Key Points
In patients with CD30+ advanced systemic mastocytosis, brentuximab vedotin was well-tolerated without signs of mast cell activation.
Brentuximab vedotin produced no responses per IWG-MRT-ECNM response criteria, nor improvements in measures of mast cell burden.
Abstract
There is an unmet need for effective therapies for advanced systemic mastocytosis (advSM). CD30 is expressed on the surface of neoplastic mast cells (MC) in more than 50% of patients with advSM. Brentuximab vedotin (BV) is a CD30-directed antibody-drug conjugate with preclinical evidence supporting both an antineoplastic effect and an attenuation of immunoglobulin E-associated mediator release. These observations are the basis for this phase 2 trial of BV monotherapy (1.8 mg/kg IV every 3 weeks up to 8 cycles) in patients with CD30-positive advSM. The primary objective was to determine the efficacy of BV according to International Working Group-Myeloproliferative Neoplasms Research and Treatment-European Competence Network on Mastocytosis (IWG-MRT-ECNM) response criteria. Secondary objectives included evaluation of safety, changes in bone marrow (BM) MC burden, serum tryptase level, flow cytometric quantification of MC surface expression of CD30, and self-reported symptom burden. The trial enrolled 10 patients with a diagnosis of CD30+ advSM (aggressive SM, SM with an associated hematologic neoplasm [SM-AHN], or mast cell leukemia [MCL]) with 1 or more signs of SM-related organ damage. According to IWG-MRT-ECNM criteria, none of the patients demonstrated better than stable disease with BV. In addition, there were no significant reductions in BM MC burden, serum tryptase levels, or MC surface expression of CD30. Self-reported symptom scores showed no durable improvement with BV treatment. We conclude that BV is not active as a single agent in CD30+ advSM. This trial was registered at www.clinicaltrials.gov as #NCT01807598.
Introduction
Systemic mastocytosis (SM) comprises a spectrum of disorders characterized by the expansion and accumulation of neoplastic mast cells (MCs) in 1 or more extracutaneous organs, including the bone marrow (BM), spleen, liver, lymph nodes, and gastrointestinal tract. The KIT D816V mutation is found in 90% of adult patients1-4 and drives the clonal expansion of neoplastic MCs.1,3,5,6
In 2016, the World Health Organization (WHO) adopted a revised classification of mastocytosis, establishing it as a stand-alone major disease category distinct from myeloproliferative neoplasms.7,8 Prognosis is generally well delineated by the WHO subvariants of SM, with indolent SM (ISM) having a normal to near normal life expectancy and smoldering SM (SSM) having an intermediate prognosis because of an increased risk for progression to aggressive SM (ASM) or leukemic transformation.9-12 Advanced SM (advSM) consists of 3 subtypes that exhibit shortened survival and are often characterized by SM-related organ damage necessitating cytoreductive therapy: ASM, SM with an associated hematologic neoplasm (SM-AHN), and MC leukemia (MCL).9,13,14
Limited treatment options are available for patients with advSM. Cladribine and (PEG)-interferon-alfa are used on an off-label basis and exhibit partial remitting activity.15-18 The oral multikinase/KIT inhibitor midostaurin was approved by the US Food and Drug Administration and European Medicines Agency in 2017, based on a phase 2 global trial that demonstrated an overall response rate (ORR) of 60% per modified Valent criteria.19 Imatinib is also approved for patients with ASM who are negative for KIT D816V or whose KIT mutation status is unknown. The selective KIT D816V inhibitor avapritinib (BLU-285) is currently under investigation in a phase 1 dose escalation and dose expansion trial, with an ORR of 83% per modified International Working Group-Myeloproliferative Neoplasms Research and Treatment-European Competence Network on Mastocytosis (IWG-MRT-ECNM) response criteria.20,21 Although KIT inhibition has become a validated therapeutic approach in advSM, exploration of novel agents is needed to improve the quality and durability of responses.
CD30 (Ki-1 antigen) is a transmembrane receptor protein of the tumor necrosis factor superfamily, predominantly expressed only on activated B or T cells. Histopathologic studies have demonstrated that surface-expressed CD30 is present on at least 10% of neoplastic MCs in more than 50% of patients with advSM.22-25 Both surface-associated and soluble forms of CD30 appear to be enriched in patients with advSM when compared with ISM or cutaneous mastocytosis.22,24,25 Its activation in MCs is associated with pleiotropic downstream effects including degranulation-independent secretion of chemokines and cytokines, regulation of immunoglobulin class switching, and increased proliferation and survival of malignant cells.22
Brentuximab vedotin (BV; cAC10-vcMMAE; SGN-35) is an antibody-drug conjugate consisting of the chimeric immunoglobulin G1 antibody cAC10, specific to human CD30, covalently attached to the microtubule-disrupting agent monomethyl auristatin E (MMAE) by a protease-cleavable linker. Its clinical anticancer activity has previously been demonstrated in diseases with prominent surface CD30-expressing cells; that is, classical Hodgkin lymphoma and systemic anaplastic large cell lymphoma.26-28 In vitro studies of BV in CD30+ human MC lines produced cell cycle arrest and apoptosis. These effects were replicated in neoplastic MC xenografts derived from patients with SM with CD30+ (but not CD30−) MC. Treatment with brentuximab vedotin also abrogated immunoglobulin E-induced histamine release from both healthy donor basophils and SM donor CD30+ MC.25
In the current study, we sought to determine the efficacy and safety of BV in patients with CD30+ advSM. Secondary objectives included evaluation of biomarkers of response, including changes in bone marrow mast cell burden, serum tryptase levels, and expression of surface CD30 on mast cells by flow cytometric immunophenotyping. The effects of BV on patient-reported symptom burden were also analyzed.
Methods
This phase 2 open-label, single-group, investigator-initiated study (NCT01807598) was conducted at 2 sites: Stanford Cancer Institute and MD Anderson Cancer Center. Patients with histologically confirmed diagnoses of advSM (ASM, SM-AHN, or MCL) with at least 1 organ damage finding per IWG-MRT-ECNM criteria were eligible. Additional inclusion criteria included age 18 years or older, Eastern Cooperative Oncology Group performance status 3 or lower, and adequate organ function. Exclusion criteria included an AHN requiring immediate therapy, treatment with interferon-α or 2-CdA within the past 30 days, treatment with hematopoietic growth factors within the past 14 days, prior treatment with BV or bleomycin, and grade 2 or worse neuropathy (see supplemental Materials for full eligibility criteria). Greater than 20% of MCs expressing surface CD30 by flow cytometry (FCM) was used as a minimal threshold to consider a patient’s advSM as CD30-positive. The study was approved by the institutional review boards at each participating institution and conducted in accordance with the principles outlined in the Declaration of Helsinki. All patients provided written informed consent before administration of study treatment or procedures.
The primary objective of the study was to determine the ORR, defined as the proportion of patients with a complete remission (CR), partial remission, or clinical improvement per IWG-MRT-ECNM consensus response criteria. Secondary objectives were to evaluate the tolerability and safety profile of BV; to analyze changes in surface CD30 expression on neoplastic MC, as well BM MC burden and serum tryptase level; to evaluate changes in mastocytosis-related symptom scores; and to determine progression-free survival and overall survival.
This phase 2 study was based on a Simon 2-stage design, with a goal accrual of 11 patients in the first stage. If 2 of 11 patients demonstrated a response, further accrual to a total of 26 patients would be undertaken. The study population included all patients who received at least 1 dose of BV, and patients were considered evaluable for efficacy after the completion of 1 cycle of therapy (≥3 weeks). BV was administered at a dose of 1.8 mg/kg IV every 3 weeks, up to a total of 8 cycles or until disease progression, unacceptable toxicity, or investigator discretion to end therapy. Spleen and liver volumes by computed tomography/magnetic resonance imaging were obtained at baseline and after every four 3-week cycles until completion of treatment, progressive disease, or withdrawal from study treatment. Disease response assessments were performed after every cycle using IWG-MRT-ECNM criteria,29 which require responses to last a minimum of 12 weeks. Responding patients who experienced subsequent loss of response could receive up to 8 additional cycles of BV on protocol (supplemental Figure 1).
Patient-reported total symptom scores (TSS) were assessed with each cycle. These scores were indexed using both the standard TSS-10 from the Myeloproliferative Neoplasm Symptom Assessment Form (MPN-SAF) and a modified 10 score inventory from the Mastocytosis Symptom Assessment Form (MSAF)30,31 (supplemental Table 2). These composite scores were performed as a post hoc exploratory analysis, as neither index has been validated in the advSM patient population.
BM histopathology, MC burden (percentage MC out of total nucleated cells on BM core biopsy), and CD30 expression for patients enrolled at both sites were centrally reviewed by one of the authors (T.I.G.). Bone marrows were scheduled at baseline, the end of cycles 4 and 8, or the time of treatment discontinuation. BM samples were processed using a standardized protocol, and CD30 expression was assessed using FCM and immunohistochemistry methods (supplemental Materials).
Adverse events (AEs) were classified and graded by the National Cancer Institute Common Toxicity Criteria for AEs (CTCAE version 4.03). Any development of drug-related grade 2 or higher peripheral neuropathy, persistent or recurrent grade 3 or higher nonhematologic toxicity, or grade 3 or 4 neutropenia despite growth factor support required BV to be held and subsequently dose reduced to 1.2 mg/kg.
Statistical analysis
The primary end point was summarized by the frequency of confirmed best responses (ORR) during the first 8 cycles. Laboratory values were summarized using median values with ranges. Overall survival and progression-free survival were summarized using Kaplan-Meier estimates and associated 95% confidence intervals. Treatment differences in TSS were tested as time series by 1-way analysis of variance with a posttest for linear trend. Pre- and posttreatment differences in MC CD30 expression, BM MC burden, and serum tryptase levels were tested using the Wilcoxon matched pairs test. Statistical analysis was conducted using GraphPad Prism (version 6.05 for Windows, GraphPad Software, La Jolla, CA).
Results
Ten patients with CD30+ advSM were enrolled between January 2014 and March 2017. Three patients were screened but did not meet eligibility criteria: 2 patients had BM MC surface CD30 expression that was low or undetectable and 1 patient lacked an eligible organ damage finding. The baseline clinicopathologic features of enrolled patients are shown in Table 1. The cohort was evenly split between treatment-naive and previously-treated patients. All patients harbored the KIT D816V mutation. The median baseline MC burden in the core biopsy was 55% (range, 15%-60%), and the median baseline serum tryptase level was 295 ng/mL (range, 81-903 ng/mL). The median baseline expression of CD30 on BM MC by FCM analysis was 70% (range, 29%-96%), whereas the median baseline BM MC CD30 expression by immunohistochemistry analysis was 5% (range, 0%-90%). At the onset of the study, the median patient-reported composite symptom burden scores were 35 (range, 14-71) and 39 (range, 6-81), as measured by the MPN-SAF TSS-10 and modified MSAF, respectively.
Baseline characteristics of patients
| Characteristics . | Overall population (N = 10) . |
|---|---|
| Median age (range), y | 72.5 (40-84) |
| Female sex, n (%) | 6 (60) |
| ECOG performance status, n (%) | |
| 0-1 | 7 (70) |
| 2-3 | 3 (30) |
| Median number of prior therapies (range), n | 0.5 (0-2) |
| Number of prior therapies per patient, n (%) | |
| 0 | 5 (50) |
| 1 | 4 (40) |
| 2 | 1 (10) |
| Type of prior therapy, n (%) | |
| Splenectomy | 3 (30) |
| Cladribine | 3 (30) |
| Midostaurin | 1 (10) |
| Imatinib | 1 (10) |
| Decitabine* | 1 (10) |
| Clinicopathologic diagnosis, n (%) | |
| ASM | 3 (30) |
| SM-AHN | 5 (50) |
| CMML-1 | 3 (30) |
| MDS/MPN-U | 1 (10) |
| CEL, NOS | 1 (10) |
| MCL | 2 (20) |
| CMML-1 | 1 (10) |
| Mutation status, n (%) | |
| KIT D816V mutation | 10 (100) |
| Median number of IWG-MRT-ECNM organ damage findings† (range), n | 1 (1-3) |
| Number of IWG-MRT-ECNM organ damage findings per patient, n (%) | |
| 1 | 6 (60) |
| 2 | 2 (20) |
| 3 | 2 (20) |
| Types of IWG-MRT-ECNM organ damage findings, n (%) | |
| Elevated alkaline phosphatase | 5 (50) |
| Symptomatic splenomegaly | 3 (30) |
| Anemia | 4 (40) |
| Transfusion-dependent | 2 (20) |
| Transfusion-independent | 2 (20) |
| Thrombocytopenia | 4 (40) |
| Transfusion-dependent | 2 (20) |
| Transfusion-independent | 2 (20) |
| Neutropenia | 1 (10) |
| Ascites | 1 (10) |
| Median serum tryptase (range), ng/mL | 295 (81-903) |
| Median bone marrow core biopsy mast cell burden‡ (range), % involvement | 55 (15-60) |
| Median bone marrow mast cell CD30+ expression¶ (range), % | 70 (29-96) |
| Characteristics . | Overall population (N = 10) . |
|---|---|
| Median age (range), y | 72.5 (40-84) |
| Female sex, n (%) | 6 (60) |
| ECOG performance status, n (%) | |
| 0-1 | 7 (70) |
| 2-3 | 3 (30) |
| Median number of prior therapies (range), n | 0.5 (0-2) |
| Number of prior therapies per patient, n (%) | |
| 0 | 5 (50) |
| 1 | 4 (40) |
| 2 | 1 (10) |
| Type of prior therapy, n (%) | |
| Splenectomy | 3 (30) |
| Cladribine | 3 (30) |
| Midostaurin | 1 (10) |
| Imatinib | 1 (10) |
| Decitabine* | 1 (10) |
| Clinicopathologic diagnosis, n (%) | |
| ASM | 3 (30) |
| SM-AHN | 5 (50) |
| CMML-1 | 3 (30) |
| MDS/MPN-U | 1 (10) |
| CEL, NOS | 1 (10) |
| MCL | 2 (20) |
| CMML-1 | 1 (10) |
| Mutation status, n (%) | |
| KIT D816V mutation | 10 (100) |
| Median number of IWG-MRT-ECNM organ damage findings† (range), n | 1 (1-3) |
| Number of IWG-MRT-ECNM organ damage findings per patient, n (%) | |
| 1 | 6 (60) |
| 2 | 2 (20) |
| 3 | 2 (20) |
| Types of IWG-MRT-ECNM organ damage findings, n (%) | |
| Elevated alkaline phosphatase | 5 (50) |
| Symptomatic splenomegaly | 3 (30) |
| Anemia | 4 (40) |
| Transfusion-dependent | 2 (20) |
| Transfusion-independent | 2 (20) |
| Thrombocytopenia | 4 (40) |
| Transfusion-dependent | 2 (20) |
| Transfusion-independent | 2 (20) |
| Neutropenia | 1 (10) |
| Ascites | 1 (10) |
| Median serum tryptase (range), ng/mL | 295 (81-903) |
| Median bone marrow core biopsy mast cell burden‡ (range), % involvement | 55 (15-60) |
| Median bone marrow mast cell CD30+ expression¶ (range), % | 70 (29-96) |
AHN-directed therapy for MDS/MPN-U.
For the full listing and definitions of eligible organ damage criteria, please see the IWG-MRT-ECNM consensus guidelines.29
Measured via tryptase, CD117, and CD25 immunohistochemistry staining on the core biopsy specimen.
Measured via multiparametric FCM methods using anti-BerH83 antibody on aspirate specimen.
CEL, chronic eosinophilic leukemia, not otherwise specified; CMML-1, chronic myelomonocytic leukemia, <10% blasts; ECOG, Eastern Cooperative Oncology Group; KIT, CD117, c-Kit receptor; MCL, mast cell leukemia; MDS/MPN-U, myelodysplastic/myeloproliferative neoplasm, unclassifiable; MPN-SAF, Myeloproliferative Neoplasm Symptom Assessment Form.
The median duration of study follow-up was 722 days (range, 18-1246 days), and the median number of cycles of BV administered was 5 (range, 1-8 cycles). No responses were observed according to IWG-MRT-ECNM criteria (Table 2). Of the 10 enrolled patients, 1 subject was not evaluable for response as a result of an early death during cycle 1 because of intracranial hemorrhage secondary to a mechanical fall, considered unrelated to the study treatment. Of the 9 remaining patients, 8 (80%) patients had stable disease and 1 (10%) patient had progressive disease (PD), characterized by a worsening red blood cell transfusion requirement. Four patients discontinued treatment early because of investigator discretion for lack of clinical benefit (n = 3) and PD (n = 1). The median progression-free survival for the evaluable cohort was 210 days (95% confidence interval, 77-343 days), and the median overall survival was not reached (supplemental Figure 2).
Efficacy summary
| IWG-MRT-ECNM response*, n (%) . | Overall population (N = 10) . |
|---|---|
| ORR (CR + PR +CI) | 0 (0) |
| Stable disease | 8 (80) |
| Progressive disease | 1 (10) |
| Not evaluable | 1 (10) |
| IWG-MRT-ECNM response*, n (%) . | Overall population (N = 10) . |
|---|---|
| ORR (CR + PR +CI) | 0 (0) |
| Stable disease | 8 (80) |
| Progressive disease | 1 (10) |
| Not evaluable | 1 (10) |
For the full listing and definitions of response criteria, please see the IWG-MRT-ECNM consensus guidelines.29
CI, clinical improvement
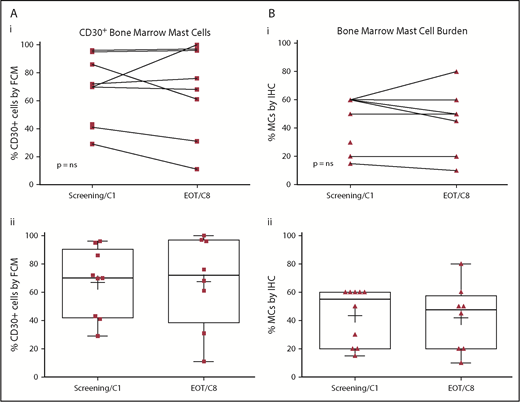
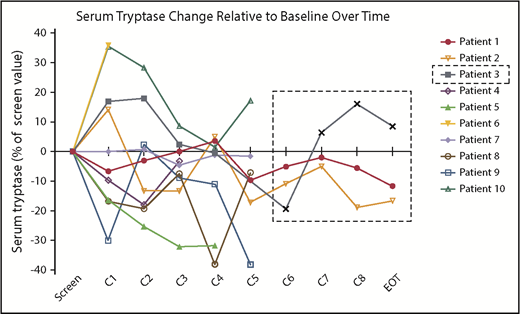
There were no significant or durable reductions in BM MC burden (Figure 1B) or serum tryptase level (Figure 2) during treatment. Although 2 patients achieved a 50% or greater reduction in CD30+ BM MC burden, these responses were not durable (Figure 1A). At specific points, there was no concordance among changes in BM MC burden, MC surface CD30 expression, and serum tryptase level. In addition, there were no durable reductions in composite symptom burden scoring over the course of treatment (Figure 3A,Bi). Two patients reported a 50% or greater reduction in composite symptom burden scores both by TSS-10 and modified MSAF; however, these responses were not durable (Figure 3A,Bii).
Baseline and on-treatment MC CD30 expression and BM MC burden. CD30+ BM MCs (A), assessed by multiparameter flow cytometry on the aspirate specimen, as well as BM MC burden (B), BM MC burden, assessed by immunohistochemistry on the core biopsy specimen, did not show significant reductions when compared with pretreatment baseline by Wilcoxon matched pairs signed rank test (i). Pre- and posttherapy assessments are represented for individual patients (i) and for the entire cohort (ii) with each biomarker. Box plot boundaries represent the 75th and 25th percentiles; whiskers denote maximum and minimum values, (+) denotes the cohort’s mean, and symbols represent individual patient values. EOT, end of treatment; FCM, multiparametric flow cytometry; IHC, immunohistochemistry.
Baseline and on-treatment MC CD30 expression and BM MC burden. CD30+ BM MCs (A), assessed by multiparameter flow cytometry on the aspirate specimen, as well as BM MC burden (B), BM MC burden, assessed by immunohistochemistry on the core biopsy specimen, did not show significant reductions when compared with pretreatment baseline by Wilcoxon matched pairs signed rank test (i). Pre- and posttherapy assessments are represented for individual patients (i) and for the entire cohort (ii) with each biomarker. Box plot boundaries represent the 75th and 25th percentiles; whiskers denote maximum and minimum values, (+) denotes the cohort’s mean, and symbols represent individual patient values. EOT, end of treatment; FCM, multiparametric flow cytometry; IHC, immunohistochemistry.
Changes in serum tryptase levels on BV therapy. Serum tryptase activity level, represented for each patient as a percentage change relative to their pretreatment baseline, did not show durable reductions over the course of treatment. Patient 3 discontinued treatment after cycle 4 because of progressive disease, with subsequent values obtained during posttherapy monitoring shown within the dashed line box.
Changes in serum tryptase levels on BV therapy. Serum tryptase activity level, represented for each patient as a percentage change relative to their pretreatment baseline, did not show durable reductions over the course of treatment. Patient 3 discontinued treatment after cycle 4 because of progressive disease, with subsequent values obtained during posttherapy monitoring shown within the dashed line box.
Changes in patient-reported symptoms on BV therapy. Composite symptom burden assessments using the MPN-SAF TSS-10* (A) and modified MSAF** (B) did not show significant change over time (r2 ∼0) by 1-way analysis of variance with a posttest for linear trend (i). Individual patients’ assessments (ii) did not show a consistent trend over time. *Composite of patient-reported scores on 10 items (0-10 scale each, with higher indicating worse): worst fatigue, concentration, early satiety, inactivity, night sweats, itching, bone/muscle pain, abdominal discomfort, weight loss, and fever. **Composite of patient-reported scores on 10 items (0-10 scale each, with higher indicating worse): pruritus, dizziness, headache, worst fatigue, flushing, abdominal discomfort, diarrhea, bone/muscle pain, concentration, and depression.
Changes in patient-reported symptoms on BV therapy. Composite symptom burden assessments using the MPN-SAF TSS-10* (A) and modified MSAF** (B) did not show significant change over time (r2 ∼0) by 1-way analysis of variance with a posttest for linear trend (i). Individual patients’ assessments (ii) did not show a consistent trend over time. *Composite of patient-reported scores on 10 items (0-10 scale each, with higher indicating worse): worst fatigue, concentration, early satiety, inactivity, night sweats, itching, bone/muscle pain, abdominal discomfort, weight loss, and fever. **Composite of patient-reported scores on 10 items (0-10 scale each, with higher indicating worse): pruritus, dizziness, headache, worst fatigue, flushing, abdominal discomfort, diarrhea, bone/muscle pain, concentration, and depression.
The most common AEs reported regardless of attribution were nonhematologic in nature and primarily grade 1 or 2, with fatigue, rash, and gastrointestinal complaints being the most prevalent (Table 3). Two patients reported recurrent infusion-related reactions. The majority of grade 3 or worse AEs were hematologic in nature, with the most common being neutropenia. Three patients required BV to be held because of grade 4 neutropenia, and 1 patient required granulocyte colony stimulating factor support and dose reduction of BV from 1.8 to 1.2 mg/kg after 6 cycles of treatment. The median duration of dose holds was 21 days (range, 7-21 days). There were no episodes of febrile neutropenia. There were no treatment discontinuations resulting from treatment-associated toxicity. Serious AEs were all considered unrelated to study treatment and included grade 5 intracranial hemorrhage, grade 3 portal vein thrombosis, grade 3 epiglottitis, and grade 3 pleural effusion (all n = 1).
Adverse events reported on study, regardless of attribution
| Overall population (N = 10) . | Any grade, n (%) . | Grade 3/4, n (%) . | Grade 5, n (%) . | |
|---|---|---|---|---|
| Nonhematologic | ||||
| Fatigue | 4 (40) | |||
| Infusion reaction | 2 (20) | |||
| Abdominal pain | 2 (20) | |||
| Anorexia | 2 (20) | |||
| Vomiting | 2 (20) | |||
| Diarrhea | 2 (20) | |||
| Constipation | 2 (20) | |||
| Cough | 2 (20) | |||
| Dyspnea | 2 (20) | |||
| Depression | 2 (20) | |||
| Rash | 1 (10) | 1 (10) | ||
| Peripheral neuropathy | 1 (10)* | |||
| Hematologic | ||||
| Neutropenia | 3 (30)† | |||
| Anemia | 2 (20) | 1 (10) | ||
| Thrombocytopenia | 1 (10) | |||
| Serious adverse events‡ | ||||
| Intracranial hemorrhage | 1 (10)¶ | |||
| Portal vein thrombosis | 1 (10) | |||
| Epiglottitis | 1 (10) | |||
| Pleural effusion | 1 (10) | |||
| Overall population (N = 10) . | Any grade, n (%) . | Grade 3/4, n (%) . | Grade 5, n (%) . | |
|---|---|---|---|---|
| Nonhematologic | ||||
| Fatigue | 4 (40) | |||
| Infusion reaction | 2 (20) | |||
| Abdominal pain | 2 (20) | |||
| Anorexia | 2 (20) | |||
| Vomiting | 2 (20) | |||
| Diarrhea | 2 (20) | |||
| Constipation | 2 (20) | |||
| Cough | 2 (20) | |||
| Dyspnea | 2 (20) | |||
| Depression | 2 (20) | |||
| Rash | 1 (10) | 1 (10) | ||
| Peripheral neuropathy | 1 (10)* | |||
| Hematologic | ||||
| Neutropenia | 3 (30)† | |||
| Anemia | 2 (20) | 1 (10) | ||
| Thrombocytopenia | 1 (10) | |||
| Serious adverse events‡ | ||||
| Intracranial hemorrhage | 1 (10)¶ | |||
| Portal vein thrombosis | 1 (10) | |||
| Epiglottitis | 1 (10) | |||
| Pleural effusion | 1 (10) | |||
Pretreatment grade 1 neuropathy worsened to grade 2 after 1 cycle of BV, but subsequently returned to baseline without dose modification.
All grade 4 events, required dose holds for 1 wk (n = 1) and 3 wk (n = 2); 1 patient required dose reduction of BV for cycle 7 after 2 successive dose holds.
All SAEs were considered unrelated to BV.
Patient died of an intracranial hemorrhage resulting from a mechanical fall during cycle 1 of BV in the setting of thrombocytopenia.
Discussion
BV, although well tolerated, did not demonstrate clinical activity in this cohort of patients with CD30+ advSM, whether assessed by improvement in SM-related organ damage, measures of mast cell burden, or durable improvement in patient-reported symptoms. Compared with prior studies19 that used (modified) Valent criteria to adjudicate responses, this study employed the more stringent, but more clinically relevant, IWG-MRT-ECNM consensus response criteria, which are now being used by the regulatory health authorities for evaluation of novel agents in patients with advSM.
These negative clinical results follow several multiple promising preclinical studies identifying CD30 as a therapeutic target on neoplastic MCs.22-25 To date, the only other report in the literature that examined the clinical responses of patients with SM receiving BV monotherapy was a retrospective case series of 4 patients enrolled in a phase 2 open-label study of BV in CD30+ nonlymphomatous malignancies (NCT01461538).32 In this series, 1 patient with ASM and severe neutropenia experienced a partial remission by IWG-MRT-ECNM criteria29 (>50% reduction in their BM MC burden and serum tryptase level after 12 cycles); however, the patient’s neutropenia response to BV therapy was confounded by concurrent granulocyte colony stimulating factor support. One patient with ISM and a high mediator symptom burden experienced a major regression of symptoms that lasted for 11 cycles. The series also included 1 patient with ASM who had PD after 3 cycles and 1 patient with SSM who demonstrated PD after 8 cycles.
Our study recapitulated that CD30 expression was more accurately measured by multiparameter FCM on BM aspirate samples.24,29,33,34 In keeping with several prior clinical studies using BV, we set a threshold for CD30 positivity at greater than 20% of neoplastic cells.35 The amount of surface-expressed CD30, as measured by FCM, was variable at baseline in our cohort and did not appear to correlate with BV’s ability to reduce the neoplastic MC burden or serum tryptase level. In other CD30+ malignancies such as classical Hodgkin lymphoma, large cell lymphoma, diffuse large B-cell lymphoma, and cutaneous T-cell lymphomas (eg, mycosis fungoides and Sézary syndrome), no clear relationship exists between pretreatment CD30 expression and treatment response.27,28,36-38
The absence of any durable reductions in CD30+ cell burden in response to BV may be a result of one or more potential mechanisms of failure or resistance seen in other CD30+ malignancies including target escape via downregulation of surface CD30 expression,39 off-target binding to non-cell surface associated or soluble CD30,40-43 or pharmacokinetic failures related to the tumor microenvironment or multidrug resistance efflux pumps.44,45 However, BV has demonstrated major, durable responses in a majority of relapsed/refractory patients with classical Hodgkin lymphoma and patients with large cell lymphoma, where many of these mechanisms were first described. Thus it appears that CD30 may not be as relevant to the induction of apoptosis or attenuation of immunoglobulin E-dependent histamine release for MCs in vivo as was the case in preclinical models.
BV was well tolerated in this cohort, with most reported AEs being low grade and requiring dose modification in only 1 patient’s case because of the known hematologic toxicity of neutropenia. There were no treatment discontinuations attributable to BV-related toxicities.
In summary, BV failed to demonstrate sufficient activity in the first stage of this phase 2, Simon 2-stage trial, thus precluding further evaluation in this population of CD30+ advSM patients. With the advent of targeted KIT inhibition and viable alternative MC surface-associated targets for antibody-drug conjugates, we do not recommend BV as monotherapy for patients with advSM.
The full-text version of this article contains a data supplement.
Acknowledgments
This work was supported by Seattle Genetics, Inc., and the Charles and Ann Johnson Foundation. This work was supported, in part, by the Anderson Cancer Center support grant P30 CA016672 from the National Institutes of Health, National Cancer Institute.
Authorship
Contribution: J.G. and S.V. were responsible for study conception and design; T.I.G. performed all central pathology review and CD30 expression analysis; J.G., P.B., and S.V. evaluated patients on the clinical trial; J.H.B., J.G., and C.P. planned and performed data analysis; J.H.B. and J.G. drafted the manuscript; and C.L., I.R., J.A., C.P., K.S., and the remaining authors contributed to collection, assembly, and interpretation of data and took part in revision and final approval of the manuscript.
Conflict-of-interest disclosure: J.G. received funding from Seattle Genetics, Inc. for the conduct of this phase 2 trial. Pathology testing (T.I.G.) was performed at TriCore Reference Laboratories (Albuquerque, NM) supported by Seattle Genetics, Inc. The remaining authors delcare no competing financial interests.
The current affiliation for T.I.G. is Department of Pathology, University of Utah, Salt Lake City, UT.
Correspondence: Jason Gotlib, Stanford Cancer Institute, 875 Blake Wilbur Dr, Room 2324, Stanford, CA 94305-6555; e-mail: jason.gotlib@stanford.edu.
References
Author notes
J.G. and J.H.B. are joint primary authors.
Deidentified enrolled participant data are available indefinitely at https://clinicaltrials.gov/ct2/show/results/NCT01807598. Proposals for access to deidentified individual level data on which these results are based should be sent to jason.gotlib@stanford.edu.