Key Points
This is the largest study describing the clinical features and outcomes, according to different therapeutic approaches, of patients with BPDCN.
NHL- or AL-like treatments, followed by a consolidation transplantation strategy, are associated with the best outcomes.
Abstract
The purpose of this study is to describe the clinical and prognostic features and to evaluate the outcome of different therapeutic approaches among patients with blastic plasmacytoid dendritic cell neoplasm (BPDCN) who have been diagnosed and treated in different institutions. A total of 398 patients from 75 centers were included in the study. Treatment consisted of non-Hodgkin lymphoma (NHL)–like regimens in 129 (32.8%) patients and acute leukemia (AL)–like regimens in 113 (23.5%) patients. In 61 (15.5%) and 16 (4.1%) patients, chemotherapy was followed by allogeneic and autologous hematopoietic stem cell transplantation (HSCT), respectively. Twenty-seven (6.9%) patients received radiotherapy, 6 (1.5%) received new agents, and 62 (15.7%) received palliative care. After a median follow-up of 12 months, median overall survival (OS) was 18 months. Patients who received NHL/AL-like regimens, followed by allogeneic HSCT, had the best outcome; median OS was not reached. OS was 65 months for patients who underwent autologous HSCT; 18 months and 14 months, respectively, for those treated with AL-like and NHL-like regimens without consolidation; and 4 months for those receiving palliative care (P < .001). In BPDCN, chemotherapy with lymphoma- or AL-like regimens, followed by transplantation, represents the therapeutic strategy associated with the best outcome. Consolidation with allogeneic HSCT, when feasible, appears superior to autologous HSCT.
Introduction
Blastic plasmacytoid dendritic cell neoplasm (BPDCN) is a clinically aggressive rare hematopoietic malignancy derived from precursor plasmacytoid dendritic cells. BPDCN was initially classified within the acute myeloid leukemia (AML)–related precursor neoplasms in the 2008 World Health Organization classification,1 although it was reclassified as a separate entity in the recently revised edition.2 Most BPDCN patients present with cutaneous lesions, followed by systemic dissemination involving lymph nodes, bone marrow (BM), and, rarely, extranodal localizations. The median age at diagnosis is 61 to 67 years.2
The expression of CD4, CD56, and CD123, along with the negativity for lineage-specific markers, is essential for diagnosis. The malignant cell population may express blood dendritic cell antigen-2 (BDCA2; CD303) and other plasmacytoid dendritic cell–associated antigens (eg, T-cell leukemia 1 [TCL1] and CD2-associated protein).3,4 The expression of other markers, such as CD33, terminal deoxynucleotidyl transferase (TdT), CD79a, CD2, and CD7, which are usually associated with other cell lineages, has also been described. Rare cases may display atypical phenotypic profiles, including the rare absence of CD56.3
A history of hematologic malignancy, such as AML, chronic myeloid leukemia, chronic myelomonocytic leukemia, or, more rarely, myelodysplastic syndrome (MDS), is observed in ∼10% to 20% of cases.5-7
BPDCN has a poor prognosis, in part because it commonly displays early drug resistance against conventional cytotoxic agents. The use of AML-like, acute lymphoblastic leukemia (ALL)–type, or non-Hodgkin lymphoma (NHL)–like chemotherapy regimens is associated with a complete remission (CR) rate of 40% to 90%8-10 ; however remissions are usually short-lived, resulting in a median overall survival (OS) of 12 to 14 months9-11
Patients who received an allogeneic hematopoietic stem cell transplant (allo-HSCT) had better survival results compared with those who received chemotherapy alone (median OS, 22.7 months vs 7.1 months; P = .03).5 In a retrospective study from the European Society for Blood and Marrow Transplantation, when feasible, allo-HSCT was associated with a 3-year OS of 41%, reaching 53% in patients who were transplanted in first CR.12 Survival benefits might also be obtained with an autologous HSCT (auto-HSCT) in first CR.13
The purpose of this multicenter retrospective study was to describe the clinical and prognostic features of patients with BPDCN diagnosed and treated in different institutions, as well as to analyze the outcome associated with different therapeutic approaches.
Patients and methods
Physicians from different international centers considered to be experts in the field were contacted individually and asked to collaborate on this retrospective series by providing data about BPDCN cases from their group or center. Participating centers enrolled consecutively diagnosed cases from January 2001 to December 2017. Questionnaires were sent to collect clinical and laboratory data for each patient, including date of diagnosis, age, sex, clinical presentation of the disease, conventional cytogenetic and/or fluorescence in situ hybridization analysis, molecular analyses, type of treatments, including chemotherapy and auto-HSCT and allo-HSCT when applicable, sites of relapse, and follow-up updates. Centers provided histopathology and flow cytometry reports to confirm the diagnosis of BPDCN, which was defined according to the 2016 World Health Organization classification.
BPDCN diagnosis requires the expression of CD4 and CD56 with ≥1 other plasmacytoid dendritic cell–associated antigen among which CD123, TCL1, and BDCA2/CD303 and the negativity of lineage-specific markers for myeloid cells (myeloperoxidase), T cells (CD3), B cells (CD20 and CD79a), and monocytes (CD11c, CD163, lysozyme). Rare cases of CD4− and/or CD56− BPDCN-related were included if they expressed CD123, BDCA2/CD303, and TCL1.
The E-box transcription factor TCF4 acts as a master regulator of the BPDCN oncogenic program. Its downregulation causes the loss of BPDCN-specific gene expression and apoptosis. TCF4 can be used as a diagnostic tool for BPDCN, but it was not evaluated in our study, which was begun prior to the identification of this marker.14,15
Patients were separated into groups according to disease presentation: isolated skin involvement, disseminated disease with cutaneous localization, disseminated disease with cutaneous and extranodal localization, disseminated disease without cutaneous localization, and disseminated disease with extranodal localizations, with the exception of the skin. Because an international consensus definition of treatment response does not exist, we considered the definition of treatment efficacy, as documented at each center, according to previously standardized criteria,16-18 based on the evaluation of the most commonly involved disease sites (cutaneous, BM, blood, lymph nodes, and imaging assessment by computed tomography). A complete response was defined as the disappearance of the disease in each initially involved site.
A novel outcome of clinical complete response was reported recently by Pemmaraju et al,19 who defined patients as having a complete response in all nonskin disease sites and marked clearance of all skin lesions from baseline. However, the persistence of residual skin abnormalities not indicative of active BPDCN was not applicable in this study, which was begun prior to the publication of this response criterion.
Treatments were separated into the following groups: chemotherapy (ALL-like, AML-like, NHL-like) + allo-HSCT, chemotherapy (ALL-like, AML-like, NHL-like) + auto-HSCT, chemotherapy without consolidation (ALL-like, AML-like, NHL-like), radiotherapy, new drugs (SL401, bortezomib, 5-azacitidine; venetoclax), and palliative care.
ALL therapy included ALL-like (multidrug associations as in ALL treatments, including central nervous system [CNS] prophylaxis), such as Hyper-CVAD (cyclophosphamide, vincristine, doxorubicin and dexamethasone/methotrexate and cytarabine) and high-dose methotrexate with asparaginase (Aspa-MTX), AML therapy included standard-dose cytarabine by continuous infusion for 7 days in combination with daunorubicin or idarubicin for 3 to 5 days, and NHL therapy included CHOP (cyclophosphamide, doxorubicin, vincristine, and prednisone) or a CHOP-like regimen and other NHL regimens (ifosfamide/etoposide/platinum-based therapy): ESHAP (etoposide, solu-medrol, high-dose ara-C, cisplatin), DEVIC (dexamethasone, etoposide, ifosfamide, carboplatin), ICE (ifosfamide, carboplatin, etoposide), DHAP (dexamethasone, high-dose ara-C, cisplatin).
The number of patients in CR or with a partial response (PR), stable disease, or progression was determined for each treatment group. The date of disease relapse or progression was documented, as well as the date of last follow-up and death and the cause of death.
Statistical analysis
Descriptive analyses summarized the characteristics of the patients globally and by country. The quantitative variables are represented by means and standard deviations if the normality of the distribution was demonstrated by the Shapiro-Wilk test, and by medians, ranges, and interquartile ranges. The Kruskal-Wallis test was used to compare quantitative variables, and Pearson’s χ2 test was used for qualitative ones.
Associations between variables of interest and OS or PFS outcomes were assessed using a Cox proportional-hazards model for univariate analyses. Multivariate models on those 2 outcomes, including variables with univariate significant effect and <25% of missing data, were generated. Kaplan-Meier survival curves were plotted using months since treatment as the time scale. Differences between survival curves were assessed using the log-rank test.
Reported P values are 2-sided, and values <.05 were considered statistically significant. The estimations of risks are presented with 95% confidence intervals (CIs). Statistical analyses were performed using R (R Project for Statistical Computing; v3.6.0). The study protocol was approved by the local ethics committee of Le Mans Hospital.
Results
Clinical presentation
A total of 398 patients from 75 centers in 5 countries (France, Italy, Japan, Austria, and United States) were included in this study; 74% were males, with a median age of 67 years (range, 18-96), and 65.3% had Eastern Cooperative Oncology Group (ECOG) score of 0 or 1 (207/317). Involvement of the skin, BM, lymph nodes, and peripheral blood at diagnosis was observed in 89%, 62%, 39%, and 15% of cases, respectively. Disease was limited to the skin (30%), disseminated with cutaneous localization (50%), disseminated with cutaneous and extranodal localization (7%), disseminated noncutaneous (11%), and disseminated with noncutaneous extranodal localization (2%) (Table 1). Splenomegaly was present in 35 (9%) patients, and hepatomegaly was seen in 22 (6%) patients. CNS involvement was seen in 10 cases (2%) at diagnosis. BPDCN was associated with MDS in 9 cases (2%).
Patient characteristics and type of treatment of the entire cohort (N = 398)
| Characteristic . | Data . |
|---|---|
| Males/females | 294 (74)/104 (26) |
| ECOG score 0-1 | 207 (65) |
| Age, median (range), y | 67 (18-96) |
| Localization | |
| Skin | 353 (89) |
| BM | 243 (62) |
| Peripheral blood | 36 (15) |
| Lymph nodes | 152 (39) |
| Forms | |
| Cutaneous isolated | 121 (30) |
| Disseminated with cutaneous localization | 200 (50) |
| Disseminated with cutaneous and extranodal localization | 26 (7) |
| Disseminated noncutaneous | 43 (11) |
| Disseminated noncutaneous with extranodal localization | 8 (2) |
| Karyotype (available for 96 patients) | |
| Normal | 44 (46) |
| Abnormal | 52 (54) |
| Complex (CK+) | 32 (33) |
| Monosomic (MK+) | 16 (17) |
| MK+MC+ ≥ 5 | 6 (6) |
| Type of treatment | 394 (100) |
| Chemotherapy without consolidation | 222 (56.3) |
| NHL-like | 129 (32.8) |
| ALL-like* | 57 (14.4) |
| AML-like | 36 (9.1) |
| Chemotherapy + allo-HSCT | 61 (15.5) |
| ALL-like + allo-HSCT | 33 (8.4) |
| AML-like + allo-HSCT | 16 (4.1) |
| NHL-like + allo-HSCT | 12 (3.0) |
| Chemotherapy + auto-HSCT | 16 (4.1) |
| ALL-like + auto-HSCT | 6 (1.5) |
| AML-like + auto-HSCT | 1 (0.3) |
| NHL-like + auto-HSCT | 9 (2.3) |
| Radiotherapy | 27 (6.9) |
| New drugs (SL401, bortezomib, azacitidine) | 6 (1.5) |
| Palliative | 62 (15.7) |
| Characteristic . | Data . |
|---|---|
| Males/females | 294 (74)/104 (26) |
| ECOG score 0-1 | 207 (65) |
| Age, median (range), y | 67 (18-96) |
| Localization | |
| Skin | 353 (89) |
| BM | 243 (62) |
| Peripheral blood | 36 (15) |
| Lymph nodes | 152 (39) |
| Forms | |
| Cutaneous isolated | 121 (30) |
| Disseminated with cutaneous localization | 200 (50) |
| Disseminated with cutaneous and extranodal localization | 26 (7) |
| Disseminated noncutaneous | 43 (11) |
| Disseminated noncutaneous with extranodal localization | 8 (2) |
| Karyotype (available for 96 patients) | |
| Normal | 44 (46) |
| Abnormal | 52 (54) |
| Complex (CK+) | 32 (33) |
| Monosomic (MK+) | 16 (17) |
| MK+MC+ ≥ 5 | 6 (6) |
| Type of treatment | 394 (100) |
| Chemotherapy without consolidation | 222 (56.3) |
| NHL-like | 129 (32.8) |
| ALL-like* | 57 (14.4) |
| AML-like | 36 (9.1) |
| Chemotherapy + allo-HSCT | 61 (15.5) |
| ALL-like + allo-HSCT | 33 (8.4) |
| AML-like + allo-HSCT | 16 (4.1) |
| NHL-like + allo-HSCT | 12 (3.0) |
| Chemotherapy + auto-HSCT | 16 (4.1) |
| ALL-like + auto-HSCT | 6 (1.5) |
| AML-like + auto-HSCT | 1 (0.3) |
| NHL-like + auto-HSCT | 9 (2.3) |
| Radiotherapy | 27 (6.9) |
| New drugs (SL401, bortezomib, azacitidine) | 6 (1.5) |
| Palliative | 62 (15.7) |
Unless otherwise noted, data are n (%).
Including Hyper-CVAD regimen and Aspa-MTX.
Immunophenotype
Phenotypic analysis showed the combination of CD4+CD56+CD123+ in 96% of cases, CD4− and/or CD56− CD123+TCL1+BDCA2+ in 13 patients (3%), and CD4+CD56+CD123−TCL1+ and/or BDCA2+ in 3 patients (1%). All cases lacked expression of cytoplasmic CD3, CD19, CD20, CD11c, lysozyme, and myeloperoxidase.
The CD4+CD56+CD123+TCL1+ (with or without BDCA2) combination was found in 192 of 227 (85%) cases tested, and 35 of 98 (36%) cases tested were positive for the 5 markers CD4+CD56+CD123+TCL1+BDCA2+.
BDCA4 expression was rarely evaluated. It was positive in 13 of the 17 (76%) patients tested.
TdT positivity was found in 82 of 230 (36%) cases; these patients had a median age of 63 years (range, 18-87), and 40% had isolated cutaneous lesions. BCL2 was positive in 16 of 22 (73%) tested cases; these patients had a median age of 74 years (range, 29-87), and 60% had disseminated disease.
Ambiguous lineage markers (CD34+ and/or CD117+) were found in 21 of 205 (10%) cases tested. These patients had a median age of 66 years (range, 28-87), and disseminated disease was noted in 85% of cases. Other lineage-specific antigens were found as previously described1-5 : HLA-DR, CD43, CD68, CD7, CD2, CD5, CD33, CD13, MX1, CD30, CD38, and S100, were positive in 119 of 121 (98%), 99 of 103 (96%), 93 of 167 (56%), 75 of 191 (39%), 47 of 146 (32%), 4 of 116 (3%), 51 of 152 (34%), 2 of 98 (2%), 27 of 47 (57%), 1 of 65 (1.5%), 42 of 56 (75%), and 13 of 63 (25%) patients, respectively (Table 2).
Immunophenotypic markers in the blastic population in BPDCN cases
| Immunophenotype . | Positive result/tested (%) . |
|---|---|
| BPDC markers | |
| CD4+ CD56+ CD123+ | 391/398 (96) |
| CD4− and/or CD56− CD123+ TCL1+ BDCA2+ | 13 (3) |
| CD4+ CD56+ CD123− TCL1+ and/or BDCA2+ | 3 (1) |
| CD4+ CD56+ CD123+ TCL1+ and/or BDCA2+ | 192/227 (85) |
| CD4+ CD56+ CD123+ TCL1+ BDCA2+ | 35/98 (36) |
| BDCA4 | 13/17 (76) |
| T-lymphoid and natural killer cell markers | |
| CD3 | 0/398 (0) |
| cCD3 | 0/398 (0) |
| CD8 | 0/130 (0) |
| CD16 | 0/82 (0) |
| TIA | 0/60 (0) |
| CD1 | 1/46 (2) |
| CD57 | 1/42 (2) |
| Granzyme | 1/56 (2) |
| CD5 | 4/116 (3) |
| CD2 | 47/146 (32) |
| CD7 | 75/ 191 (39) |
| B-lymphoid markers | |
| CD19 | 0/142 (0) |
| CD20 | 0/348 (0) |
| cCD22 | 0/65 (0) |
| PAX5 | 0/15 (0) |
| CD138 | 1/41 (2) |
| cCD79a | 6/201 (3) |
| Myeloid and monocyte markers | |
| Myeloperoxidase | 0/398 (0) |
| CD11c | 0/169 (0) |
| Lysozyme | 0/77 (0) |
| CD14 | 0/72 (0) |
| CD15 | 0/64 (0) |
| CD64 | 1/40 (2) |
| CD13 | 2/98 (2) |
| CD117 | 16/107 (15) |
| CD33 | 51/152 (34) |
| CD68 | 93/167 (56) |
| CD36 | 31/36 (86) |
| Immature and other markers | |
| CD34 | 5/205 (2) |
| CD30 | 1/65 (1.5) |
| CD45Ro | 1/43 (2) |
| CD10 | 4/92 (4) |
| S100 | 13/63 (20) |
| CD38 | 14/56 (25) |
| TdT | 82/230 (36) |
| MX1 | 27/47 (57) |
| BCL2 | 16/22 (73) |
| CD2AP | 8/10 (80) |
| CD43 | 99/103 (96) |
| CD45RA | 124/128 (97) |
| HLA-DR | 119/121 (98) |
| Immunophenotype . | Positive result/tested (%) . |
|---|---|
| BPDC markers | |
| CD4+ CD56+ CD123+ | 391/398 (96) |
| CD4− and/or CD56− CD123+ TCL1+ BDCA2+ | 13 (3) |
| CD4+ CD56+ CD123− TCL1+ and/or BDCA2+ | 3 (1) |
| CD4+ CD56+ CD123+ TCL1+ and/or BDCA2+ | 192/227 (85) |
| CD4+ CD56+ CD123+ TCL1+ BDCA2+ | 35/98 (36) |
| BDCA4 | 13/17 (76) |
| T-lymphoid and natural killer cell markers | |
| CD3 | 0/398 (0) |
| cCD3 | 0/398 (0) |
| CD8 | 0/130 (0) |
| CD16 | 0/82 (0) |
| TIA | 0/60 (0) |
| CD1 | 1/46 (2) |
| CD57 | 1/42 (2) |
| Granzyme | 1/56 (2) |
| CD5 | 4/116 (3) |
| CD2 | 47/146 (32) |
| CD7 | 75/ 191 (39) |
| B-lymphoid markers | |
| CD19 | 0/142 (0) |
| CD20 | 0/348 (0) |
| cCD22 | 0/65 (0) |
| PAX5 | 0/15 (0) |
| CD138 | 1/41 (2) |
| cCD79a | 6/201 (3) |
| Myeloid and monocyte markers | |
| Myeloperoxidase | 0/398 (0) |
| CD11c | 0/169 (0) |
| Lysozyme | 0/77 (0) |
| CD14 | 0/72 (0) |
| CD15 | 0/64 (0) |
| CD64 | 1/40 (2) |
| CD13 | 2/98 (2) |
| CD117 | 16/107 (15) |
| CD33 | 51/152 (34) |
| CD68 | 93/167 (56) |
| CD36 | 31/36 (86) |
| Immature and other markers | |
| CD34 | 5/205 (2) |
| CD30 | 1/65 (1.5) |
| CD45Ro | 1/43 (2) |
| CD10 | 4/92 (4) |
| S100 | 13/63 (20) |
| CD38 | 14/56 (25) |
| TdT | 82/230 (36) |
| MX1 | 27/47 (57) |
| BCL2 | 16/22 (73) |
| CD2AP | 8/10 (80) |
| CD43 | 99/103 (96) |
| CD45RA | 124/128 (97) |
| HLA-DR | 119/121 (98) |
BPDC, blastic plasmacytoid dendritic cell; cCD22, cytoplasmic CD22; cCD3, cytoplasmic CD3; cCD79a, cytoplasmic CD79a; CD2AP, CD2-associated protein.
Cytogenetics
Information about karyotype was available for 96 patients. An abnormal karyotype was found in 52 cases (54%). Major chromosomal abnormalities known to be associated with BPDCN, on 5q (70%), 12p (62%), 13q (60%), 6q (52%), and 15q (39%), as well as monosomy 9 (20%), were identified in our series, as previously reported.20,21
Monosomal karyotype (MK+) and/or complex karyotype (CK+) was found in 35 cases (36%), MK+ in 16 cases (17%), CK+ in 32 cases (33%), and MK+ with ≥5 clonal abnormalities (MK + CK ≥ 5) in 6 cases (6%). Monosomy 13 was noted in 6 cases (38%). Monosomy 15, 17, 9, 8, 7, and 5 was found in 3 (19%), 2 (13%), 1 (6%), 1 (6%), 1 (6%), and 1 (6%) case, respectively. MK+ and/or CK+ patients had disseminated disease in 86% of cases.
Fluorescence in situ hybridization was performed in 15 cases with normal karyotype and identified an ETV6 rearrangement on 12p13 in 3 patients (20%).
Molecular studies were performed in 14 cases. No patient presented with an NPM1 mutation. Three patients with a normal karyotype had the FLT3-ITD mutation (21%).
Treatment and response
Data about treatment were available for 394 patients. Treatment consisted of NHL-like chemotherapy in 129 (32.8%) patients, ALL-like regimens in 57 (14.4%) patients, AML-like regimens in 36 (9.1%) patients, and NHL- or AL-type treatment, followed by allo-HSCT or auto-HSCT in 61 (15.5%) and 16 (4.1%) patients, respectively. Six (1.5%) patients received new agents (azacitidine, bortezomib, venetoclax, and SL-401 [a CD123-targeted therapy]), 27 (6.9%) patients were treated with radiotherapy, and 62 (15.7%) patients received only palliative care (Table 1).
NHL therapy included CHOP or CHOP-like regimens in 70% of cases and other NHL-like regimens in 30% of cases. ALL therapy included ALL-like, Aspa-MTX, and HCVAD in 47%, 30%, and 23% of cases, respectively. AML therapy included standard-dose cytarabine continuous infusion for 7 days + daunorubicin or idarubicin for 3 to 5 days in all cases.
Disease response was reported for 387 patients. It included 66% CRs and 12% PRs, whereas 22% progressed. Three hundred and one patients (76%) relapsed. The site of relapse was documented in 103 cases. Localization was limited to the skin in 21 cases (20%) and was disseminated in 82 cases (80%). Skin, BM, lymph nodes, and CNS was involved in 75%, 65%, 22%, and 17% of patients, respectively.
After a median follow-up of 12 months (range, 0.2-137), 151 patients (38%) were alive, and 247 (62%) had died. Causes of death were documented in 227 cases and were due to disease progression in 211 cases (93%).
The type of treatment that led to a relapse was documented in 89 cases: NHL-like (35%), leukemia-like (19%), palliative care (19%), NHL-like or AL-like + allo-HSCT (17%), radiotherapy (5%), ALL-like + auto-HSCT (3%), and new agents (2%).
Patients who underwent allo-HSCT had a median age of 50 years (range, 18-70). Disease was disseminated with cutaneous localization, disseminated noncutaneous, and cutaneous isolated in 60%, 20%, and 20% of cases, respectively. ALL therapy, AML therapy, or NHL therapy was provided in 53%, 27%, or 20% of cases, and first CR, PR, or progression disease (PD) was achieved before allo-HSCT in 94%, 3%, or 3% of cases, respectively. The relapse rate was 27% in this subgroup (Table 3).
Patient characteristics according to treatment
| . | Chemotherapy+ allo-HSCT (n = 61) . | Chemotherapy+ auto-HSCT (n = 16) . | Chemotherapy without consolidation (n = 222) . |
|---|---|---|---|
| Age, median (range), y | 50 (18-70) | 63 (19-68) | 68 (18-87) |
| Disseminated with cutaneous involvement | 37 (60) | 12 (75) | 133 (60) |
| Disseminated noncutaneous | 12 (20) | 1 (6) | 20 (9) |
| Cutaneous isolated | 12 (20) | 3 (19) | 69 (31) |
| ALL-type | 33 (53) | 6 (38) | 57 (26) |
| AML-type | 16 (27) | 1 (6) | 36 (16) |
| NHL-type | 12 (20) | 9 (56) | 129 (58) |
| Response to treatment | |||
| CR | 57 (94) | 16 (100) | 153 (69) |
| PR | 2 (3) | 0 | 31 (14) |
| PD | 2 (3) | 0 | 38 (17) |
| Relapse | 16/60 (27) | 5/16 (31) | 131/168 (78) |
| . | Chemotherapy+ allo-HSCT (n = 61) . | Chemotherapy+ auto-HSCT (n = 16) . | Chemotherapy without consolidation (n = 222) . |
|---|---|---|---|
| Age, median (range), y | 50 (18-70) | 63 (19-68) | 68 (18-87) |
| Disseminated with cutaneous involvement | 37 (60) | 12 (75) | 133 (60) |
| Disseminated noncutaneous | 12 (20) | 1 (6) | 20 (9) |
| Cutaneous isolated | 12 (20) | 3 (19) | 69 (31) |
| ALL-type | 33 (53) | 6 (38) | 57 (26) |
| AML-type | 16 (27) | 1 (6) | 36 (16) |
| NHL-type | 12 (20) | 9 (56) | 129 (58) |
| Response to treatment | |||
| CR | 57 (94) | 16 (100) | 153 (69) |
| PR | 2 (3) | 0 | 31 (14) |
| PD | 2 (3) | 0 | 38 (17) |
| Relapse | 16/60 (27) | 5/16 (31) | 131/168 (78) |
Unless otherwise noted, data are n (%). Patients treated with new drugs (n = 6), radiotherapy (n = 27), or palliative approaches (n = 62) were excluded.
Among the 33 patients who received induction ALL therapy followed by allo-HSCT, 94% achieved CR and 13% relapsed. Among the 16 patients who received AML therapy followed by allo-HSCT, 88% achieved CR and 58% relapsed.
Twelve patients received induction NHL therapy, followed by allo-HSCT; the CR and relapse rates were 100% and 33%.
Stem cell source was BM or peripheral blood in 76% or 12% of cases, respectively. The allogeneic transplant donor was a relative in 43% of patients, unrelated BM was used in 19% of patients, unrelated peripheral blood was used in 9% of patients, cord blood was used in 24% of patients, and haploidentical in 5% of patients. Among patients who underwent allo-HSCT, myeloablative conditioning or reduced-intensity conditioning regimens were used in 79% or 21% of patients, respectively.
Patients who underwent auto-HSCT (median age, 63 years; range, 19-68) were older than patients treated by allo-HSCT. Disease was disseminated with cutaneous involvement, disseminated noncutaneous, or cutaneous isolated in 75%, 6%, or 19% of cases, respectively. They received NHL therapy, ALL therapy, or AML therapy in 56%, 38%, or 6% of cases, respectively. Despite achieving first CR in 100% of cases before auto-HSCT, the relapse rate was 31%.
Patients treated with polychemotherapy, without allogeneic or autologous consolidation, had a median age of 68 years (range, 18-87). Disease was disseminated with cutaneous involvement, disseminated noncutaneous, or cutaneous isolated in 60%, 9%, or 31% of cases, respectively. They received NHL therapy, ALL therapy, or AML therapy in 58%, 26%, or 16% of cases. They achieved first CR, PR, or PD in 69%, 14%, or 17% of cases, respectively. The relapse rate was 78%.
The relapse rate was 72%, 91%, or 78% in patients treated with ALL-like, AML-like, or NHL-like chemotherapy without consolidation, respectively.
Median duration of treatment was 9 months (range, 0.5-136). It was 20 months (range, 1-136), 28 months (range, 7-79), 7 months (range, 1-46), 5 months (range, 0.7-33), 5 months (range, 0.5-31), or 5 months (range, 0.5-33) for NHL-type or AL-type treatment + allo-HSCT, NHL-type or AL-type treatment + auto-HSCT, AL-type treatment, NHL-type treatment, radiotherapy, or palliative treatment, respectively (P < .001, Kruskal-Wallis test).
Survival
After a median follow-up of 12 months (range, 0.2-137), the median OS was 18 months (95% CI, 15-22). OS probabilities at 24, 36, and 60 months were 39% (95% CI, 34.1-45), 27.5% (95% CI, 22.5-33.5), or 16.8% (95% CI, 12-23.5), respectively.
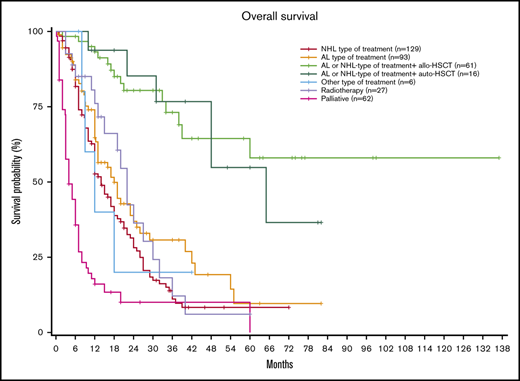
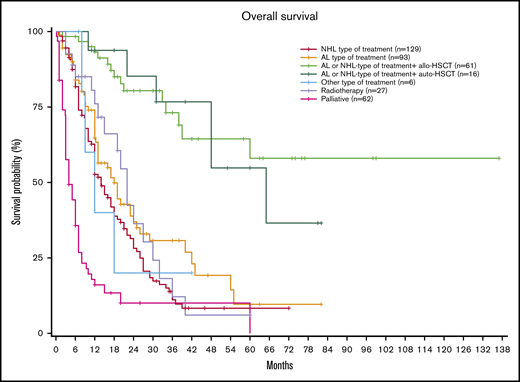
Interestingly, when stratifying based on the type of treatment, patients receiving NHL-type or leukemia-type treatments followed by allo-HSCT obtained the best results, with a median OS not reached (NR) (95% CI, 39 months to NR) vs 65 months (95% CI, 48 months to NR) for patients receiving chemotherapy followed by auto-HSCT, 18 months (95% CI, 13-25) for those receiving AL-type treatment, 14 months (95% CI, 12-18) for those receiving NHL-type treatment without consolidation, and 4 months (95% CI, 3-7) for those receiving palliative treatment (P < .001; Figure 1).
OS probability at 3 years was 72.4% (95% CI, 59-88) in patients receiving NHL-type or leukemia-type treatments followed by allo-HSCT, 76% (95% CI, 56-100) in patients receiving chemotherapy followed by auto-HSCT, 30.7% (95% CI, 21-45) for those receiving AL-type treatment, 11.1% (95% CI, 6.1-20) for those receiving NHL-type treatment without consolidation, and 10% for those receiving palliative treatment (95% CI, 4.1-24.5).
OS probability was 56.2% (95% CI, 39.3-80.4) at 5 years in patients receiving NHL-type or leukemia-type treatments followed by allo-HSCT, 54.2% (95% CI, 31.3-95.9) in patients receiving chemotherapy followed by auto-HSCT, 9.60% (95% CI, 2.9-31.3) for those receiving AL-type treatment, 8.3% (95% CI, 4.1-16.9) for those receiving NHL-type treatment without consolidation, and 0.0% for those receiving palliative treatment.
Within the allo-HSCT group, we evaluated OS based on the type of treatment received before transplant. Median OS was NR when using an NHL-like regimen (n = 12) (95% CI, 38 to not applicable [NA]) or an AML-like regimen (n = 16) (95% CI, 21 to NA), and it was 60 months (95% CI, 39 to NA) when using an ALL-like regimen (n = 33) (P = .587, log-rank test). The 3-year OS was 88.8% (95% CI, 70.5-100), 73.6% (95% CI, 55.3-97.9), and 59.5% (95% CI, 37.05-95.64) when using an NHL-like, ALL-like, or AML-like-regimen followed by allo-HSCT, respectively. The 5-year OS was 71.1% (95% CI, 43.3-100), 47.3% (95% CI, 23.4-95.5), or 59.5% (95% CI, 37.1-95.6) when using an NHL-like, ALL-like, or AML-like regimen followed by allo-HSCT, respectively.
Within the AL-type group, 17 patients were treated with Aspa-MTX (median age, 59 years; range, 35-82). The median OS and PFS were 18 months (95% CI, 12-NA) and 8.9 months (95% CI, 7.8-11), respectively.
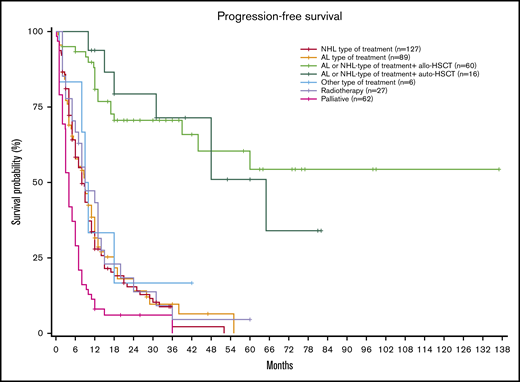
Median PFS for the entire population was 10 months (95% CI, 8.7-11). Median PFS was NR (95% CI, 44 to NR) in patients receiving chemotherapy followed by allo-HSCT vs 65 months (95% CI, 48 to NR) for patients receiving chemotherapy followed by auto-HSCT vs 9 months (95% CI, 6-12) for those receiving AL-type treatment vs 8 months (95% CI, 8.7-11) for those receiving NHL-type treatment without consolidation, and 4 months (95% CI, 3-6) for those receiving palliative treatment (P < .001; Figure 2). PFS probability at 3 years was 70.6%, 71.3%, 9.6%, 2.2%, and 0% respectively.
Presentation by country
The observations made in this study were also analyzed by country. There appeared to be a better survival rate in Japanese cases (P = .002), United States cases (P = .018), and Austrian cases (P = .024) as opposed to those in Italy (P = .676) and France (reference; P = 1.00), which reflected differences in age and type of treatment received. The median age was 61.5, 64.5, 65, and 67 years in Japan, the United States, Austria, and Italy, respectively, vs 70 years in France (P = .003).
The proportion of patients who received a leukemia-type chemotherapy + allo-HSCT was 21.7% in Japan, 23% in the United States, 21% in Austria, 9.4% in Italy, and 8.2% in France (P < .001).
Prognostic factors
In univariate analyses, 6 factors were found to have a significant impact on OS: age, ECOG score >1, peripheral involvement, disseminated disease with or without skin involvement, and disseminated disease with skin involvement. CD4+CD56+CD123+TCL1+BDCA2+ phenotype and palliative treatment had a negative impact on prognosis, whereas country of diagnosis, expression of TdT, and use of AL-like treatment with or without transplantation were associated with a better prognosis. There was no impact of sex, abnormal cytogenetics, or expression of CD34 and/or CD117 or BCL2 (Table 4).
Univariate and multivariate analysis of prognostic factors for OS
| Variable . | Modality . | Reference . | Univariate analysis . | Multivariate analysis . | ||||
|---|---|---|---|---|---|---|---|---|
| HR . | 95% CI . | P . | HR . | 95% CI . | P . | |||
| Age | Continuous variable | 1.05 | 1.04-1.06 | <.001 | 1.02 | 1.01-1.03 | <.001 | |
| ECOG (0-1) | Yes | No | 0.23 | 0.17-0.30 | <.001 | 0.44 | 0.29-0.66 | <.001 |
| Sex | Female | Male | 0.86 | 0.64-1.16 | .326 | |||
| Localization | Disseminated with or without cutaneous localization | Cutaneous isolated | 1.57 | 1.17-2.11 | .003 | 2.31 | 1.65-3.23 | <.001 |
| Disseminated with cutaneous localization | 2.09 | 1.30-3.35 | .002 | 5.37 | 3.15-9. | <.001 | ||
| Extranodal disease | 1.47 | 0.87-2.51 | .154 | 4.68 | 2.61-8.40 | <.001 | ||
| Abnormal karyotype | Yes | No | 0.78 | 0.47-1.32 | .360 | |||
| CK | 1.02 | 0.59-1.77 | .938 | |||||
| MK | 0.71 | 0.33-1.50 | .362 | |||||
| Type of treatment | AL-type treatment | NHL-type treatment | 0.71 | 0.51-0.99 | .047 | 0.88 | 0.59-1.32 | .547 |
| AL- or NHL-type treatment + allo-HSCT | 0.17 | 0.10-0.30 | <.001 | 0.21 | 0.12-0.39 | <.001 | ||
| AL- or NHL-type treatment + auto-HSCT | 0.20 | 0.09-0.046 | <.001 | 0.24 | 0.10-0.59 | .002 | ||
| Radiotherapy | 0.74 | 0.45-1.22 | .242 | 1.32 | 0.74-2.35 | .353 | ||
| Palliative | 2.96 | 2.10-4.16 | <.001 | 2.23 | 1.48-3.34 | <.001 | ||
| Immunophenotype | CD4+ CD56+ CD123+ TCL1+ BDCA2+ | No | 1.90 | 1.15-3.13 | .013 | |||
| TdT+ | No | 0.65 | 0.46-0.93 | .019 | ||||
| CD34+ and/or CD117+ | No | 1.19 | 0.65-2.17 | .573 | ||||
| BCL2+ | No | 2.75 | 0.55-13.73 | .218 | ||||
| Country | Austria | France | 0.59 | 0.37-0.93 | .024 | 0.67 | 0.42-1.07 | .092 |
| Italy | 0.92 | 0.64-1.34 | .676 | 0.94 | 0.62-1.42 | .752 | ||
| Japan | 0.58 | 0.41-0.83 | .002 | 0.66 | 0.45-0.97 | .032 | ||
| United States | 0.63 | 0.43-0.92 | .018 | 0.62 | 0.40-0.97 | .035 | ||
| Variable . | Modality . | Reference . | Univariate analysis . | Multivariate analysis . | ||||
|---|---|---|---|---|---|---|---|---|
| HR . | 95% CI . | P . | HR . | 95% CI . | P . | |||
| Age | Continuous variable | 1.05 | 1.04-1.06 | <.001 | 1.02 | 1.01-1.03 | <.001 | |
| ECOG (0-1) | Yes | No | 0.23 | 0.17-0.30 | <.001 | 0.44 | 0.29-0.66 | <.001 |
| Sex | Female | Male | 0.86 | 0.64-1.16 | .326 | |||
| Localization | Disseminated with or without cutaneous localization | Cutaneous isolated | 1.57 | 1.17-2.11 | .003 | 2.31 | 1.65-3.23 | <.001 |
| Disseminated with cutaneous localization | 2.09 | 1.30-3.35 | .002 | 5.37 | 3.15-9. | <.001 | ||
| Extranodal disease | 1.47 | 0.87-2.51 | .154 | 4.68 | 2.61-8.40 | <.001 | ||
| Abnormal karyotype | Yes | No | 0.78 | 0.47-1.32 | .360 | |||
| CK | 1.02 | 0.59-1.77 | .938 | |||||
| MK | 0.71 | 0.33-1.50 | .362 | |||||
| Type of treatment | AL-type treatment | NHL-type treatment | 0.71 | 0.51-0.99 | .047 | 0.88 | 0.59-1.32 | .547 |
| AL- or NHL-type treatment + allo-HSCT | 0.17 | 0.10-0.30 | <.001 | 0.21 | 0.12-0.39 | <.001 | ||
| AL- or NHL-type treatment + auto-HSCT | 0.20 | 0.09-0.046 | <.001 | 0.24 | 0.10-0.59 | .002 | ||
| Radiotherapy | 0.74 | 0.45-1.22 | .242 | 1.32 | 0.74-2.35 | .353 | ||
| Palliative | 2.96 | 2.10-4.16 | <.001 | 2.23 | 1.48-3.34 | <.001 | ||
| Immunophenotype | CD4+ CD56+ CD123+ TCL1+ BDCA2+ | No | 1.90 | 1.15-3.13 | .013 | |||
| TdT+ | No | 0.65 | 0.46-0.93 | .019 | ||||
| CD34+ and/or CD117+ | No | 1.19 | 0.65-2.17 | .573 | ||||
| BCL2+ | No | 2.75 | 0.55-13.73 | .218 | ||||
| Country | Austria | France | 0.59 | 0.37-0.93 | .024 | 0.67 | 0.42-1.07 | .092 |
| Italy | 0.92 | 0.64-1.34 | .676 | 0.94 | 0.62-1.42 | .752 | ||
| Japan | 0.58 | 0.41-0.83 | .002 | 0.66 | 0.45-0.97 | .032 | ||
| United States | 0.63 | 0.43-0.92 | .018 | 0.62 | 0.40-0.97 | .035 | ||
The multivariate analysis on OS showed a negative impact for age (hazard ratio [HR], 1.02; 95% CI, 1.01-1.03; P < .001), disseminated disease with or without skin involvement (HR, 2.31; 95% CI, 1.65-3.23; P < .001), disseminated disease with skin involvement (HR, 5.37; 95% CI, 3.15-9.17; P < .001), and extranodal disease (HR, 4.68; 95% CI, 2.61-8.40; P < .001). A positive impact was observed with allo-HSCT (HR, 0.21; 95% CI, 0.12-0.39; P < .001), auto-HSCT (HR, 0.24; 95% CI, 0.10-0.59; P = .002), and country (Japan: HR, 0.66; 95% CI, 0.45-0.97; P = .032 and United States: HR, 0.62; 95% CI, 0.40-0.97; P = .035).
The multivariate analysis on PFS showed a negative impact for age (HR, 1.01; 95% CI, 1.00-1.02; P = .019), disseminated disease with or without skin involvement (HR, 1.41; 95% CI, 1.05-1.89; P = .022), disseminated disease with skin involvement (HR, 2.56; 95% CI, 1.60-4.09; P < .001), and extranodal disease (HR, 1.96; 95% CI, 1.16-3.32; P = .012).
A positive impact was observed with allogeneic-HSCT (HR, 0.18; 95% CI, 0.10-0.31; P < .001) and auto-HSCT (HR, 0.19; 95% CI, 0.08-0.43; P < .001). Country of treatment had no impact on PFS. The role of the conditioning regimen and type of donor for allo-HSCT could not be analyzed because of the small sample size.
Discussion
This work represents the largest series describing the clinical and biological features and outcome of patients with BPDCN, which remains an aggressive malignancy usually associated with a short survival.22
Our data show that older age and disseminated disease at diagnosis have a negative impact on OS and PFS, whereas NHL/AL-type treatment followed by allo-HSCT or auto-HSCT has a positive impact on OS and PFS. In selected patients, the use of chemotherapy followed by allo-HSCT and, to a lesser extent, auto-HSCT, was associated with significantly better outcomes.8,9,12,13,23-26
On the other hand, in view of the retrospective nature of our study, we were not able to determine with absolute certainty the rationale for clinical decisions when allocating treatment strategy. However, it is plausible that autografted patients in our study were deemed ineligible for an allo-HSCT as a result of advanced age or associated comorbidities. Interestingly, those patients had better outcomes compared with those receiving a nontransplantation regimen, with 3-year OS and PFS of 76% and 71.3%, respectively. It is of note that patients who received auto-HSCT may have been selected for transplant, because all of them were in CR before transplant. All other treatments options had significantly lower efficacy. Acute leukemia–like chemotherapy seemed to be more effective compared with a lymphoma-like regimen, radiotherapy, or palliative care.
With regard to the role of HSCT in BPDCN therapy, several reports suggested better results in terms of enduring remissions and relapse rates with allo-HSCT compared with auto-HSCT. These studies demonstrated durable CRs with allo-HSCT, with OS rates ranging from 40% at 10 years to 58% at 3 years, depending on the follow-up period.8,12
In general, allo-HSCT consolidation seems to yield the best results when performed in first CR, with OS rates reaching 74% to 82% at 3 to 4 years.8,13 In the allo-HSCT settings, reduced-intensity conditioning was shown to be equivalent to myeloablative regimens in terms of relapse rates.8 Eligible patients should be considered for allo-HSCT consolidation in first CR whenever feasible. However, these patients represent the minority of BPDCN patients, because the disease normally affects elderly patients, with a median age of 68 years,9 which is also very close to the median age in our study (67 years), resulting in only 15.5% who received allo-HSCT. On the other hand, patients not eligible for transplant could benefit more from intensive induction regimens that are considered to be more effective compared with standard therapies (eg, CHOP-like).3 The inclusion of L-asparaginase in ALL-like regimens could be an alternative option in this setting, because it showed clinical activity in BPDCN in combination with single-agent methotrexate in previous studies25,27 and is confirmed in our series. However, we should be careful considering the risk of toxicity, especially in elderly patients, as reported in several series of AL.28-31
Thus, Pagano et al reported a therapeutic algorithm according to the age and the clinical condition of patients with BPDCN.32 They proposed to treat adults younger than 65 years of age and/or without comorbidities with newly diagnosed BPDCN with ALL-like chemotherapy and consolidation with allo-HSCT in first remission. They suggest reserving auto-HSCT for patients who are not eligible for allogeneic procedures or when a suitable donor is not available, in the early course of the disease.
In direct agreement with this, the SEER-18 registry reported 219 patients with BPDCN from the year 2008 onward and on active follow-up. The median OS declined with increasing age, consistent with prior reports suggesting better outcomes in pediatric patients.33
Previous studies reported that >60% of patients with BPDCN have an abnormal karyotype, primarily a complex karyotype, but specific chromosomal aberrations are lacking.20,21 In our study, cytogenetic analysis confirmed that most patients had unfavorable cytogenetics (54%), and one third of patients had a complex karyotype. However, cytogenetics had no impact on survival.
As reported in the literature, BPDCN patients do not express NPM1 mutations. Our range of cases also confirmed these data.34 It is relevant that 21% of our patients had FLT3-ITD mutations. The detection of this mutation is difficult to interpret, and the occurrence in BPDCN is well debated. No other clinical study has supported this observation, probably because the FLT3-ITD mutation was not searched for in large numbers of cases of BPDCN. On the other hand, the expression of FLT3-ITD and a previous myelodysplastic phase could suggest the myeloid origin of the neoplastic clone of BPDCN.35,36
As for the immunophenotype profile, TdT+ cases showed a lower median age at onset (63 years vs 69 years; P = .02, Mann-Whitney U test), as reported previously,37 and a greater, but not significant, frequency of isolated cutaneous lesions (40% vs 38%; P = .789, proportion test). TdT was predictive of survival in univariate analyses (HR, 0.65; 95% CI, 0.45-0.92, P = .016), as shown in previous studies.38,39
On the other hand, the expression of CD34 and/or CD117 (ambiguous lineage leukemia aspect) did not have any effect on survival (HR, 1.19; 95% CI, 0.65-2.17; P = .573),1 even if it was associated with disseminated disease in 85% of cases, as reported previously.4 However, the low number of positive cases tested (21 of 205; 10%) reminds us to consider these results with caution.
BCL2+ cases showed a higher, but nonsignificant, median age at onset. BCL2 expression also did not have any impact on survival (HR, 2.75; 95% CI, 0.55-13.73; P = .218), despite the fact that 50% of them were treated with a palliative approach, probably because of the low number of cases tested.
Multivariate analysis of OS revealed a negative impact of age (P < .001) and a positive impact of NHL-type or AL-type treatment followed by allo-HSCT (P < .001) or by auto-HSCT (P = .002), as discussed above.
We also identified a negative impact of disseminated disease (P < .001) and extranodal disease (P < .001), as reported in previous studies.40 In addition, there was a difference in survival rate depending upon where patients were treated (Japan, P = .032; United States, P = .035) that was related, in part, to younger age (P = .003) and higher transplantation rate (P < .001).
Recently, Taylor et al reported a longer median OS from diagnosis (24 months) in a series of 59 patients with BPDCN.26 In this study, 55% of patients received intensive chemotherapy, and 42% of patients underwent stem cell transplantation, a much higher percentage than in our series, in which only 15.5% of patients received allo-HSCT. Intensive first-line therapy and “lymphoid-type” chemotherapy regimens were associated with better outcomes, whereas age older than 60 years and TdT negativity in BPDCN cells were associated with poor outcome, as reported in our series. In this series, BPDCN with “skin only” involvement was not associated with better outcomes, contrary to what we reported. On the other hand, abnormal karyotype was associated with poor outcome, which we were not able to demonstrate.26
In view of the unsatisfactory results with low-intensity treatments and the decreased ability to use intensive therapies followed by allo-HSCT consolidation as a result of advanced age and comorbidities, there is strong rationale for the use of novel targeted agents for the treatment of BPDCN.
Tagraxofusp (SL-401), a novel recombinant protein including components of diphtheria toxin fused to interleukin-3, showed promising results in a phase 1 study.41
Pemmaraju et al19 recently reported the final results of their pivotal phase 2 study evaluating tagraxofusp in 47 patients with BPDCN. Among the 29 previously untreated patients, the primary outcome occurred in 72% of patients, and the overall response rate was 90%. These results were superior to our findings with a CR rate and OR rate of 66% and 78%, respectively. However, this comparison should be weighted, given the difference in the patients studied and the assessment of response criteria. Indeed, in our study, a complete response was defined as the disappearance of the disease in each initially involved site, whereas in the study by Pemmaraju et al, the primary outcome combined complete response and clinical complete response. The clinical complete response described patients who had complete response in all non-skin disease and had significant clearance of all skin lesions but still had residual skin abnormalities not indicative of active BPDCN.
Survival rates at 18 and 24 months were 59% and 52%, respectively. Capillary leak syndrome was an important toxic effect and occurred in 19% of patients with 2 deaths, leading the investigators to amend the protocol and to implement additional preventive measures.
These results led to US Food and Drug Administration approval of tagraxofusp in December 2018 for patients aged ≥2 years with BPDCN.19
Another promising and growing field of cellular therapy is the use of chimeric antigen receptor (CAR) T cells, with interesting results in AML and BPDCN.42 In the same settings, the use of the BCL2 inhibitor venetoclax has been successful in myeloid malignancies, either alone or in combination with hypomethylating agents. However, available evidence comes from small series, and its use is still limited in the case of BPDCN.43-47 Montero et al showed that primary BPDCN cells were dependent on the antiapoptotic protein BCL2 and demonstrated in vivo clinical activity of venetoclax in patient-derived xenografts and in 2 patients with relapsed chemotherapy-refractory BPDCN.45
Based on these findings, Pemmaraju et al initiated ongoing an phase 1 trial of venetoclax in patients with BPDCN (ClinicalTrials.gov number, NCT03485547).
Venetoclax was also investigated in combination with cytotoxic chemotherapy. Three patients received Hyper-CVAD plus venetoclax (frontline treatment in 1 patient and treatment of relapsed or refractory disease in 2 patients). All achieved CR with no major toxicity shown. Both patients with relapsed or refractory disease were successfully bridged to allogeneic stem cell transplantation, thus bringing new treatment opportunities.48
The same group initiated the ongoing phase 2 study of venetoclax in combination with hypomethylating agents in AML, high-risk MDS, and BPDCN (NCT03404193).
In conclusion, NHL- or AL-type therapy, followed by consolidation transplantation strategies, showed the best outcomes, with the superiority of allo-HSCT over auto-HSCT. The study was limited by its retrospective nature, the nonuniform response criteria for BPDCN, and heterogeneity of the treatments used.
In unfit patients, the use of novel agents could be a better option, because traditional chemotherapy and other treatments are still associated with poor results.
Data sharing requests should be sent to Kamel Laribi (klaribi@ch-lemans.fr).
Acknowledgments
The authors greatly appreciate the contributions of the many physicians and data managers who made this analysis possible, as well as the contributions made by the patients themselves.
Authorship
Contribution: T.P. and K.L. conceived, designed, directed, and supervised the study and revised the manuscript; A.B.d.M. collected the data; K.L. analyzed the data and wrote the first draft of the manuscript; M.S. analyzed the data, created the figures, and critically reviewed the manuscript; and all authors read, revised, and approved the final manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Kamel Laribi, Service Hématologie Clinique, Centre Hospitalier Le Mans, 194 Ave Rubillard, 72037 Le Mans, France; e-mail: klaribi@ch-lemans.fr.