Key Points
DECADE is the first prospective trial to investigate the effect of complement inhibition by eculizumab in CAD.
Eculizumab reduced hemolysis and transfusion dependency in the majority of patients but had no impact on cold-induced circulatory symptoms.
Abstract
Cold agglutinin disease (CAD) is a complement-dependent disorder, with extravascular and intravascular hemolysis resulting from initial or terminal complement activation, respectively. We tested the efficacy and safety of eculizumab, an inhibitor of the terminal complement pathway. Treatment-requiring patients received 600 mg eculizumab weekly for 4 weeks, followed 1 week later by 900 mg every other week through week 26. The primary end point was the difference in the lactate dehydrogenase level between the first and the last day of therapy. Twelve patients with chronic CAD and 1 patient with an acute cold agglutinin syndrome were included. The median lactate dehydrogenase level decreased from 572 U/L (interquartile range [IQR], 534-685) to 334 U/L (IQR, 243-567; P = .0215), paralleled by an increase in hemoglobin from 9.35 g/dL (IQR, 8.80-10.80) to 10.15 g/dL (IQR, 9.00-11.35; P = .0391; Wilcoxon signed-rank test). Three patients maintained and 8 patients acquired transfusion independence, and 1 patient each showed a reduced or increased transfusion requirement, respectively (P = .0215; exact McNemar’s test). Patients with cold agglutinins with a thermal amplitude of 37°C tended to have less pronounced lactate dehydrogenase responses than patients with cold agglutinins with narrower thermal amplitudes. In the latter, responses were observed at lower serum levels of eculizumab than they were in the former. In contrast to hemolysis, cold-induced circulatory symptoms remained unaffected. In conclusion, eculizumab significantly reduced hemolysis and transfusion requirement in patients with CAD. Suppression of hemolysis caused by cold agglutinins with a wide thermal amplitude may require higher eculizumab doses than used here. The trial is registered with EudraCT (#2009-016966-97) and www.clinicaltrials.gov (#NCT01303952).
Introduction
Cold agglutinin disease (CAD) is a rare form of hemolytic anemia caused by immunoglobulin M (IgM) autoantibodies against red blood cell (RBC) antigens. These antibodies bind to the red cell surface at low temperature, followed by erythrocyte agglutination and activation of the initial complement pathway. At higher temperature, the antibodies dissociate from the cells. Complement activation leads to deposition of C3b and initiation of the terminal complement cascade. C3b-coated erythrocytes are engulfed by macrophages of the reticuloendothelial system, consistent with extravascular hemolysis. Terminal complement activation may result in the formation of a membrane attack complex with intravascular hemolysis. However, because erythrocytes express CD59, an inhibitor of terminal complement activation, it is assumed that the major route of hemolysis is extravascular.1
There are 2 types of the disease.2 The most common form is chronic CAD, a B-cell lymphoproliferative disorder with production of a monoclonal hemagglutinin. Most patients suffer from mild or moderate anemia, with exacerbation at cold temperature. Circulatory symptoms resulting from erythrocyte agglutination may also be present. The second type is cold agglutinin syndrome (CAS), which is attributable to production of a hemagglutinin as a by-product of infection, cancer, or other diseases. Hemolysis may be severe, but, in most instances, the disorder is transient subsiding when the underlying cause is eliminated.
In chronic CAD, the mainstay of treatment is avoidance of cold.1 Drug treatments aim at reducing the pathogenic B-cell clone. Glucocorticoids are of limited value, but rituximab, a B-cell-targeting antibody, induces remissions in 50%,3 and rituximab combined with chemotherapy may induce remissions in up to 75% of patients.4,5 However, because CAD is a disorder of the elderly, intensive cytoreductive therapies may not be feasible.
Terminal complement activation can be inhibited by eculizumab, a monoclonal antibody against complement factor C5. Eculizumab is approved for the treatment of paroxysmal nocturnal hemoglobinuria, an acquired deficiency of the complement inhibitors CD55 and CD59,6 and atypical hemolytic uremic syndrome, which results from other acquired or, more often, hereditary defects of complement regulation.7 Despite the notion that terminal complement activation is of minor importance in CAD, we and others observed long-lasting responses to eculizumab in individual patients.8-10 To investigate this further, we performed a prospective phase 2 trial.
Patients, materials, and methods
Study design and participants
This prospective, bicentric, nonrandomized phase 2 study was designed to demonstrate the efficacy and safety of terminal complement inhibition in patients with cold agglutinin disease using eculizumab (DECADE). Patients with CAD requiring treatment of anemia received eculizumab for a total of 26 weeks according to a schedule originally developed for the treatment of paroxysmal nocturnal hemoglobinuria.6 Efficacy was demonstrated by a reduction in the activity of the serum lactate dehydrogenase which mirrors the intensity of hemolysis. The trial was performed at the University Hospitals of Essen and Ulm, both in Germany, and approved by the Federal Institute for Drugs and Medical Devices and the ethics committees at both participating sites. The trial is registered with EudraCT (#2009-016966-97) and www.clinicaltrials.gov (#NCT01303952).
Patients with CAD who were 18 years of age or older, required treatment because of anemia-related symptoms or transfusion dependency, and had a serum lactate dehydrogenase level greater than or equal to twice the upper limit of normal were eligible. CAD was defined by hemolysis with a cold agglutinin titer ≥64 at 4°C and a monospecific direct antiglobulin test with strong reactivity against C3d (a cleavage product of C3b) and negative or only weak reactivity against IgG. Treatment with alkylating agents, rituximab, human immunoglobulins, or plasmapheresis was prohibited at least 4 weeks before screening. All patients gave written informed consent. The patients were registered at the Center for Clinical Studies of the University of Duisburg-Essen who monitored the study at both sites.
Procedures
The trial consisted of 3 phases: a 2-week enrollment phase with screening (medical and transfusion history, laboratory investigations, cold agglutinin titer, and thermal amplitude) and vaccination against Neisseria meningitidis (Menveo, Novartis Vaccines); a 26-week treatment phase during which the patients received infusions of 600 mg eculizumab weekly for 4 weeks, followed 1 week later by 900 mg every other week through week 26 without any premedication (especially no steroids); and an 8-week posttreatment observation phase. Patients completing the treatment phase were given the option to continue eculizumab beyond week 26. The investigations performed at screening were repeated at predefined time points during the subsequent phases of the trial. Hemagglutination was tested at 4°C, 20°C, 30°C, and 37°C, using patient serum and pooled group 0 erythrocytes. The thermal amplitude was defined as the highest temperature at which agglutination occurred.
Outcomes
The primary end point was the difference in the lactate dehydrogenase level between the first and the last day of treatment. For the primary analysis, missing values were imputed by carrying the last observation forward. Therapy response was defined as a decrease in the lactate dehydrogenase level between the first and the last day of treatment ≥250 U/L.
Secondary end points included changes in indicators of hemolysis (hemoglobin, haptoglobin, hemopexin, free hemoglobin, reticulocytes), transfusion requirement (number of transfusion-dependent patients and number of RBC units transfused, compared with the expected requirement based on the preceding 12 months), thromboembolic events (compared with the previous medical history, d-dimers), exercise tolerance (6-minute walk test), quality of life (as assessed by the SF-36v2 QLQ tool), fatigue (Functional Assessment of Chronic Illness Therapy–Fatigue Scale version 4), and pharmacokinetics. The statistical analysis of the secondary end points was performed without imputation of missing values. At both trial sites, an individual transfusion algorithm based on each patient’s own transfusion history was developed, and the trigger for transfusion remained unchanged for each patient, as compared with his or her care before entry into the study. Trough and peak serum levels of eculizumab were determined on the first day and after 4, 12, and 26 weeks of treatment with an enzyme-linked immunosorbent assay that detects both free and C5-bound eculizumab.11
Assessment of safety included treatment-related adverse events and laboratory tests, using the Common Terminology Criteria for Adverse Events (CTCAE, version 3.0; https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/ctcaev3.pdf).
Statistical analysis
Using a paired Student t test and assuming a standard deviation of the difference between the first and the last day of treatment of 275 U/L, we calculated that 12 patients would be required to have 80% power to demonstrate a difference of 250 U/L at a 2-sided significance level α of 0.05. We estimated that, using the Wilcoxon signed-rank test, which was used throughout the investigation to test changes in paired continuous variables, 13 patients would be required to show the expected result.
All analyses of secondary end points and subgroups were exploratory. Proportions were tested using Fisher’s exact test, unpaired continuous variables were tested using the Wilcoxon rank-sum test, and changes in transfusion requirement were tested using the exact McNemar’s test. The statistical analysis was performed using version 9.4 of SAS proprietary software (SAS Institute, Cary, NC). All authors had full access to the primary data and participated in their analysis.
Results
Patient characteristics
Between 6 January 2011 and 14 January 2014, 10 women and 3 men were entered into the DECADE trial (Table 1). The median age was 74 years (range, 64-80). Twelve patients had chronic CAD, with a median disease duration of 42 months (range, 5-221). Of these, 6 were treatment naïve, and 6 were pretreated, with a median of 3 prior lines of therapy (range, 2-5), including cyclophosphamide (5 patients); prednisone (4 patients); azathioprine, rituximab (3 patients each); and chlorambucil, bendamustine, fludarabine, and human immunoglobulins (1 patient each). One patient had an acute CAS for which she had received prednisone.
Pretreatment characteristics of 13 patients with CAD and effect of eculizumab on frequency of RBC transfusion and levels of lactate dehydrogenase and hemoglobin
| Patient identification number . | Pretreatment characteristics . | Treatment results . | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Age, y/sex . | Disease duration, mo . | No. of prior therapies . | No. of RBC units expected* . | No. of RBC units received† . | Lactate dehydrogenase, U/L . | Hemoglobin, g/dL . | |||||
| Week 0 . | Week 26 . | Week 34 . | Week 0 . | Week 26 . | Week 34 . | ||||||
| Lactate dehydrogenase decrease <250 U/L, chronic CAD | |||||||||||
| 1‡ | 77/female | 42 | 0 | 1.3 | 0 | 545 | 378 | 268 | 10.8 | 11.5 | 9.9 |
| 3 | 72/male | 16 | 0 | 1.3 | 0 | 572 | 567 | 471 | 9.0 | 10.4 | 9.8 |
| 6 | 75/female | 87 | 2 | 0 | 0 | 797 | (800)§ | (899)§ | 11.6 | (11.5)§ | (13.0)§ |
| 11 | 78/female | 61 | 2 | 20.9 | 4 | 450 | 334 | 539 | (8.5)║ | 9.1 | 9.1 |
| 12 | 74/female | 7 | 0 | 2.6 | 0 | 514 | 327 | 809 | 8.3 | 8.5 | 8.6 |
| 13 | 64/female | 193 | 0 | 3.9 | 0 | 638 | 1127 | 919 | 10.8 | 10.1 | (11.1)¶ |
| Lactate dehydrogenase decrease ≥250 U/L, chronic CAD | |||||||||||
| 4‡ | 73/female | 5 | 3 | 3.9 | 0 | 625 | 243 | 231 | 8.8 | 8.9 | 9.6 |
| 7 | 80/female | 221 | 5 | 5.2 | 0 | 685 | 353 | 305 | 8.3 | 9.4 | 9.6 |
| 9 | 74/male | 42 | 3 | 2.1 | 0 | 534 | (211)# | n.d.# | 9.5 | (8.1)# | n.d.# |
| 10 | 69/female | 15 | 0 | 0 | 0 | 566 | (282)** | 683 | 9.5 | 12.1 | 11.8 |
| 14 | 73/male | 80 | 4 | 0 | 12 | 719 | 243 | 414 | (8.8)†† | 8.8 | 10.5 |
| 15 | 76/female | 10 | 0 | 0 | 0 | 453 | 194 | 486 | 9.2 | 11.2 | 9.5 |
| Lactate dehydrogenase decrease ≥250 U/L, acute CAS | |||||||||||
| 5 | 73/female | 1 | 1 | 5.2 | 0 | 2980 | 144 | 154 | 9.6 | 10.2 | 11.4 |
| Patient identification number . | Pretreatment characteristics . | Treatment results . | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Age, y/sex . | Disease duration, mo . | No. of prior therapies . | No. of RBC units expected* . | No. of RBC units received† . | Lactate dehydrogenase, U/L . | Hemoglobin, g/dL . | |||||
| Week 0 . | Week 26 . | Week 34 . | Week 0 . | Week 26 . | Week 34 . | ||||||
| Lactate dehydrogenase decrease <250 U/L, chronic CAD | |||||||||||
| 1‡ | 77/female | 42 | 0 | 1.3 | 0 | 545 | 378 | 268 | 10.8 | 11.5 | 9.9 |
| 3 | 72/male | 16 | 0 | 1.3 | 0 | 572 | 567 | 471 | 9.0 | 10.4 | 9.8 |
| 6 | 75/female | 87 | 2 | 0 | 0 | 797 | (800)§ | (899)§ | 11.6 | (11.5)§ | (13.0)§ |
| 11 | 78/female | 61 | 2 | 20.9 | 4 | 450 | 334 | 539 | (8.5)║ | 9.1 | 9.1 |
| 12 | 74/female | 7 | 0 | 2.6 | 0 | 514 | 327 | 809 | 8.3 | 8.5 | 8.6 |
| 13 | 64/female | 193 | 0 | 3.9 | 0 | 638 | 1127 | 919 | 10.8 | 10.1 | (11.1)¶ |
| Lactate dehydrogenase decrease ≥250 U/L, chronic CAD | |||||||||||
| 4‡ | 73/female | 5 | 3 | 3.9 | 0 | 625 | 243 | 231 | 8.8 | 8.9 | 9.6 |
| 7 | 80/female | 221 | 5 | 5.2 | 0 | 685 | 353 | 305 | 8.3 | 9.4 | 9.6 |
| 9 | 74/male | 42 | 3 | 2.1 | 0 | 534 | (211)# | n.d.# | 9.5 | (8.1)# | n.d.# |
| 10 | 69/female | 15 | 0 | 0 | 0 | 566 | (282)** | 683 | 9.5 | 12.1 | 11.8 |
| 14 | 73/male | 80 | 4 | 0 | 12 | 719 | 243 | 414 | (8.8)†† | 8.8 | 10.5 |
| 15 | 76/female | 10 | 0 | 0 | 0 | 453 | 194 | 486 | 9.2 | 11.2 | 9.5 |
| Lactate dehydrogenase decrease ≥250 U/L, acute CAS | |||||||||||
| 5 | 73/female | 1 | 1 | 5.2 | 0 | 2980 | 144 | 154 | 9.6 | 10.2 | 11.4 |
Imputed values are given in parentheses.
n.d., not done; no., number.
Number of RBC units expected to be transfused within the 34-week study period, based on the transfusion requirement in the preceding 12 months.
Number of RBC units received within the 34-week study period.
After the end of the treatment phase, eculizumab was continued for another 38 weeks (patient 1) or 24 weeks (patient 4), respectively.
Imputed from week 18 (premature trial termination after 12 eculizumab infusions because of persistent Raynaud syndrome); week 34 values were obtained 8 weeks after the last eculizumab dose (ie, in week 26).
Imputed from week −2 (no reliable hemoglobin value available in week 0).
Imputed from week 30 (no reliable hemoglobin value available in week 34).
Imputed from week 4 (premature trial termination after 5 eculizumab infusions because of complications of concomitant liver cirrhosis), no follow-up visits.
Imputed from week 24 (no lactate dehydrogenase value available in week 26).
Imputed from week −1 (no reliable hemoglobin value available in week 0).
Nine patients were transfusion dependent, with a median of 6 units transfused in the preceding 12 months (range, 2-32). Two patients suffered from acrocyanosis, 1 suffered from Raynaud syndrome with digital necrosis, and 1 had been diagnosed with thromboangiitis obliterans.
Hemolysis was demonstrated by reduced concentrations of haptoglobin and hemopexin, as well as increased levels of free hemoglobin, lactate dehydrogenase, bilirubin, and reticulocytes. The monospecific direct agglutination test against C3d was strongly positive in all patients, and the polyspecific direct agglutination test was positive in 11. Weak agglutination with anti-IgG was found in 4 patients. The median cold agglutinin titer at 4°C was 1024 (range, 64 to >2048; normal range, <64). The thermal amplitude was 4°C in 1, 20°C in 2, 30°C in 4, and 37°C in 6 patients.
All patients with chronic CAD had a monoclonal immunoglobulin (IgM κ, 10; IgM λ, 1; biclonal IgM/IgA/κ/λ, 1), with a median IgM concentration of 2.46 g/L (range, 1.26-6.65; normal range, 0.40-2.30). Bone marrow aspiration was performed in 12 patients, and bone marrow biopsy in 9. Although monoclonal B-cell populations ranging from 0.4% to 3% were demonstrable by flow cytometry in 5 cases, and nodular lymphocyte aggregates were seen on histology in 3, the findings were insufficient to establish a diagnosis of B-cell lymphoma.
Efficacy
Eleven patients completed the 26-week treatment phase with receipt of all 16 scheduled eculizumab infusions, and 2 patients elected to continue treatment of another 24 or 38 weeks, respectively (Table 1). One patient with concomitant liver cirrhosis discontinued treatment after 5 infusions because of hemorrhoidal hemorrhage and clinical suspicion of peritonitis, and another patient elected to stop treatment after 12 infusions because her major problem, Raynaud syndrome, failed to respond to eculizumab. Treatment was generally delivered on schedule, except for 2 treatment delays by 3 days each.
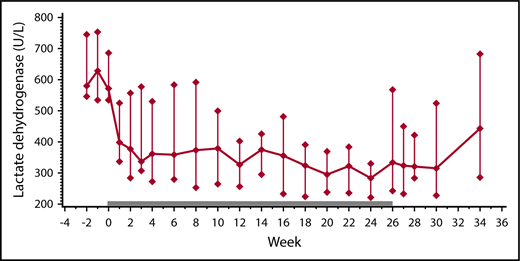
Between the first and the last day of therapy, the median lactate dehydrogenase level decreased from 572 U/L to 334 U/L (P = .0215), with 7 of 13 patients showing a lactate dehydrogenase decrease ≥250 U/L (Table 1). This was accompanied by increases in hemoglobin and hemopexin, and decreases in bilirubin and reticulocytes (Table 2). In the posttreatment observation phase, most patients experienced a rise in their lactate dehydrogenase levels (Figure 1). In patients continuing eculizumab beyond week 26, the lactate dehydrogenase level remained low (Table 1).
Impact of eculizumab on indicators of hemolysis in 13 patients with CAD
| Laboratory test . | Normal range . | Week 0 . | Week 26 . | P . |
|---|---|---|---|---|
| Lactate dehydrogenase, U/L | 120-247 | 572 (534-685) | 334† (243-567) | .0215* |
| Free hemoglobin, mg/dL | <22 | 46.2 (18.2-124.0) | 30.0 (19.5-80.6) | .8438 |
| Bilirubin, µmol/L | 5.1-20.5 | 46.0 (34.2-68.4) | 43.0 (20.0-61.6) | .0117 |
| Haptoglobin, g/L | 0.3-2.0 | 0.04 (0.03-0.05) | 0.03 (0.02-0.05) | .8438 |
| Hemopexin, g/L | 0.5-1.2 | 0.47 (0.18-0.58) | 0.85 (0.45-1.09) | .0127 |
| Hemoglobin, g/dL | 11.6-16.1 | 9.35 (8.80-10.80) | 10.15 (9.00-11.35) | .0391 |
| Reticulocytes, ×109/L | 22-76 | 160.2 (99.1-185.5) | 111.7 (67.4-142.6) | .0625 |
| Laboratory test . | Normal range . | Week 0 . | Week 26 . | P . |
|---|---|---|---|---|
| Lactate dehydrogenase, U/L | 120-247 | 572 (534-685) | 334† (243-567) | .0215* |
| Free hemoglobin, mg/dL | <22 | 46.2 (18.2-124.0) | 30.0 (19.5-80.6) | .8438 |
| Bilirubin, µmol/L | 5.1-20.5 | 46.0 (34.2-68.4) | 43.0 (20.0-61.6) | .0117 |
| Haptoglobin, g/L | 0.3-2.0 | 0.04 (0.03-0.05) | 0.03 (0.02-0.05) | .8438 |
| Hemopexin, g/L | 0.5-1.2 | 0.47 (0.18-0.58) | 0.85 (0.45-1.09) | .0127 |
| Hemoglobin, g/dL | 11.6-16.1 | 9.35 (8.80-10.80) | 10.15 (9.00-11.35) | .0391 |
| Reticulocytes, ×109/L | 22-76 | 160.2 (99.1-185.5) | 111.7 (67.4-142.6) | .0625 |
Values are displayed as median (interquartile range [IQR]) unless otherwise noted.
For the primary end point “lactate dehydrogenase” statistical analysis was done after imputation of missing values for week 26 (P = .0537 without imputation); all other analyses were done without imputation of missing values (see “Methods”).
Therapy-related changes in lactate dehydrogenase levels. Lactate dehydrogenase levels before (weeks −2 and −1), during (weeks 0 through 26; solid bar), and after eculizumab treatment (weeks 27 through 34) in 13 patients with CAD (median ± IQR).
Therapy-related changes in lactate dehydrogenase levels. Lactate dehydrogenase levels before (weeks −2 and −1), during (weeks 0 through 26; solid bar), and after eculizumab treatment (weeks 27 through 34) in 13 patients with CAD (median ± IQR).
The hemoglobin rise was paralleled by a significant reduction in RBC transfusion, with 3 patients maintaining and 8 patients acquiring transfusion independence, and 1 patient each showing a reduced or an increased transfusion need, respectively (P = .0215) (Table 1). The median number of units transfused during the 34-week treatment and posttreatment phase was 0 (range, 0-12), instead of an expected 2.6 (range, 0-20.9; P = .0625). Exercise tolerance, quality of life, or fatigue did not significantly change during the study period. Differences between lactate dehydrogenase responders and nonresponders were not observed (data not shown).
Before enrollment, 5 patients had experienced venous thromboembolism (5 deep vein thromboses, 1 superficial vein thrombosis, 1 pulmonary embolism). Most thromboembolic events had occurred only months before enrollment into the trial. During therapy with eculizumab, no such events were recorded. The median d-dimer concentration decreased from 0.79 mg/L (IQR, 0.75-1.89; normal range, <0.55) at enrollment to 0.53 mg/L (IQR, 0.36-0.93) on the last day of treatment (P = .0938). Cold-induced circulatory symptoms, such as acrocyanosis or Raynaud syndrome, remained unaffected by eculizumab.
Response prediction in chronic CAD
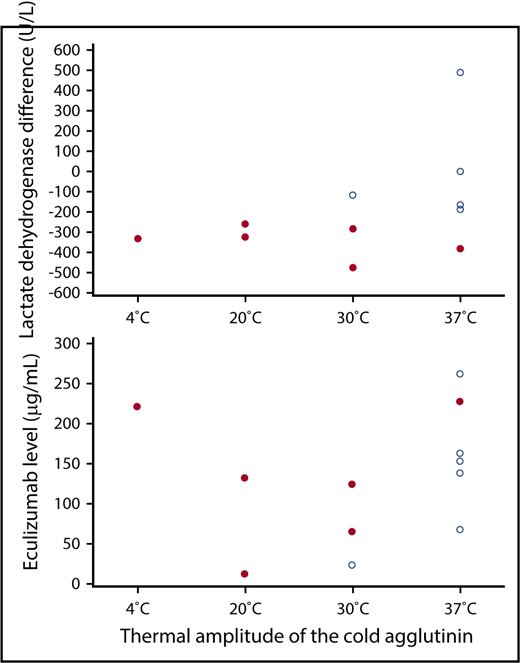
To identify factors predicting a response to eculizumab, we focused on the 12 patients with chronic CAD. Six of these had a decrease in the lactate dehydrogenase level ≥250 U/L (range, −259 to −476), 4 had a decrease <250 U/L (range, −187 to −5), and 2 had an increase (+3 and +489 U/L, respectively). There were no statistically significant differences between treatment-naïve and pretreated patients. The cold agglutinin titer and the intensity of C3d coating of the erythrocytes remained unchanged during the trial period. The lactate dehydrogenase response was not related to the cold agglutinin titer at 4°C, but it showed a nonsignificant correlation with the thermal amplitude, with 5 of 6 patients with an amplitude of 4°C, 20°C, or 30°C, but only 1 of 6 patients with an amplitude of 37°C responding (P = .0801). Patients with cold agglutinins with a thermal amplitude of 37°C tended to have less pronounced lactate dehydrogenase responses than patients with cold agglutinins with a narrower thermal amplitude (Figure 2; P = .0927 for the comparison 37°C vs 4°C to 30°C).
Relationship between lactate dehydrogenase response, eculizumab serum levels, and the thermal amplitude of the cold agglutinin. Difference in the lactate dehydrogenase level between the first and the last day of treatment (upper) and median eculizumab trough level between week 4 and week 26 of the treatment phase (lower) in relation to the thermal amplitude of the cold agglutinin in 12 patients with chronic CAD. Patients with or without a lactate dehydrogenase decrease ≥250 U/L are represented by solid or open circles, respectively.
Relationship between lactate dehydrogenase response, eculizumab serum levels, and the thermal amplitude of the cold agglutinin. Difference in the lactate dehydrogenase level between the first and the last day of treatment (upper) and median eculizumab trough level between week 4 and week 26 of the treatment phase (lower) in relation to the thermal amplitude of the cold agglutinin in 12 patients with chronic CAD. Patients with or without a lactate dehydrogenase decrease ≥250 U/L are represented by solid or open circles, respectively.
Acute CAS
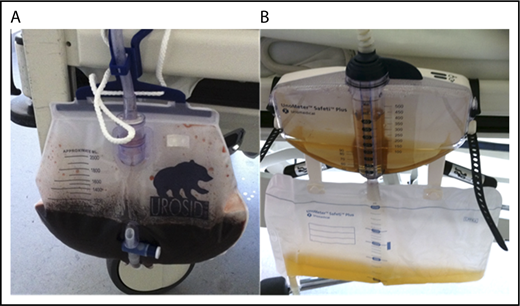
A life-threatening CAS occurred in a previously healthy 73-year-old patient who was hospitalized for acute hemolytic anemia (hemoglobin, 5.6 g/dL; lactate dehydrogenase, 439 U/L; cold agglutinin titer, 512; thermal amplitude, 30°C). The Donath-Landsteiner test was negative. The cold agglutinin was of the IgM class, but on immunofixation no monoclonal immunoglobulin was detected. After administration of 4 RBC units using an in-line blood warmer, the hemolysis dramatically increased (lactate dehydrogenase, 2980 U/L) with concomitant hemoglobinuria. Within 24 hours of the first dose of eculizumab, hemoglobinuria ceased (Figure 3), and the patient’s condition improved. Hemolysis completely resolved after 4 weeks, and the cold agglutinin became undetectable after 12 weeks. Extensive investigations including a search for a malignant disease and serologic testing for Mycoplasma pneumoniae, Epstein-Barr virus, Treponema pallidum, and other infectious agents failed to uncover the cause of the CAS.
Eculizumab response in a patient with an acute CAS. Urine of patient 5 with severe intravascular hemolysis and hemoglobinuria immediately before (A) and 24 hours after (B) the first dose of eculizumab.
Eculizumab response in a patient with an acute CAS. Urine of patient 5 with severe intravascular hemolysis and hemoglobinuria immediately before (A) and 24 hours after (B) the first dose of eculizumab.
Pharmacokinetics
In weeks 4, 12, and 26 of the treatment phase, median trough and peak levels of eculizumab were 141 µg/mL (range, 12-331) and 307 µg/mL (range, 13-532), respectively. Differences between lactate dehydrogenase responders and nonresponders were not observed. Patients with chronic disease and a cold agglutinin with a thermal amplitude of 4°C or 20°C responded to eculizumab irrespective of the trough level attained. By contrast, when the thermal amplitude was 30°C, only patients with eculizumab levels >65 µg/mL responded, and at a thermal amplitude of 37°C, the only response was observed in a patient with a trough level of 228 µg/mL (Figure 2). Similar results were obtained with eculizumab peak levels (data not shown). The patient with the acute CAS had median trough and peak levels of 230 and 466 µg/mL, respectively.
Safety
During treatment and in the posttreatment observation phase, a total of 37 adverse events were recorded in 13 patients. Thirteen adverse events (occurring in 4 patients) were classified as possibly related, and 1 (occurring in another patient) was classified as probably related to treatment (Table 3). Meningococcal infections were not observed.
Adverse events with possible or probable relationship to eculizumab treatment
| Severity/possibly related to treatment . | Probably related to treatment . |
|---|---|
| Severe | |
| Peritonitis* | Pneumonia† |
| Moderate | |
| Hemorrhoidal hemorrhage* | - - - |
| Fatigue‡ | |
| Muscle cramps‡ | |
| Arterial stenosis‡ | |
| Hypertension | |
| Pruritus | |
| Urinary tract infection | |
| Mild | |
| Limb pain‡ | - - - |
| Oral herpes infection | |
| Fatigue | |
| Creatinine increase (2 episodes) |
| Severity/possibly related to treatment . | Probably related to treatment . |
|---|---|
| Severe | |
| Peritonitis* | Pneumonia† |
| Moderate | |
| Hemorrhoidal hemorrhage* | - - - |
| Fatigue‡ | |
| Muscle cramps‡ | |
| Arterial stenosis‡ | |
| Hypertension | |
| Pruritus | |
| Urinary tract infection | |
| Mild | |
| Limb pain‡ | - - - |
| Oral herpes infection | |
| Fatigue | |
| Creatinine increase (2 episodes) |
Patient 9 with preexisting liver cirrhosis and clinical suspicion of spontaneous peritonitis (no bacterial pathogen demonstrated).
Full recovery without delay of eculizumab administration (patient 14).
Patient 11 with preexisting atherosclerosis and atrial fibrillation, pain in left foot, and cramps in left calf starting in the first month of eculizumab therapy, diagnosis of a stenosis of the left superficial femoral artery in the posttreatment phase.
Discussion
CAD is a complement-dependent disorder, with extravascular and intravascular hemolysis resulting from initial or terminal complement activation, respectively.2 There are no licensed treatments for this rare disease. In the DECADE trial, inhibition of terminal complement activation by eculizumab led to a significant reduction in hemolysis. Seven of 13 patients responded to treatment as defined by a drop in the lactate dehydrogenase level ≥250 U/L, and this was accompanied by a significant decrease in transfusion dependency. The increase in hemoglobin (median, 0.8 g/dL) was only modest, but, interestingly, it was still higher than the increase in patients with paroxysmal nocturnal hemoglobinuria treated with eculizumab in the TRIUMPH trial (0.1 g/dL).6 Similar to the last named disease, extravascular hemolysis remained unaffected by treatment with eculizumab, which explained why reticulocytes and haptoglobin failed to reach normal levels. There was no significant improvement in exercise tolerance or quality of life. Importantly, however, during treatment with eculizumab the hemoglobin level was maintained without transfusion in 11 of 13 patients, as compared with 4 of 13 patients in the preceding 12 months. These observations challenge the view that intravascular hemolysis is of minor or no importance in CAD.1
Patients with cold agglutinins with a thermal amplitude of 37°C tended to have less pronounced lactate dehydrogenase responses than patients with cold agglutinins with narrower thermal amplitudes. In CAD, the thermal amplitude is considered the most relevant measure of disease severity. It is correlated with the intensity of complement activation: the higher the temperature at which the antibody can react with the cell, the greater the ability to fix complement and the greater the ensuing damage.12,13 Our findings suggest that eculizumab was able to control hemolysis in patients with weak, but not in patients with strong, complement activation. The eculizumab schedule used was adopted from paroxysmal nocturnal hemoglobinuria,6 and the serum levels attained were similar in both diseases.14 The role of complement, however, differs in the 2 disorders. In paroxysmal nocturnal hemoglobinuria, low-level complement activation is sufficient to cause intravascular hemolysis, because a defect in CD59 renders the cells exquisitely sensitive to the action of complement. By contrast, CAD is characterized by significant complement activation which may overrun the protection afforded by CD59.2 It is tempting to speculate that the eculizumab dose used in the DECADE trial was too low to suppress hemolysis caused by cold agglutinins with a wide thermal amplitude. This assumption is supported by the observation that, in patients with cold agglutinins with a narrow thermal amplitude, responses occurred at much lower eculizumab levels than in patients with cold agglutinins with a wide thermal amplitude. In paroxysmal nocturnal hemoglobinuria, trough levels of 35 µg/mL are sufficient to suppress hemolysis under everyday conditions.14 States of increased complement activation, however, such as infection or pregnancy, necessitate higher eculizumab doses.15,16
The advent of eculizumab has prolonged survival in patients with paroxysmal nocturnal hemoglobinuria, mainly because of a reduced risk of thromboembolic events.17 Autoimmune hemolytic anemia is an established risk factor for venous thromboembolism.18 Five of our patients had experienced thromboembolic events in the past. While on treatment, no such complications occurred, and the d-dimer levels decreased. Because the duration of the trial was too short to allow any firm conclusions, further studies are required to ascertain whether eculizumab reduces the risk of venous thromboembolism in CAD.
Treatment with eculizumab did not improve cold-induced circulatory symptoms, which caused significant morbidity in 4 patients participating in the trial. In fact, 1 patient elected to stop treatment prematurely because her Raynaud syndrome did not respond to eculizumab. The failure of eculizumab to affect erythrocyte agglutination was expected and in keeping with its mechanism of action, which targets complement activation and not erythrocyte cross-linking by IgM autoantibodies.
The patient with the acute CAS experienced severe exacerbation of intravascular hemolysis after transfusion of RBCs. Explanations include provision of exogenous complement fueling erythrocyte destruction, and the nonopsonized state of the transfused cells, which enhances their ability to react with cold agglutinins.12 Within 24 hours of eculizumab administration, hemoglobinuria ceased, and the patient recovered. The situation is similar to ABO-incompatible blood transfusion where eculizumab efficiently prevents IgM-mediated hemolysis.19
In conclusion, eculizumab was well tolerated and significantly reduced hemolysis in CAD. Patients with cold agglutinins with a wide thermal amplitude may benefit from higher eculizumab doses than used here. Recently developed strategies to inhibit the early steps of complement activation, which are expected to prevent both intravascular and extravascular hemolysis, may even be more potent, but they may also increase the risk of infection or even lupuslike disease.1,20 Drug treatment of chronic CAD has so far been based on cytoreduction, using approaches derived from the treatment of lymphoma. Application of such therapies to elderly patients, however, is limited by toxicity. Complement inhibition is a well-tolerated alternative in patients ineligible for or not responding to B-cell-directed therapies, when hemolysis is the leading cause of morbidity. It is also an attractive therapeutic option in CAS.
Presented in abstract form at the 57th annual meeting of the American Society of Hematology, Orlando, FL, 7 December 2015.
Acknowledgments
The authors would like to thank the patients who participated in the trial.
This work was supported by Alexion Pharmaceuticals, which provided research funding to A.R. and helped with the pharmacokinetic analysis.
Authorship
Contribution: A.R., N.K., and U.D. designed the trial; A.R., M.B., A.H., D.H.-T., and U.D. directed the clinical activities at the participating study centers; V.L. and H.S. directed the activities at the participating Institutes of Transfusion Medicine; N.K. collected the data; N.K. and J.R. performed the statistical analysis; A.R., J.R., and U.D. designed the figures; U.D. wrote the first draft of the manuscript; and all authors contributed to data interpretation, reviewed the draft, and approved the final version of this report.
Conflict-of-interest disclosure: A.R. and U.D. received honoraria from Alexion Pharmaceuticals and Roche Pharma. H.S. received honoraria from Alexion Pharmaceuticals. The remaining authors declare no competing financial interests.
Correspondence: Alexander Röth, Department of Hematology, University Hospital Essen, Hufelandstr 55, 45147 Essen, Germany; e-mail: alexander.roeth@uk-essen.de.