Key Points
Haploidentical NK-cell infusions given with rhIL-15 achieved remission in 35% of patients with refractory acute myeloid leukemia.
SC dosing of rhIL-15 after lymphodepletion prolongs drug exposure leading to cytokine release syndrome and neurotoxicity.
Abstract
In vivo expansion of haploidentical natural killer (NK) cell infusions with interleukin-2 (IL-2) can induce remission of refractory acute myeloid leukemia, but efficacy may be hampered by concurrent stimulation of host regulatory T cells. To overcome this limitation, we substituted the NK homeostatic factor IL-15 in 2 phase 1/2 trials. Forty-two patients received either intravenous (IV) (NCT01385423) or subcutaneous (SC) (NCT02395822) recombinant human IL-15 (rhIL-15) after lymphodepleting chemotherapy and haploidentical NK cells. Escalating doses of rhIL-15 (0.3-1.0 μg/kg) were given on 12 consecutive days in a phase 1 trial. Of 26 patients, 36% had robust in vivo NK-cell expansion at day 14, and 32% achieved complete remission. Hypothesizing that SC dosing of rhIL-15 would be safer and better tolerated, 16 patients received 10 once per day doses of SC rhIL-15 at 2.0 μg/kg on a phase 2 trial. NK-cell expansion at day 14 was seen in 27% of the patients, and 40% achieved remission. rhIL-15 induced better rates of in vivo NK-cell expansion and remission compared with previous trials with IL-2, but it was associated with previously unreported cytokine release syndrome (CRS) after SC but not IV dosing. CRS was observed in 56% of patients given SC rhIL-15 (with concurrent neurologic toxicity in 5 of 9 patients) and was responsive to steroids and tocilizumab. SC administration was associated with slower pharmacokinetic clearance and higher levels of IL-6 than IV dosing. These novel trials testing the use of IL-15 to potentiate cell therapy suggest that dosing schedules based on pharmacokinetics and pharmacodynamics will preserve the therapeutic benefits of IL-15 and minimize CRS. These trials were registered at www.clinicaltrials.gov as #NCT01385423 and #NCT02395822.
Introduction
Natural killer (NK) cells are lymphocytes in the innate immune system that mediate antitumor surveillance and cytotoxicity modulated by balanced expression of activating and inhibitory receptors.1 Interleukin-2 (IL-2)–activated haploidentical NK (haplo-NK) cell therapy induces complete remission (CR) in 30% to 50% of patients with relapsed and/or refractory acute myeloid leukemia (AML) and is an effective bridge to potentially curative allogeneic hematopoietic stem cell transplantation (alloHSCT).2,3 However, use of IL-2 is limited by activation-induced cell death and induction of immunosuppressive host T regulatory cells (Tregs).4 -7 Strategies to augment in vivo expansion of donor NK cells include lymphodepletion with cyclophosphamide/fludarabine (Cy/Flu) before NK-cell infusion, depletion of Tregs with an IL-2 diphtheria toxin fusion protein, and in vivo use of recombinant cytokines.3
IL-15 is a member of the four-helix-bundle family of cytokines that stimulates both NK cells and T cells. IL-15 binds to a heterotrimeric receptor (IL-15R) containing a specific IL-15R-α subunit as well as a common IL-2R/IL-15R-β receptor shared with IL-2 and a common γc receptor.8,9 IL-15 in complex with its IL-15R-α receptor induces a potent proliferative signal that expands NK-cell and CD8+ T-cell populations in murine models.10,11 Intravenous (IV) recombinant human IL-15 (rhIL-15) has been tested in human clinical trials but not in a cell therapy setting. Dose-limiting toxicities (DLTs) include hypotension and vascular leak.12 A phase 1 trial testing subcutaneous (SC) delivery of rhIL-15 as monotherapy for advanced solid tumors reported lower peak serum IL-15 levels and significant in vivo expansion of endogenous NK cells.13 Given the limitations of IL-2, IL-15 has been identified as a prime candidate for a combination with chemotherapy-induced lymphodepletion to potentiate in vivo activation of NK-cell therapy. Although many ex vivo cell expansion methods have been explored, they are expensive, labor intensive, and may induce cytokine dependence. Thus, in vivo expansion using cytokines may be optimal for adoptive cell transfer. We report the results of our first-in-human phase 1 and phase 2 trials of IV and SC rhIL-15 given with haplo-NK-cell therapy after lymphodepletion to treat relapsed/refractory AML.
Methods
Patient eligibility and clinical protocols
A total of 26 patients with relapsed/refractory AML were treated from 2011 to 2015 on a phase 1/2 dose-escalation study of IV rhIL-15 dosed after lymphodepleting chemotherapy and haploidentical donor NK-cell infusion. Subsequently, 16 patients were treated from 2015 to 2016 on a similar phase 2 study using SC rhIL-15. The rhIL-15 was produced in Escherichia coli by the Biopharmaceutical Development Program at the National Cancer Institute (NCI) under current good manufacturing practices. Eligible patients with relapsed/refractory AML had active leukemia with adequate performance status and organ function as previously reported.3 Four dose levels of rhIL-15 given as IV bolus on 12 consecutive days were tested: (1) 0.25 μg/kg (n = 3), (2) 0.5 μg/kg (n = 3), (3) 1 μg/kg (n = 4), and (2.5) 0.75 μg/kg (n = 16). SC rhIL-15 was given at 2 μg/kg for 10 doses (5 days on, 2 days off, 5 days on) using the maximum tolerated dose (MTD), which had been determined by a previous phase 1 study (CITN-02) in patients with advanced solid tumors.13 All patients received the same lymphodepleting chemotherapy regimen of fludarabine 25 mg/m2 IV for 5 doses on days −6 to −2 and cyclophosphamide 60 mg/kg IV on days −5 and −4 before the NK-cell infusion.
The primary end point of the IV trial was to determine the MTD of IV rhIL-15. The DLTs were defined as any treatment-related nonhematologic, noninfectious grade 4 to 5 toxicity except for fever alone through day 14 and as an absolute neutrophil count of <500 and a bone marrow that was <5% cellular (marrow aplasia) persisting at day 42 in the absence of leukemia. The secondary end point of the IV trial and the primary end point of the SC trial were to determine the CR rate or the CR with incomplete blood count recovery (CRi) rate by day 42.14 The Lee revised cytokine release syndrome (CRS) grading system was used to grade cytokine-associated toxicity.15
Preparation of NK-enriched cell products
In both cohorts, nonmobilized donor peripheral blood mononuclear cells were collected with the COBE Spectra Apheresis System (Terumo BCT, Lakewood, CO) over 5 hours. The apheresis products were T-cell (CD3) and B-cell (CD19) depleted using the Miltenyi Biotec CliniMACS Cell Selection System and CD3 and CD19 MicroBeads and reagent (Miltenyi Biotec, Auburn, CA). The NK-cell enriched product was activated by overnight incubation with 10 ng/mL rhIL-15 under good manufacturing practices conditions.16 The final product was analyzed by flow cytometry to determine the number of T, B, and NK cells by using conjugated antibodies against CD3, CD14, CD56, CD19, or CD20 and tested in a 4-hour Cr-release cytotoxicity assay against the K562 cell line.17
Informed consent
The protocol and consent procedures were approved by the University of Minnesota Institutional Review Board (clinicaltrials.gov #NCT01385423 and #NCT02395822). Both studies were approved by the US Food and Drug Administration under BB-IND 14448 (J. Miller, sponsor). All patients and donors gave informed consent for treatment and prospective data collection in accordance with the Declaration of Helsinki.
Immunophenotyping and enzyme-linked immunosorbent assay
Lymphocytes were immunophenotyped by multicolor fluorescent antibodies directed against CD45, CD56, CD3, CD4, CD20, and Ki-67 (BD PharMingen, San Diego, CA) before chemotherapy and at days 7 and 14. Plasma IL-15, IL-6, and interferon-γ concentrations were determined by commercial enzyme-linked immunosorbent assay (R&D Systems, Minneapolis, MN). Chimerism studies (day 7 and day 14) were performed using informative short tandem repeat markers to differentiate between donor and recipient DNA in whole blood samples. Successful in vivo donor NK-cell expansion at day 14 was defined as an absolute circulating donor-derived NK-cell count of >100 cells per μL at day +14 after NK-cell infusion, calculated by the following formula: (absolute lymphocytes [determined by complete blood cell count] × % donor chimerism) × (% CD56+/CD3– NK cells [based on lymphocyte gate]).
Statistical analysis
Simple proportions were used to describe in vivo donor NK-cell expansion and rates of CR. When calculated, P values from categorical covariates on binary responses were generated using a χ2 test or Fisher’s exact test. Comparisons from continuous factors on binary responses were assessed using logistic regression. Overall survival and progression-free survival were estimated with Kaplan-Meier curves with Hall-Wellner 95% confidence bands.
Results
Patient and product characteristics
Two separate cohorts of patients with relapsed/refractory AML received identical Cy/Flu lymphodepleting chemotherapy and haploidentical donor NK-cell products followed by IV (n = 26) vs SC (n = 16) administration of rhIL-15 to promote in vivo NK-cell expansion. Overall patient characteristics are shown in Table 1 with individual patient and disease characteristics in supplemental Table 1A-B. Compared to patients receiving IV dosing, patients admistered SC rhIL-15 were older (median age, 52 vs 63 years; P = .05) and had a higher incidence of secondary AML (15% vs 56%; P < .01). Both groups had received several previous therapies (mean, 3 in the IV group vs 2.3 in the SC group; P = .06) and had similar rates of previous alloHSCT (12% in the IV group vs 19% in the SC group; P = not significant). Patients received significantly more planned doses of rhIL-15 in the IV than in the SC cohort with an average administration of 11.6 of the 12 planned IV doses (range, 9-12 doses; 96% of planned doses) vs 7.5 of the 10 planned SC doses (range, 1-10 doses; 75% of planned doses) (P < .01). Karnofsky performance status, pretreatment blast count, cytogenetic and molecular risk group, and cytomegalovirus (CMV) status of the donors and recipients were similar between the 2 groups.
Patient characteristics
| Characteristic . | IV IL-15 . | SC IL-15 . | P . |
|---|---|---|---|
| N | 26 | 16 | |
| Time period | 2011-2015 | 2015-2016 | |
| Median age (range), y | 52 (22-68) | 63 (20-71) | .05 |
| Male sex | 14 (54) | 12 (75) | NS |
| KPS <80 | 2 (8) | 2 (13) | NS |
| Blasts by morphology | NS | ||
| Mean (SD) | 35.5 (29.5) | 25.1 (24.8) | NS |
| ELN risk group | NS | ||
| Favorable | 1 | 2 (13) | |
| Intermediate | 14 (50) | 4 (25) | |
| Adverse | 10 (50) | 10 (63) | |
| Molecular mutations | |||
| FLT3-ITD+ | 7 (27) | 2 (13) | NS |
| NPM1+ | 1 (4) | 1 (6) | NS |
| AML subtype | <.01 | ||
| De novo | 21 (81) | 5 (31) | |
| Secondary AML | 4 (15) | 9 (56) | |
| t-AML | 1 (4) | 2 (13) | |
| Mean no. of prior therapies (SD) | 3.0 (1.3) | 2.3 (0.6) | NS |
| No. of patients receiving prior hematopoietic cell transplantation | 3 (12) | 3 (19) | NS |
| Seropositive CMV | |||
| Recipient | 13 (50) | 9 (56) | NS |
| Donor | 9 (35) | 4 (25) | NS |
| KIR mismatch | NS | ||
| Unknown | 0 | 1 | |
| No | 20 (78) | 9 (60) | |
| Yes | 6 (22) | 6 (40) |
| Characteristic . | IV IL-15 . | SC IL-15 . | P . |
|---|---|---|---|
| N | 26 | 16 | |
| Time period | 2011-2015 | 2015-2016 | |
| Median age (range), y | 52 (22-68) | 63 (20-71) | .05 |
| Male sex | 14 (54) | 12 (75) | NS |
| KPS <80 | 2 (8) | 2 (13) | NS |
| Blasts by morphology | NS | ||
| Mean (SD) | 35.5 (29.5) | 25.1 (24.8) | NS |
| ELN risk group | NS | ||
| Favorable | 1 | 2 (13) | |
| Intermediate | 14 (50) | 4 (25) | |
| Adverse | 10 (50) | 10 (63) | |
| Molecular mutations | |||
| FLT3-ITD+ | 7 (27) | 2 (13) | NS |
| NPM1+ | 1 (4) | 1 (6) | NS |
| AML subtype | <.01 | ||
| De novo | 21 (81) | 5 (31) | |
| Secondary AML | 4 (15) | 9 (56) | |
| t-AML | 1 (4) | 2 (13) | |
| Mean no. of prior therapies (SD) | 3.0 (1.3) | 2.3 (0.6) | NS |
| No. of patients receiving prior hematopoietic cell transplantation | 3 (12) | 3 (19) | NS |
| Seropositive CMV | |||
| Recipient | 13 (50) | 9 (56) | NS |
| Donor | 9 (35) | 4 (25) | NS |
| KIR mismatch | NS | ||
| Unknown | 0 | 1 | |
| No | 20 (78) | 9 (60) | |
| Yes | 6 (22) | 6 (40) |
All data are n (%), except as noted.
ELN, European LeukemiaNet; ITD, internal tandem duplication; KIR, killer cell immunoglobulin-like receptor; KPS, Karnofsky performance status; NS, not significant; SD, standard deviation; t-AML, therapy-related AML.
The NK-cell products manufactured for the IV and SC cohorts from healthy related donors of similar ages contained comparable doses of total nucleated cells (mean for the IV group, 3.7 × 107/kg vs mean for the SC group, 3.9 × 107/kg), B cells (mean for the IV group, 18 × 103/kg vs mean for the SC group, 11 × 103/kg), and T cells (mean for the IV group, 6.2 × 104/kg vs mean for the SC group, 1.7 × 104/kg; P = .06). The IV rhIL-15 group had modestly higher NK-cell doses (mean for the IV group, 1.9 × 107/kg vs mean for the SC group, 1.2 × 107/kg; P = .02) and lower monocyte cell doses (mean for the IV group, 1.8 × 107/kg vs mean for the SC group, 2.7 × 107/kg; P = .01). This variation likely reflects chance donor-to-donor variability. Killer cell immunoglobulin–like receptor ligand mismatch in the graft-versus-host disease (GVHD) direction was seen in 29% (12 of 42) of the products, with similar rates in both groups.
MTD of IV rhIL-15
The phase 1 dose-escalation trial of IV dosing reached predetermined DLT at dose level 3 (1 μg/kg; n = 4) consisting of grade 4 pulmonary toxicity (diffuse alveolar hemorrhage) in 1 patient and prolonged neutropenia (beyond 42 days) in 2 of 4 patients at this dose. Although no DLT was observed in dose levels 1 (0.25 μg/kg; n = 3) and 2 (0.5 μg/kg; n = 3), no patients had in vivo expansion of donor-derived NK cells at day 14. With approval by the US Food and Drug Administration, 16 additional patients were treated on an unplanned cohort (dose level 2.5) of 0.75 μg/kg. Thus, a total of 26 IV patients were included for analysis.
With the intent of delivering more rhIL-15 with less toxicity, we opened a subsequent phase 2 trial using the 2-μg/kg SC rhIL-15 MTD dosing schedule (10 doses) determined in the NCI solid tumor monotherapy trial.13 One of the 17 patients enrolled on the SC rhIL-15 trial was not evaluable because that patient received neither the NK-cell infusion nor any rhIL-15 because of toxicity caused by the lymphodepleting chemotherapy. Thus a total of 16 SC patients were included for analysis.
IV or SC rhIL-15 induces functional in vivo donor NK-cell persistence and expansion
Persistence of DNA from the NK-cell donors, as measured by chimerism at day 7, was detected in 100% of the patients with available samples from both cohorts (IV, n = 24; SC, n = 13; median chimerism, 37%; range, 11%-89%). At day 14, NK-donor chimerism persisted in 42% (10 of 24 IV patients) and 33% (5 of 15 SC patients) (median, 90%; range, 2% to 100%; Figure 1A). In vivo expansion of donor NK cells, defined by detection of >100 donor-derived NK cells per μL of blood at day 14 (based on the percentage of DNA chimerism, the absolute lymphocyte count, and percentage of NK cells by flow cytometry), was detected in 35% of patients in the IV group. At lower IV doses (levels 1 and 2) 0 of 6 patients had donor NK-cell expansion, but at the MTD of 0.75 μg/kg, 7 (44%) of 16 had donor NK-cell expansion as did 2 of 4 patients (50%) at the highest (1 μg/kg) dose level. In the SC cohort, a similar rate of successful in vivo NK-cell expansion was detected in 27% (4 of 15) evaluable patients (1 did not have an informative polymorphism for chimerism testing). The rates of in vivo donor NK-cell expansion induced by either IV or SC rhIL-15 were significantly higher (P = .02) than the 10% rate reported in historical controls from trials using IL-2 in similar patients with relapsed or refractory AML.2,3,5 Adoptively transferred NK cells can be long-lived as evidenced by flow cytometric identification of a predominant population of polyclonal donor-derived NK cells in a pleural fluid sample collected at day 60 from a patient with prolonged neutropenia who developed pneumonia with a pleural effusion (supplemental Figure 1). Donor/recipient CMV mismatch, peak IL-15 levels, baseline absolute lymphocyte counts, and infused NK-cell doses did not correlate with in vivo NK-cell expansion at day 14 (data not shown).
rhIL-15 promotes donor chimerism 14 days after haplo-NK-cell infusion with highly functional donor NK cells. (A) Percentages of peripheral blood NK-cell detectable donor chimerism at days 7 and 14 in the IV and SC rhIL-15 cohorts. (B) Cytotoxicity of K562 cells was measured using NK cells isolated from peripheral blood mononuclear cells at day 14 after haplo-NK-cell infusion from patients who successfully in vivo expanded donor NK cells vs nonexpanders. Cytotoxicity was similar in IV or SC IL-15–stimulated NK cells (not shown).
rhIL-15 promotes donor chimerism 14 days after haplo-NK-cell infusion with highly functional donor NK cells. (A) Percentages of peripheral blood NK-cell detectable donor chimerism at days 7 and 14 in the IV and SC rhIL-15 cohorts. (B) Cytotoxicity of K562 cells was measured using NK cells isolated from peripheral blood mononuclear cells at day 14 after haplo-NK-cell infusion from patients who successfully in vivo expanded donor NK cells vs nonexpanders. Cytotoxicity was similar in IV or SC IL-15–stimulated NK cells (not shown).
The function of in vivo expanded donor NK cells was tested by using peripheral blood mononuclear cells collected at day 14 from patients with or without successful NK-cell expansion. Cytotoxicity against K562 targets was significantly better using blood from patients who had in vivo expanded donor NK cells after IV or SC rhIL-15 compared with those who did not (Figure 1B). Notably, proliferative index (as measured by the percentage of Ki-67 positivity) in in vivo expanded donor NK cells was higher in the SC compared with the IV cohort (85% vs 50%; P = .0002); this was similar between clinical responders (those who achieved CR/CRi) and nonresponders (62% vs 66%; P = not significant). There was a nonsignificant trend toward a higher proportion of recipient Ki-67+CD8+ T cells in the IV compared with the SC rhIL-15 cohort (92% vs 81%; P = .06). As expected, the percentage of Tregs at day 14 was low in both groups.
Haplo-NK-cell infusions with rhIL-15 induce CR in patients with relapsed/refractory AML
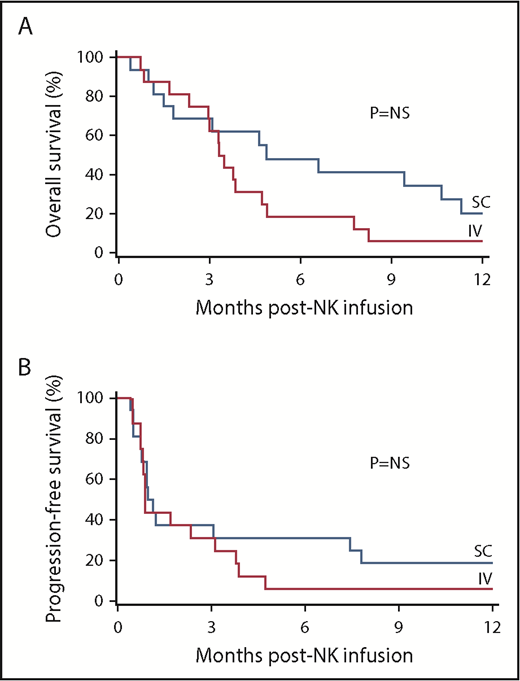
Clinical responses by day 42 after haplo-NK-cell treatment with rhIL-15 for 40 evaluable patients with relapsed/refractory AML are shown in Table 2. Two patients were not evaluable for clinical response; 1 IV patient died on day 17 (cerebral infarct with intracranial aspergilloma), and 1 SC patient died on day 11 (neurotoxicity with concurrent CRS). The overall rate of CR/CRi was 35% (14 of 40) for both cohorts: 8 (32%) of 25 in the IV group and 6 (40%) of 15 in the SC group. Similar numbers of patients in each group with CRs proceeded to consolidation with haploidentical alloHSCT after treatment (5 in the IV group and 6 in the SC group). The median durations of response were 107 days in the IV group and 278 days in the SC group. Overall survival at 1 year was similar in both cohorts: 19% (95% confidence interval [CI], 7%-36%) after IV rhIL-15, and 21% (95% CI, 5%-43%) after SC rhIL-15 (Figure 2A-B). Rates of progression-free survival at 1 year in the IV and SC rhIL-15 cohorts were not significantly different: 12% and 19%, respectively. Univariable analysis of donor/recipient CMV mismatch, killer cell immunoglobulin–like receptor mismatch, previous alloHSCT, number of previous therapies, bone marrow blast count at baseline, presence or absence of CRS, and donor NK-cell expansion and cytotoxicity were not associated with disease response. No correlation between in vivo NK-cell expansion was observed because 23% percent of expanders achieved CR/CRi compared with 41% of nonexpanders (P = .61) with similar rates in the IV and SC groups.
Disease response to IV and SC IL-15 with haplo-NK-cell infusion
| . | IV IL-15 . | SC IL-15 . | P . |
|---|---|---|---|
| No. of evaluable patients | 25 | 15 | |
| Overall response | 8 (32) | 6 (40) | NS |
| CR | 6 (24) | 1 (6) | NS |
| CRi | 2 (8) | 5 (33) | |
| PD | 17 (68) | 9 (60) | |
| NE | 1 | 1 | |
| Overall survival (95% CI) | NS | ||
| 1 y | 19 (7-36) | 21 (5-43) | |
| Median time to death (IQR), mo | 3.68 (2.73-8.37) | 4.93 (1.67-11.47) | |
| Progression-free survival (95% CI) | NS | ||
| 1 y | 12 (3-27) | 19 (5-40) | |
| Median time to progression or death (IQR), mo | 0.93 (0.73-3.83) | 1.05 (0.75-7.72) | |
| No. of patients bridged to alloHSCT | 5 | 6 | NS |
| Median time from transplantation to death (IQR), mo | 24.6 (20.7-28.4) | 14.4 (6.4-22.3) | NS |
| . | IV IL-15 . | SC IL-15 . | P . |
|---|---|---|---|
| No. of evaluable patients | 25 | 15 | |
| Overall response | 8 (32) | 6 (40) | NS |
| CR | 6 (24) | 1 (6) | NS |
| CRi | 2 (8) | 5 (33) | |
| PD | 17 (68) | 9 (60) | |
| NE | 1 | 1 | |
| Overall survival (95% CI) | NS | ||
| 1 y | 19 (7-36) | 21 (5-43) | |
| Median time to death (IQR), mo | 3.68 (2.73-8.37) | 4.93 (1.67-11.47) | |
| Progression-free survival (95% CI) | NS | ||
| 1 y | 12 (3-27) | 19 (5-40) | |
| Median time to progression or death (IQR), mo | 0.93 (0.73-3.83) | 1.05 (0.75-7.72) | |
| No. of patients bridged to alloHSCT | 5 | 6 | NS |
| Median time from transplantation to death (IQR), mo | 24.6 (20.7-28.4) | 14.4 (6.4-22.3) | NS |
All data are n (%), except as noted. Testing for distribution of responses used CR vs CRi vs progressive disease (PD).
NE, not evaluable; IQR, interquartile range; .
rhIL-15 with haplo-NK-cell infusion is associated beneficial clinical outcomes. Kaplan-Meier curves of overall survival (A) and progression-free survival (B) of patients who received IV (n = 26) and SC (n = 16) rhIL-15 with haplo-NK-cell infusion.
rhIL-15 with haplo-NK-cell infusion is associated beneficial clinical outcomes. Kaplan-Meier curves of overall survival (A) and progression-free survival (B) of patients who received IV (n = 26) and SC (n = 16) rhIL-15 with haplo-NK-cell infusion.
SC but not IV rhIL-15 is associated with CRS
Similar to our previous trials using IL-2 with haplo-NK-cell infusions after lymphodepleting Cy/Flu, no CRS was observed in the 26 patients in the IV rhIL-15 cohort. However, unexpectedly 9 (56%) of 16 patients in the SC cohort had documented CRS (any grade), 4 (25%) of whom had grade 3 to 5 CRS (Table 3). Manifestations of CRS included fevers (n = 6), rash (n = 4), hypotension (n = 4), and concurrent neurotoxicity (n = 5). The median time to onset of CRS was 8 days (range, 2 to 21 days) from the first dose of rhIL-15. Ferritin, C-reactive protein (CRP), and IL-6 levels were increased in the 8 patients with available samples; median peak ferritin of 16 478 ng/mL (range, 5944 to 133 439 ng/mL), median peak CRP of 185 mg/L (range, 37 to 420 mg/L), and median peak IL-6 of 347 pg/mL (range, 125 to 32 275 pg/mL). However, these biomarkers did not correlate with the grade of CRS, because patients with grade 1 to 2 CRS (n = 5) compared with grade 3 to 5 CRS (n = 4) showed similar median serum values of peak ferritin (14 006 vs 16 478 ng/mL), CRP (180 vs 275 mg/L), and IL-6 (219 vs 234 pg/mL). CRS was treated with steroids (n = 7) and/or tocilizumab (n = 4), with resolution in 7 of 9 patients. Univariable analysis of risk factors for grade 1 to 5 or grade 3 to 5 CRS did not show associations with maximum IL-6 level, maximum IL-15 level, soluble IL-2-2R-α level, granzyme B, number of rhIL-15 doses, baseline absolute peripheral blood lymphocytes, T-cell (CD4 or CD8) or monocyte count, presence or absence of in vivo NK-cell expansion at day 14, donor chimerism, CMV status of donor, or baseline bone marrow blast count. Furthermore, neither the doses of NK cells, T cells, B cells, or monocytes in NK-cell products nor the absolute peripheral blood CD8+ T-cell counts at day 14 correlated with the development of CRS. Importantly, development of CRS had no impact on disease response.
Clinical and laboratory characteristics of CRS and neurotoxicity in SC IL-15 with haplo-NK-cell infusion
| Patient . | No. of IL-15 doses . | CRS . | Neurotoxicity . | Ferritin, ng/mL . | CRP, mg/L . | IL-6 , pg/mL . | Steroids . | Tocilizumab . | Disease response . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Onset (day) . | Grade . | Symptoms . | Response . | Onset (day) . | Grade . | Symptoms . | Response . | ||||||||
| 1 | 10 | 17 | 2 | Fever, rash | Yes | 44 | 3 | Confusion | Yes | 5 944 | 37 | 1 240 | Yes | Yes | CRi |
| 2 | 10 | 21 | 3 | Hypotension | Yes | — | — | — | — | 16 108 | 380 | 32 275 | Yes | Yes | PD |
| 3 | 8 | 11 | 2 | Hypoxia, rash | Yes | 11 | 2 | Confusion | Yes | 18 761 | 280 | 191 | No | No | CRi |
| 4 | 1 | 2 | 4 | Fever | No | 2 | 4 | Confusion, dysarthria, status epilepticus | Yes | 16 848 | 170 | 226 | Yes | No | PD |
| 5 | 9 | 8 | 1 | Fever, rash | Yes | — | — | — | — | — | — | — | No | No | PD |
| 6 | 6 | 7 | 1 | Fever, hypotension | Yes | — | — | — | — | 9 252 | 200 | 159 | Yes | No | PD |
| 7 | 7 | 8 | 4 | Fever, rash, hypotension | Yes | 22 | 4 | Confusion, dysarthria, status epilepticus | Yes | 133 439 | 150 | 125 | Yes | Yes | PD |
| 8 | 5 | 9 | 2 | Fever, hypotension | Yes | — | — | — | — | 50 408 | 160 | 248 | Yes | No | CRi |
| 9 | 10 | 7 | 4 | Hypoxia | No | 7 | 5 | Confusion, hemiparesis, intracranial hemorrhage | No | 13 711 | 420 | 243 | Yes | Yes | NE |
| Patient . | No. of IL-15 doses . | CRS . | Neurotoxicity . | Ferritin, ng/mL . | CRP, mg/L . | IL-6 , pg/mL . | Steroids . | Tocilizumab . | Disease response . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Onset (day) . | Grade . | Symptoms . | Response . | Onset (day) . | Grade . | Symptoms . | Response . | ||||||||
| 1 | 10 | 17 | 2 | Fever, rash | Yes | 44 | 3 | Confusion | Yes | 5 944 | 37 | 1 240 | Yes | Yes | CRi |
| 2 | 10 | 21 | 3 | Hypotension | Yes | — | — | — | — | 16 108 | 380 | 32 275 | Yes | Yes | PD |
| 3 | 8 | 11 | 2 | Hypoxia, rash | Yes | 11 | 2 | Confusion | Yes | 18 761 | 280 | 191 | No | No | CRi |
| 4 | 1 | 2 | 4 | Fever | No | 2 | 4 | Confusion, dysarthria, status epilepticus | Yes | 16 848 | 170 | 226 | Yes | No | PD |
| 5 | 9 | 8 | 1 | Fever, rash | Yes | — | — | — | — | — | — | — | No | No | PD |
| 6 | 6 | 7 | 1 | Fever, hypotension | Yes | — | — | — | — | 9 252 | 200 | 159 | Yes | No | PD |
| 7 | 7 | 8 | 4 | Fever, rash, hypotension | Yes | 22 | 4 | Confusion, dysarthria, status epilepticus | Yes | 133 439 | 150 | 125 | Yes | Yes | PD |
| 8 | 5 | 9 | 2 | Fever, hypotension | Yes | — | — | — | — | 50 408 | 160 | 248 | Yes | No | CRi |
| 9 | 10 | 7 | 4 | Hypoxia | No | 7 | 5 | Confusion, hemiparesis, intracranial hemorrhage | No | 13 711 | 420 | 243 | Yes | Yes | NE |
Neurologic toxicities
As with the CRS, unexpected significant neurologic toxicity was observed in 6 patients who received SC but not IV dosing of rhIL-15. One event (subdural hematoma in a patient with a preexisting gait abnormality and thrombocytopenia with a hospital fall) was not attributed to the study elements. The remaining 5 neurotoxicity events (5 [33%] of 15) were seen only in patients with concurrent CRS. The median time to onset of neurologic toxicity was 7 days with a wide range (2 to 44 days). Neurologic toxicity consisted of confusion (n = 5), status epilepticus (n = 2), dysarthria (n = 2), and intracranial hemorrhage (n = 1). Neurotoxicity was severe (grade 3-5) in 4 of 5 patients. Neurologic symptoms were treated with steroids and tocilizumab (n = 4), steroids only (n = 3), or discontinuation of SC rhIL-15 and no additional therapy (n = 1), and they resolved in 4 of 5 patients. Grade 4 CRS was observed in 3 of the patients with neurologic toxicity, and it resolved in 1 of the 3 patients. One patient with grade 4 neurologic toxicity manifesting status epilepticus and encephalopathy was treated initially with steroids without noticeable response but had dramatic improvement in mental status within a few hours of receiving 1 dose of tocilizumab. Evaluation of patients with CRS and neurologic toxicity included computed tomography scans (n = 4; normal in 3 patients and abnormal in 1 patient with intracranial hemorrhage [ICH]), brain magnetic resonance imaging scans (n = 4; abnormal in 1 patient), and electroencephalograph scans (n = 3; normal in 1 patient and abnormal in 2 patients with status epilepticus). The patient with fatal ICH had a magnetic resonance imaging scan for mental status changes 1 day before the acute bleed, which showed focal leptomeningeal and cortical enhancement with restricted diffusion, consistent with but not definitive for centeral nervous system leukemia. Cerebrospinal fluid was analyzed in 3 of 5 patients with neurologic CRS and was negative for leukemia, infection, and NK cells.
Other toxicities
Transient low-grade rhIL-15–related symptoms were common in both the IV and SC cohorts (Table 4). Frequent (>50% of patients) grade 1 to 3 adverse events in all groups included hypertension (56%-100%), febrile neutropenia (63%-100%), rash (60%-88%), chills (63%-81%), edema (50%-63%), and headache (13%-70%). In the SC cohort, 63% had reactions at the injection site (none in the IV cohort), and increased creatinine was more common (63% vs ∼12% in the IV cohort). In both cohorts, grade 1 to 2 QT prolongation was frequent (40%-50%), but only 1 patient had grade 3 QT prolongation, and there were no cases of torsades de pointes.
Adverse events associated with IV and SC IL-15 with haplo-NK-cell infusion as measured by CTCAE 4.03
| Adverse event . | IL-15 . | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0.25, 0.5, 1 μg/kg IV non-MTD . | 0.75 μg/kg IV . | 2.0 μg/kg SC . | ||||||||||||||||
| Grade 1-3 . | Grade 4 . | Grade 5 . | Grade 1-3 . | Grade 4 . | Grade 5 . | Grade 1-3 . | Grade 4 . | Grade 5 . | ||||||||||
| n . | % . | n . | % . | n . | % . | n . | % . | n . | % . | n . | % . | n . | % . | n . | % . | n . | % . | |
| Hypertension | 10 | 100 | 13 | 81 | 9 | 56 | ||||||||||||
| Hypotension | 3 | 30 | 5 | 31 | 8 | 50 | ||||||||||||
| Hypoxia | 3 | 30 | 1 | 10 | 5 | 31 | 5 | 31 | ||||||||||
| Dyspnea/cough | 2 | 20 | 2 | 20 | 5 | 31 | 7 | 44 | ||||||||||
| Pulmonary infiltrates | 2 | 20 | 5 | 31 | 1 | 6 | 2 | 13 | ||||||||||
| Pulmonary edema | 2 | 20 | 0 | 6 | 38 | |||||||||||||
| Respiratory failure | 0 | 0 | 1 | 6 | 0 | 1 | 6 | |||||||||||
| Diffuse alveolar hemorrhage | 0 | 1 | 10 | 0 | 0 | |||||||||||||
| Febrile neutropenia | 10 | 100 | 13 | 81 | 10 | 63 | ||||||||||||
| Infection/sepsis | 0 | 1 | 6 | 1 | 6 | 2 | 13 | 2 | 13 | 2 | 13 | |||||||
| Marrow aplasia | 0 | 3 | 30 | 1 | 10 | 0 | 0 | |||||||||||
| Injection site reaction | 0 | 0 | 10 | 63 | ||||||||||||||
| Rash | 6 | 60 | 10 | 63 | 14 | 88 | ||||||||||||
| Myalgia/arthralgia | 0 | 3 | 19 | 0 | ||||||||||||||
| Chills | 8 | 80 | 10 | 63 | 13 | 81 | ||||||||||||
| Edema | 5 | 50 | 8 | 50 | 10 | 63 | ||||||||||||
| Flu-like symptoms | 0 | 2 | 13 | 1 | 6 | |||||||||||||
| Fatigue | 0 | 1 | 6 | 3 | 19 | |||||||||||||
| Pain | 0 | 3 | 19 | 1 | 6 | 3 | 19 | |||||||||||
| Arrhythmia | 0 | 1 | 10 | 1 | 6 | 0 | ||||||||||||
| Prolonged QTc | 4 | 40 | 7 | 44 | 8 | 50 | ||||||||||||
| Increased troponin | 1 | 10 | 3 | 19 | 0 | |||||||||||||
| Decreased LVEF | 0 | 1 | 6 | 1 | 6 | 0 | ||||||||||||
| Increased creatinine | 1 | 10 | 2 | 13 | 1 | 6 | 1 | 6 | 10 | 63 | ||||||||
| Multiorgan failure | 0 | 1 | 10 | 0 | 0 | |||||||||||||
| Hypernatremia | 0 | 0 | 3 | 19 | ||||||||||||||
| Increased bilirubin | 0 | 0 | 6 | 38 | ||||||||||||||
| Increased AST/ALT | 3 | 30 | 2 | 13 | 6 | 38 | ||||||||||||
| Diarrhea | 0 | 2 | 13 | 0 | ||||||||||||||
| Vomiting | 1 | 10 | 1 | 6 | 0 | |||||||||||||
| Dizziness | 0 | 1 | 6 | 0 | ||||||||||||||
| Headache | 7 | 70 | 2 | 13 | 5 | 31 | ||||||||||||
| Stroke/intracranial hemorrhage/subdural hematoma | 1 | 10 | 0 | 0 | 2 | 13 | ||||||||||||
| Gait disturbance | 0 | 0 | 1 | 6 | ||||||||||||||
| Cytokine release syndrome | 0 | 0 | 4 | 25 | 2 | 13 | ||||||||||||
| Encephalopathy | 0 | 0 | 1 | 6 | 1 | 6 | ||||||||||||
| Seizure | 0 | 0 | 0 | 2 | 13 | |||||||||||||
| Confusion | 0 | 2 | 13 | 6 | 38 | |||||||||||||
| Adverse event . | IL-15 . | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0.25, 0.5, 1 μg/kg IV non-MTD . | 0.75 μg/kg IV . | 2.0 μg/kg SC . | ||||||||||||||||
| Grade 1-3 . | Grade 4 . | Grade 5 . | Grade 1-3 . | Grade 4 . | Grade 5 . | Grade 1-3 . | Grade 4 . | Grade 5 . | ||||||||||
| n . | % . | n . | % . | n . | % . | n . | % . | n . | % . | n . | % . | n . | % . | n . | % . | n . | % . | |
| Hypertension | 10 | 100 | 13 | 81 | 9 | 56 | ||||||||||||
| Hypotension | 3 | 30 | 5 | 31 | 8 | 50 | ||||||||||||
| Hypoxia | 3 | 30 | 1 | 10 | 5 | 31 | 5 | 31 | ||||||||||
| Dyspnea/cough | 2 | 20 | 2 | 20 | 5 | 31 | 7 | 44 | ||||||||||
| Pulmonary infiltrates | 2 | 20 | 5 | 31 | 1 | 6 | 2 | 13 | ||||||||||
| Pulmonary edema | 2 | 20 | 0 | 6 | 38 | |||||||||||||
| Respiratory failure | 0 | 0 | 1 | 6 | 0 | 1 | 6 | |||||||||||
| Diffuse alveolar hemorrhage | 0 | 1 | 10 | 0 | 0 | |||||||||||||
| Febrile neutropenia | 10 | 100 | 13 | 81 | 10 | 63 | ||||||||||||
| Infection/sepsis | 0 | 1 | 6 | 1 | 6 | 2 | 13 | 2 | 13 | 2 | 13 | |||||||
| Marrow aplasia | 0 | 3 | 30 | 1 | 10 | 0 | 0 | |||||||||||
| Injection site reaction | 0 | 0 | 10 | 63 | ||||||||||||||
| Rash | 6 | 60 | 10 | 63 | 14 | 88 | ||||||||||||
| Myalgia/arthralgia | 0 | 3 | 19 | 0 | ||||||||||||||
| Chills | 8 | 80 | 10 | 63 | 13 | 81 | ||||||||||||
| Edema | 5 | 50 | 8 | 50 | 10 | 63 | ||||||||||||
| Flu-like symptoms | 0 | 2 | 13 | 1 | 6 | |||||||||||||
| Fatigue | 0 | 1 | 6 | 3 | 19 | |||||||||||||
| Pain | 0 | 3 | 19 | 1 | 6 | 3 | 19 | |||||||||||
| Arrhythmia | 0 | 1 | 10 | 1 | 6 | 0 | ||||||||||||
| Prolonged QTc | 4 | 40 | 7 | 44 | 8 | 50 | ||||||||||||
| Increased troponin | 1 | 10 | 3 | 19 | 0 | |||||||||||||
| Decreased LVEF | 0 | 1 | 6 | 1 | 6 | 0 | ||||||||||||
| Increased creatinine | 1 | 10 | 2 | 13 | 1 | 6 | 1 | 6 | 10 | 63 | ||||||||
| Multiorgan failure | 0 | 1 | 10 | 0 | 0 | |||||||||||||
| Hypernatremia | 0 | 0 | 3 | 19 | ||||||||||||||
| Increased bilirubin | 0 | 0 | 6 | 38 | ||||||||||||||
| Increased AST/ALT | 3 | 30 | 2 | 13 | 6 | 38 | ||||||||||||
| Diarrhea | 0 | 2 | 13 | 0 | ||||||||||||||
| Vomiting | 1 | 10 | 1 | 6 | 0 | |||||||||||||
| Dizziness | 0 | 1 | 6 | 0 | ||||||||||||||
| Headache | 7 | 70 | 2 | 13 | 5 | 31 | ||||||||||||
| Stroke/intracranial hemorrhage/subdural hematoma | 1 | 10 | 0 | 0 | 2 | 13 | ||||||||||||
| Gait disturbance | 0 | 0 | 1 | 6 | ||||||||||||||
| Cytokine release syndrome | 0 | 0 | 4 | 25 | 2 | 13 | ||||||||||||
| Encephalopathy | 0 | 0 | 1 | 6 | 1 | 6 | ||||||||||||
| Seizure | 0 | 0 | 0 | 2 | 13 | |||||||||||||
| Confusion | 0 | 2 | 13 | 6 | 38 | |||||||||||||
ALT, alanine aminotransferase; AST, aspartate aminotransferase; CTCAE, Common Terminology Criteria for Adverse Events; LVEF, left ventricular ejection fraction.
Grade 4 to 5 toxicities in the IV cohorts included diffuse alveolar hemorrhage, hypoxia, dyspnea, arrhythmia, multiorgan failure, and prolonged marrow aplasia. Grade 4 to 5 toxicities in the SC cohort included infection, respiratory failure, CRS, seizures, encephalopathy, ICH, and subdural hematoma. Of 3 patients with grade 5 toxicities in the SC rhIL-15 arm, 2 deaths were the result of progressive disease, and 1 death was the result of CRS with neurologic toxicity and ICH.
After lymphodepleting chemotherapy, serum clearance of SC rhIL-15 is slow compared with IV dosing
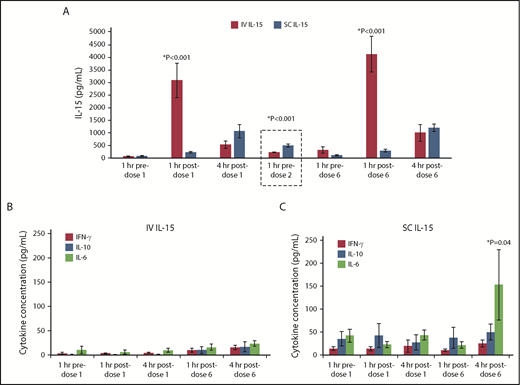
Pharmacokinetic data demonstrated greater peak levels of IL-15 after IV vs SC dosing. Levels of IL-15 measured 1 hour after dose 1 were 10-fold higher after IV compared with SC administration (P < .001). Measurable IL-15 decreased rapidly by 4 hours after dose 1 in the IV cohort but was increased in the SC cohort, which suggests accumulation. Although the peak level of IL-15 after SC dosing was likely missed at the time points measured, the clearance was significantly slower as shown by higher trough levels of IL-15 at 24 hours, just before dose 2 (501 vs 233 pg/mL; P < .001) (Figure 3A). In contrast, patients with solid tumors who received SC rhIL-15 on the same dose and schedule that were used for treatment on the NCI’s monotherapy study who did not receive lymphodepleting chemotherapy (and thus were lymphoreplete), had IL-15 concentrations that were much lower (range, 30-113 pg/mL at 24 hours after dose 1).13 Thus, SC administration of rhIL-15 after lymphodepletion with high-dose Cy/Flu markedly prolonged clearance, thus increasing the accumulation of and exposure to rhIL-15, presumably because of less absorption of IL-15 by lymphocytes and elimination of the cytokine sink.18
Pharmacokinetics of rhIL-15 and inflammatory cytokines. (A) Mean and serum IL-15 levels measured in IV and SC rhIL-15 cohorts at the specified time points are compared (standard error of the mean shown). Peak IL-15 levels were significantly higher 1 hour after dosing in the IV vs SC cohorts. Immediately before dose 2 (dashed box), patients in the SC cohort had significantly higher residual IL-15 levels than those in the IV cohort. Cytokine levels associated with IV rhIL-15 (B) and SC rhIL-15 (C). IL-6 levels are significantly increased at 4 hours after dose 6 in SC compared with IV dosing (P = .04).
Pharmacokinetics of rhIL-15 and inflammatory cytokines. (A) Mean and serum IL-15 levels measured in IV and SC rhIL-15 cohorts at the specified time points are compared (standard error of the mean shown). Peak IL-15 levels were significantly higher 1 hour after dosing in the IV vs SC cohorts. Immediately before dose 2 (dashed box), patients in the SC cohort had significantly higher residual IL-15 levels than those in the IV cohort. Cytokine levels associated with IV rhIL-15 (B) and SC rhIL-15 (C). IL-6 levels are significantly increased at 4 hours after dose 6 in SC compared with IV dosing (P = .04).
IFN-γ, IL-10, and IL-6 levels measured at several time points before and after rhIL-15 dosing are shown in Figure 3B-C. The route of administration affects cytokine production, because we observed higher mean IL-6 levels at 4 hours after dose 6 in the SC vs IV rhIL-15 cohort (153.2 vs 24.1 pg/mL; P = .04). Importantly, the IL-6 levels in the SC cohort were 15-fold higher than those measured in the patients with solid tumors who received the same dose and schedule, suggesting that prior lymphodepletion significantly affects the downstream IL-6 kinetics as well.13 More patients had IL-6 levels >100 pg/mL in the SC compared with the IV rhIL-15 cohort (62.5% vs 6.7%; P < .01) and more had higher mean peak IL-6 levels (SC group, 375 pg/mL vs IV group, 47 pg/mL; P = .03).
Discussion
We demonstrated that rhIL-15 is associated with an antileukemic effect in combination with lymphodepleting chemotherapy and haplo-NK-cell infusion. CR/CRi was achieved in 32% of patients with relapsed/refractory AML who received IV rhIL-15 and 40% of patients with SC rhIL-15 at 2.0 μg/kg. The clinical efficacy of this approach compares favorably with other single-agent salvage regimens, including BCL2 inhibitors (venetoclax) and FLT3 or IDH1/2 inhibitors, in which the CR/CRi rates are reported to be 10% to 40%19 and higher rates (51%) with newer strategies using BCL2 inhibitors in combination with hypomethylating agents.20 Compared with our historical experience using IL-2, rhIL-15 supported significantly higher rates of in vivo expansion of adoptively transferred haploidentical donor NK cells after lymphodepleting chemotherapy.2,3,5 However, unlike our previous experience with IL-2, in vivo donor NK-cell expansion with IL-15 did not correlate with clinical efficacy.2,3,5 It is possible that the function of the IL-15–activated NK cells was so enhanced that the previously reported correlation with absolute NK-cell numbers became less relevant. It is also possible that stimulation of host CD8+ T cells, while increasing rejection of the donor NK cells, contributed to both antileukemia activity and the clinical benefit. These possibilities should be addressed in a future randomized trial.
We observed a high frequency of CRS (56%) and neurotoxicity (33%) after treatment with SC rhIL-15. These previously unreported toxicities developed after lymphodepleting Cy/Flu and adoptive cellular therapy and were not seen in 26 patients who received IV dosing of rhIL-15. Furthermore, no such toxicity was seen after monotherapy with SC rhIL-15 in the phase 1 CITN-02 solid tumor trial that established 2.0 μg/kg as the MTD.13 Thus, we conclude that the Cy/Flu lymphodepletion seems to be a key variable in IL-15 clearance and the development of CRS. Our data support the hypothesis that depletion of peripheral lymphocytes leads to greater accumulation of IL-15 by eliminating the cytokine sink, which results in more inflammation, higher IL-6 levels, and CRS.21-24 Although CRS did not diminish the CR/CRi rate in the SC vs IC cohorts, it is possible that the steroids required in 7 of 9 patients may have diminished NK-cell function and the potential for added clinical benefit. Future rhIL-15 studies should aim to maximize NK-cell activity balanced with adjusted dosing intervals to account for the lack of cellular sinks induced by lymphodepleting chemotherapy that affects IL-15 levels.
CRS has been reported in 70% of patients with B-lymphoid malignancies (n = 133) who received CD19 chimeric antigen receptor (CAR) T-cell therapy.25 We observed milder manifestations of systemic CRS in this cohort of SC rhIL-15 patients. There was no association between higher NK-cell doses or pretherapy tumor burden with the development of CRS, as has been reported in CAR T-cell therapy. Lymphodepleting chemotherapy with Cy/Flu has been reported as a risk factor for CRS in CAR T-cell studies,25 suggesting a common theme that more circulating lymphocytes may protect patients from cytokine excess.
Neurotoxicity after CAR T-cell therapy for B-lymphoblastic leukemia was reported in 40% of patients (grade ≥4 in 5%), with earlier onset of CRS predictive of more severe neurotoxicity.26,27 In contrast to our generally unrevealing neuroimaging studies, CRS associated with CAR T-cell therapy can be associated with T2-weighted-fluid-attenuated inversion recovery changes, which indicate vasogenic edema. Fortunately, CRS and neurotoxicity did not obviate the clinical benefit of haplo-NK-cell adoptive transfer for patients with relapsed/refractory AML. The lack of correlation between clinical efficacy and toxicity in these trials differs from the CAR T-cell experience in which, in general, the development of CRS can be a marker for effector cell expansion and subsequent response. Our data suggest that although it is serious, the CRS associated with this platform can be managed and is reversible with steroids and tocilizumab. Future studies using SC rhIL-15 after lymphodepletion should be informed by this experience and follow a more traditional dose-escalation design with prospective monitoring for CRS and neurotoxicity using proactive treatment strategies modeled after the CAR T-cell experience.
Shah et al28 have described an immune activation syndrome characterized as GVHD with adoptive transfer of donor-derived IL-15/4-1BBL–activated NK cells, but we and others have not observed CRS or GVHD in >200 patients with myeloid, lymphoid, and solid tumors treated with lymphodepleting chemotherapy and adoptive haplo-NK-cell therapy with IL-2.4,5,29,30 The pathophysiology of CAR T-cell–induced CRS and neurotoxicity has been dissected with murine models and human data. Highly activated T cells stimulate myeloid macrophage cells, which in turn release IL-6 and IL-1, which contribute to CRS and neurotoxicity, respectively.31-33 Thus, we do not believe that the NK cells were directly responsible for the CRS. Rather, our data support the hypothesis that the accumulation of rhIL-15 after SC but not IV dosing is exacerbated by the lack of lymphocyte sinks because of the preceding Cy/Flu and that the IL-15 either directly stimulates host monocytes, activates host T cells, or both. The development of CRS and neurotoxicity did not correlate with peripheral blood T-cell or monocyte counts, but it is likely that tissue-resident macrophages secrete the IL-6 and IL-1.
To exploit the promising anticancer immunostimulatory effect of IL-15, strategies to best administer this drug are still being studied with the premise that prolonged exposure to SC or continuous infusion dosing optimizes biologic effects while minimizing high peak concentrations associated with toxicity.34 In addition to the NCI’s monomeric rhIL-15 protein, several IL-15 products have been developed that focus on the IL-15R-α stabilization of IL-15 such as IL-15/IL-15R-α-Fc (ALT-803) or heterodimeric IL-15.35,36 The toxicity profiles of the various agents will differ. For example, we recently reported that SC injections of ALT-803 resulted in significant skin reactions from a lymphoid infiltrate, but no large skin reactions were seen in this study of SC dosing of rhIL-15.37 Each IL-15 product may also have different effects on NK cells and CD8+ T cells. Although IL-15 may be superior to IL-2 for in vivo stimulating and expanding donor NK cells, it also stimulates host CD8+ T cells. Thus, after lymphodepletion, the IL-15–driven expansion of recovering host CD8+ T cells may reject donor NK cells, which may explain why NK-cell persistence at day 14 was not seen in all patients.
In conclusion, IL-15 should be preferable to IL-2 for use in adoptive cell therapy for refractory/relapsed AML because it avoids Treg stimulation. However, further strategies to selectively and transiently blunt recovering host CD8+ T cells need to be developed to further enhance this effect. Thus, novel methods to prevent host rejection of adoptively transferred cells will be required.
The full-text version of this article contains a data supplement.
Acknowledgments
The authors thank the Flow Cytometry Shared Resource, the Clinical Trials Office, the Translational Therapy Laboratory, the Cancer Research Translational Initiative, and the Clinical Informatics Shared Services.
This work was supported by National Institutes of Health, National Cancer Institute (NCI) grants CA111412 (S.C., D.J.W., and J.S.M.), CA65493 (S.C., B.R.B., and J.S.M.), CA197292 (J.S.M.), 5P30CA077598-18 (funding for the University of Minnesota Masonic Cancer Center), Intramural Research Program (T.A.W. and K.C.C.), and National Heart, Lung, and Blood Institute grant R01 HL56067 (B.R.B.). The authors also acknowledge the NCI's Experimental Therapeutics (NExT) Program (189344) and CTEP for their support of this study.
Authorship
Contribution: S.C., F.H., D.J.W., and J.S.M. conceptualized the study, collected, assembled, analyzed, and interpreted the data, and wrote the manuscript; T.E.D., J.M.C., and P.R. collected, assembled, and analyzed the data; and V.B., G.M.V., B.G., K.C.C., T.A.W., D.H.M., and B.R.B. reviewed and edited the manuscript.
Conflict-of-interest disclosure: S.C., who contributed to this work while on faculty at University of Minnesota, is now an employee of Fate Therapeutics. The remaining authors declare no competing financial interests.
Correspondence: Jeffrey S. Miller, University of Minnesota, 420 Delaware St SE, Minneapolis, MN 55455; e-mail: mille011@umn.edu.
References
Author notes
S.C. and F.H. are joint first authors.