothrop St, Pittsburgh, PA 15261; e-mail: tak77@pitt.edu.
Appendix: study group members
The NHLBI Recipient Epidemiology Donor Evaluation Study-III (REDS-III), Red Blood Cell (RBC)-Omics Study, is the responsibility of the following persons: Hubs: A. E. Mast, J. L. Gottschall, W. Bialkowski, L. Anderson, J. Miller, A. Hall, Z. Udee, and V. Johnson, BloodCenter of Wisconsin, Milwaukee, WI; D. J. Triulzi, J. E. Kiss, and P. A. D'Andrea, The Institute for Transfusion Medicine (ITXM), Pittsburgh, PA; E. L. Murphy and A. M. Guiltinan, University of California, San Francisco, San Francisco, CA; R. G. Cable, B. R. Spencer, and S. T. Johnson, American Red Cross Blood Services, Farmington, CT; Data coordinating center: D. J. Brambilla, M. T. Sullivan, S. M. Endres, G. P. Page, Y. Guo, N. Haywood, D. Ringer, and B. C. Siege, RTI International, Rockville, MD; Central and testing laboratories: M. P. Busch, M. C. Lanteri, M. Stone, and S. Keating, Blood Systems Research Institute, San Francisco, CA; T. Kanias and M. Gladwin, Pittsburgh Heart, Lung, Blood, and Vascular Medicine Institute, Division of Pulmonary, Allergy and Critical Care Medicine, University of Pittsburgh, Pittsburgh, PA; Steering committee chairman: S. H. Kleinman, University of British Columbia, Victoria, BC, Canada; National Heart, Lung, and Blood Institute, National Institutes of Health: S. A. Glynn, K. B. Malkin, and A. M. Cristman.