Key Points
In this phase 3, open-label, randomized trial, azacitidine maintenance did not improve RFS after transplant in high-risk AML/MDS.
We believe that posttransplant maintenance strategy merits further study to decrease the risk of relapse in AML/MDS patients.
Abstract
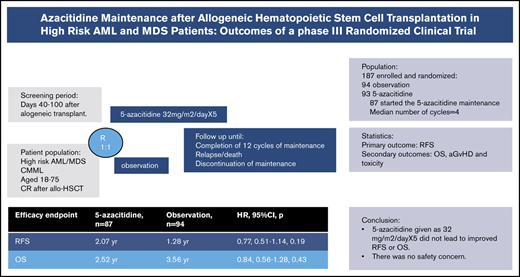
This study investigated the efficacy and safety of azacitidine maintenance in the posttransplant setting based on the encouraging phase 1/2 reports for azacitidine maintenance in patients with acute myeloid leukemia/myelodysplastic syndrome (AML/MDS). Between 2009 and 2017, a total of 187 patients aged 18 to 75 years were entered into a randomized controlled study of posttransplant azacitidine if they were in complete remission. Patients randomized to the treatment arm (n = 93) were scheduled to receive azacitidine, given as 32 mg/m2 per day subcutaneously for 5 days every 28 days for 12 cycles. The control arm (n = 94) had no intervention. Eighty-seven of the 93 patients started azacitidine maintenance. The median number of cycles received was 4; a total of 29 patients relapsed on study, and 23 patients withdrew from the study due to toxicity, patient’s preference, or logistical reasons. Median relapse-free survival (RFS) was 2.07 years in the azacitidine group vs 1.28 years in the control group (P = .43). There was also no significant difference for overall survival, with a median of 2.52 years vs 2.56 years in the azacitidine and control groups (P = .85), respectively. Multivariate Cox regression analysis revealed no improvement in RFS or overall survival with the use of azacitidine as maintenance compared with the control group (hazard ratios of 0.73 [95% confidence interval, 0.49-1.1; P = .14] and 0.84 [95% confidence interval, 0.55-1.29; P = .43]). This randomized trial with azacitidine maintenance showed that a prospective trial in the posttransplant setting was feasible and safe but challenging. Although RFS was comparable between the 2 arms, we believe the strategy of maintenance therapy merits further study with a goal to reduce the risk of relapse in patients with AML/MDS. This trial was registered at www.clinicaltrials.gov as #NCT00887068.
Introduction
Allogeneic hematopoietic stem cell transplantation (HSCT) is a potentially curative treatment for patients with myelodysplastic syndromes (MDS) or acute myeloid leukemia (AML). However, disease relapse accounts for ∼ 40% of treatment failures, with a preponderance of failures occurring in the first year after HSCT despite the significant therapeutic advances in HSCT over the last decade.1-3
The administration of posttransplant “maintenance” therapy, with the ability to either augment a graft-versus-leukemia effect or deliver direct antitumor activity after transplantation, represents a promising strategy to reduce the risk of disease relapse. There has long been an interest in identifying an effective and tolerable pharmacologic agent to be used as maintenance treatment to decrease the risk of relapse after HSCT. Low-dose azacitidine was the first to be investigated as posttransplant maintenance therapy, given its significant activity in AML/MDS4,5 and potential to promote hypomethylation even with low doses.6 Its potential to increase expression of epigenetically silenced leukemia antigens to induce a CD8+ T-cell response that might augment the graft-versus-leukemia effect7,8 and to reduce graft-versus-host disease (GVHD) through induction of immunomodulatory regulatory T cells9,10 made azacitidine a promising drug to be investigated as maintenance therapy.
We previously conducted a dose- and schedule-finding study of posttransplant azacitidine.11 We found that azacitidine, 32 mg/m2 given subcutaneously for 5 days, was safe and could be administered after allogeneic transplant for at least 4 cycles to heavily pretreated AML/MDS patients; 1‐year event‐free and overall survival (OS) rates were 58% and 77%, respectively. Those results justified a randomized clinical trial to determine if there was a clinical benefit of azacitidine given after hematopoietic transplantation in high-risk patients with AML/MDS. Herein, we report the outcomes of a randomized controlled study of posttransplant azacitidine for the prevention of AML and MDS relapse (ClinicalTrials.gov identifier #NCT00887068).
Methods
Patients
Patients 18 to 75 years of age who had AML or MDS and underwent HSCT were screened for the randomized clinical trial. Patients with AML were eligible for the trial if they had high-risk features, induction failure, relapsed disease, or were in a second remission or beyond at HSCT. Patients with AML in first complete remission (CR1) were eligible if they had high-risk features including chromosome 5 or 7 abnormalities or complex karyotype or FLT3 mutations. Patients with MDS had to have intermediate-1 or higher risk disease features according to the International Prognostic Scoring System12 to be eligible. Patients with therapy-related AML or MDS or biphenotypic acute leukemia were eligible.
Patients were screened and entered into the trial posttransplant if they achieved engraftment, were in morphologic CR by day 28 bone marrow aspiration, and met the following eligibility criteria: a bilirubin level <1.6 mg/dL, alanine aminotransferase levels <3 times the upper limit of normal range, creatinine <40% above the upper normal level, and absence of major coexisting transplant complications, including active GVHD or infections. CR was defined as a cellular marrow with <5% blasts, no Auer rods, no evidence of extramedullary leukemia, absence of circulating blasts, absolute neutrophil count ≥1.0 × 109/L, and platelet count ≥20 × 109/L.
Patients were allowed to enroll between 42 and 100 days post-HSCT. All patients provided written informed consent. The trial was reviewed and approved by the institutional review board of the University of Texas MD Anderson Cancer Center and performed in accordance with the principles of the Declaration of Helsinki and the International Conference on Harmonisation Good Clinical Practice guidelines.
Randomization and treatment
Enrolled patients were randomly assigned, in a 1:1 ratio, to 12 monthly cycles of azacitidine (at a dose of 32 mg/m2 per day, administered by subcutaneous injections for 5 days every 4 weeks) or to observation (no further treatment) (Figure 1). For randomization, the Pocock-Simon13 method was used to balance the randomization on (AML, MDS, biphenotypic leukemia). For initiation of the first cycle of azacitidine, patients were required to have a platelet count >20 × 109/L without transfusion for at least 2 days and an absolute neutrophil count >1.5 × 109/L without growth factor for at least 2 days. For subsequent azacitidine cycles to start, these criteria were >20 × 109/L and 1 × 109/L for platelets and absolute neutrophil count, respectively. The trial allowed the treatment within a cycle to be delayed for up to 4 weeks due to organ toxicity or hematologic toxicity. All patients routinely had bone marrow evaluation 1 month, 3 months, 6 months, and 1 year after allogeneic stem cell transplantation per departmental guidelines to monitor disease status. Additional bone marrow examinations were performed if clinically indicated for suspected relapse.
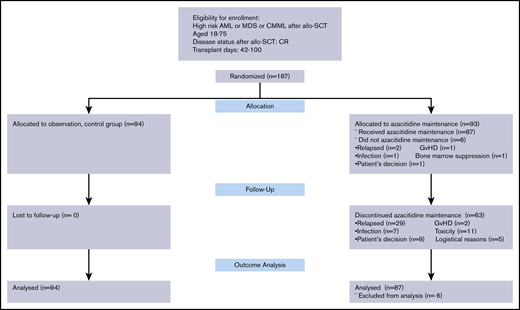
Of 93 patients randomized to azacitidine maintenance, 87 started the treatment. The plan was to complete 12 cycles of maintenance treatment for patients randomized to azacitidine maintenance. Patients randomized to the control arm (n = 94) did not receive any intervention. allo-SCT, allogeneic stem cell transplantation; CMML, chronic myelomonocytic leukemia.
Of 93 patients randomized to azacitidine maintenance, 87 started the treatment. The plan was to complete 12 cycles of maintenance treatment for patients randomized to azacitidine maintenance. Patients randomized to the control arm (n = 94) did not receive any intervention. allo-SCT, allogeneic stem cell transplantation; CMML, chronic myelomonocytic leukemia.
Statistical analysis, criteria of response, and evaluation of outcome
The primary outcome of this study was relapse-free survival (RFS), defined as time from HSCT to relapse or death from any cause. The planned sample size required to achieve the necessary numbers of events required by the group sequential tests was between 213 and 246. The goal was to test the hypothesis that azacitidine provided at least a 50% improvement in median RFS from 6 to 9 months. A 2-sided group sequential design was used with an overall type I error of 0.05 and power of 0.80 to detect the alternative median = 9 months, with up to 2 interim tests and one final test, including both outer bounds for superiority and inner bounds for futility. Secondary outcomes included OS, incidence of acute GVHD (aGVHD), and toxicity. Additional outcome analyses, including incidence of relapse, transplant-related mortality, and chronic GVHD, that were not planned at design were also performed. Efficacy analyses did not include the 6 patients who were randomized to receive azacitidine maintenance but did not start the treatment.
The cumulative incidence of aGVHD was assessed in a competing risks framework based on the events disease relapse and death without relapse. Patients who had not experienced either of these events by day 100 were censored at day 100 after transplant. Distributions were compared between treatment arms by using the Fine and Gray method.14 Cox models with covariate adjustment were built for RFS and OS, respectively. The variables included in the model were treatment groups and well-established disease and transplant-related risk factors: disease type, cytogenetics risk group, disease status at transplant, hematopoietic stem cell source, and presence of previous allogeneic transplant. All statistical analyses were performed by using R version 3.4.3. All statistical tests used a significance level of 5%. No adjustments for multiple testing were made.
Results
From April 2009 through January 2017, total of 187 patients were enrolled in the trial and randomized to a study arm. Reasons for screening failures were not addressed prospectively until February 2011. From February 2011 through April 2017, the data of 561 screened patients were collected. The most common reasons for nonenrollment, after failing eligibility (233 of 561 [41%]), were lack of interest of the patient, typically due to concerns about receiving an additional year of chemotherapy (129 of 561 [23%]), followed by concerns of the treating physician (n = 84/561 [15%]) (Table 1). There were 62 patients who refused study entry but received azacitidine as maintenance in the posttransplant setting, off the clinical trial, during the study period.
Causes of screen failures (February 2011 through April 2017), N = 561
| Cause . | n/N . | % . |
|---|---|---|
| Failed eligibility criteria | 233/561 | 41 |
| Multiple comorbidity | 135/233 | |
| Thrombocytopenia | 32/233 | |
| Neutropenia | 3/233 | |
| Pancytopenia | 15/233 | |
| Relapsed or graft failure | 29/233 | |
| Secondary malignancy | 5/233 | |
| Other | 14/233 | |
| Patient was not interested | 129/561 | 23 |
| Physician was not interested (some received AZA off-study) | 84/561 | 15 |
| Detectable minimal residual disease after transplant (received AZA off-study) | 26/561 | 5 |
| No clinical trial benefit | 28/561 | 5 |
| Outside physician refused | 10/561 | 2 |
| On competing IND study | 10/561 | 2 |
| Cause . | n/N . | % . |
|---|---|---|
| Failed eligibility criteria | 233/561 | 41 |
| Multiple comorbidity | 135/233 | |
| Thrombocytopenia | 32/233 | |
| Neutropenia | 3/233 | |
| Pancytopenia | 15/233 | |
| Relapsed or graft failure | 29/233 | |
| Secondary malignancy | 5/233 | |
| Other | 14/233 | |
| Patient was not interested | 129/561 | 23 |
| Physician was not interested (some received AZA off-study) | 84/561 | 15 |
| Detectable minimal residual disease after transplant (received AZA off-study) | 26/561 | 5 |
| No clinical trial benefit | 28/561 | 5 |
| Outside physician refused | 10/561 | 2 |
| On competing IND study | 10/561 | 2 |
AZA, azacitidine; IND, investigational new drug.
Of 187 patients enrolled in the study, 93 were randomized to the intervention arm with azacitidine maintenance, and 87 of these started the first cycle of azacitidine. The remaining 94 patients were randomized to the control arm and did not receive maintenance treatment. Patient age, cytogenetic risk classification, disease status at HSCT, donor type, hematopoietic stem cell source, and conditioning intensity were well balanced between the azacitidine and control arms. There were more patients with a hematopoietic comorbidity index15 ≥4 in the azacitidine arm compared with the control subjects (42.5% vs 21.3%) (Table 2).
Characteristic of comparison groups
| Characteristic . | Observation (n = 94) . | AZA maintenance (n = 87) . |
|---|---|---|
| Age, median (range), y | 57.5 (20-75) | 57 (19-72) |
| Male sex, n (%) | 57 (60.6) | 51 (58.6) |
| MDS, n (%) | 25 (26.6) | 22 (25.3) |
| Cytogenetics, n (%) | ||
| Good risk | 15 (16) | 8 (9.2) |
| Intermediate risk | 42 (44.7) | 33 (37.9) |
| Poor risk | 37 (39.3) | 46 (52.9) |
| Disease status at HSCT, AML, n (%) | ||
| CR1/CR2 with count recovery | 23 (33.3) | 33 (50.8) |
| CR without count recovery | 7 (10.2) | 17 (26.2) |
| Active disease | 39 (56.5) | 15 (23) |
| Disease status at HSCT, MDS, n (%) | ||
| CR | 6 (24) | 4 (18.2) |
| Active disease | 19 (76) | 18 (81.8) |
| Hematopoietic stem cell source, n (%) | ||
| Bone marrow | 32 (34) | 31 (35.6) |
| PBSC | 60 (63.8) | 55 (63.2) |
| Cord blood | 2 (2.2) | 1 (1.2) |
| Donor type, n (%) | ||
| Matched related | 31 (33) | 33 (37.9) |
| Matched unrelated | 53 (56.4) | 44 (50.6) |
| Haploidentical | 5 (5.3) | 4 (4.6) |
| Reduced intensity conditioning | 18 (19.1) | 14 (16.1) |
| GVHD prophylaxis, n (%) | ||
| PTCy | 9 (9.5) | 4 (4.5) |
| Tacro/MTX | 82 (87.2) | 82 (94.2) |
| Tacro/MMF | 3 (3.2) | 1 (1.1) |
| HCT-CI, n (%) | ||
| 0-1 | 37 (39.4) | 28 (32.2) |
| 2-3 | 37 (39.4) | 22 (25.3) |
| ≥4 | 20 (21.3) | 37 (42.5) |
| Characteristic . | Observation (n = 94) . | AZA maintenance (n = 87) . |
|---|---|---|
| Age, median (range), y | 57.5 (20-75) | 57 (19-72) |
| Male sex, n (%) | 57 (60.6) | 51 (58.6) |
| MDS, n (%) | 25 (26.6) | 22 (25.3) |
| Cytogenetics, n (%) | ||
| Good risk | 15 (16) | 8 (9.2) |
| Intermediate risk | 42 (44.7) | 33 (37.9) |
| Poor risk | 37 (39.3) | 46 (52.9) |
| Disease status at HSCT, AML, n (%) | ||
| CR1/CR2 with count recovery | 23 (33.3) | 33 (50.8) |
| CR without count recovery | 7 (10.2) | 17 (26.2) |
| Active disease | 39 (56.5) | 15 (23) |
| Disease status at HSCT, MDS, n (%) | ||
| CR | 6 (24) | 4 (18.2) |
| Active disease | 19 (76) | 18 (81.8) |
| Hematopoietic stem cell source, n (%) | ||
| Bone marrow | 32 (34) | 31 (35.6) |
| PBSC | 60 (63.8) | 55 (63.2) |
| Cord blood | 2 (2.2) | 1 (1.2) |
| Donor type, n (%) | ||
| Matched related | 31 (33) | 33 (37.9) |
| Matched unrelated | 53 (56.4) | 44 (50.6) |
| Haploidentical | 5 (5.3) | 4 (4.6) |
| Reduced intensity conditioning | 18 (19.1) | 14 (16.1) |
| GVHD prophylaxis, n (%) | ||
| PTCy | 9 (9.5) | 4 (4.5) |
| Tacro/MTX | 82 (87.2) | 82 (94.2) |
| Tacro/MMF | 3 (3.2) | 1 (1.1) |
| HCT-CI, n (%) | ||
| 0-1 | 37 (39.4) | 28 (32.2) |
| 2-3 | 37 (39.4) | 22 (25.3) |
| ≥4 | 20 (21.3) | 37 (42.5) |
HCT-CI, hematopoietic comorbidity index; MTX, methotrexate; MMF, mycophenolate mofetil; PBSC, peripheral blood stem cell; PTCy, posttransplant cyclophosphamide.
The median time to enrollment in the trial was 54 days after transplant, and median time to first cycle of azacitidine was 62 days (range, 42-100 days). The median time to enrollment after the first bone marrow biopsy confirming the disease status as CR after transplant was 28 days (range, 7-69 days). A second cycle of azacitidine was administered in 71 of 87 patients. The median time between the first and second cycles was 28 days, as dictated by the protocol. The median number of azacitidine treatment cycles was 4. Twenty-four (27.6%) of the 87 patients completed the planned 12 cycles of azacitidine. A total of 63 patients were taken off the study for the following reasons: disease relapse (n = 29 [47%]), toxicity (n = 11 [18%]), patient’s preference not to continue (n = 9 [15%]), infection (n = 7 [11%]), logistical reasons (n = 5 [8%]), and GVHD (n = 2 [4%]).
Median RFS was not improved with azacitidine maintenance compared with observation
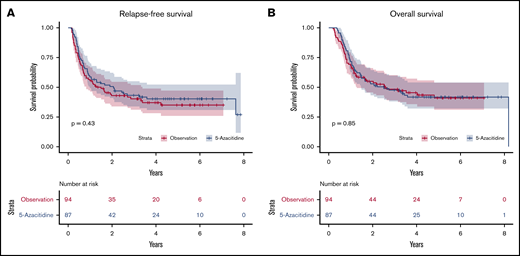
Among the 80 patients who survived, the median follow-up was 4.6 years in the azacitidine arm and 4.06 years in the control arm. Median RFS times were not significantly different between the groups: 2.07 years in the azacitidine arm and 1.28 years in the control arm (P = .43) (Figure 2A). Comparative analyses in different disease risk subgroups showed no benefit of azacitidine maintenance after transplant (data not shown). There was also no significant difference in OS, with a median of 2.52 vs 2.56 years in the azacitidine group (P = .85) (Figure 2B) and control group (HR, 0.96; 95% CI, 0.65-1.4; P = .85), respectively.
Relapse free and overall survial. The use of subcutaneous 5-azacitidine as posttransplant maintenance strategy was not associated with improved relapse-free survival (A) and overall survival (B) compared with observation arm.
Relapse free and overall survial. The use of subcutaneous 5-azacitidine as posttransplant maintenance strategy was not associated with improved relapse-free survival (A) and overall survival (B) compared with observation arm.
Cox regression analyses were performed both for RFS and OS. The use of azacitidine as posttransplant maintenance improved neither RFS nor OS compared with observation. The only significant factor that improved RFS and OS was transplant in CR compared with active disease (HR of 0.48 [95% CI, –0.3 to 0.75; P = .001] and HR of 0.53 [95% CI, 0.33 to 0.84; P = .007], respectively) (Table 3).
Multivariate regression for RFS and OS
| Variable . | n . | RFS . | OS . | ||||
|---|---|---|---|---|---|---|---|
| HR . | 95% CI . | P . | HR . | 95% CI . | P . | ||
| Observation | 94 | Ref | |||||
| AZA maintenance | 87 | 0.73 | 0.49-1.10 | .14 | 0.84 | 0.55-1.29 | .43 |
| AML | 134 | Ref | Ref | ||||
| MDS | 47 | 0.38 | 0.22-0.67 | <.001 | 0.39 | 0.22-0.71 | .002 |
| Cytogenetic risk groups | |||||||
| Good | 23 | Ref. | Ref | ||||
| Intermediate | 75 | 1.97 | 0.84-4.66 | .12 | 1.5 | 0.63-3.57 | .36 |
| Poor | 83 | 2.43 | 1.06-5.61 | .04 | 2.13 | 0.92-4.9 | .08 |
| Active disease | 115 | Ref | Ref | ||||
| Complete remission* | 66 | 0.48 | 0.3-0.75 | .001 | 0.53 | 0.33-0.84 | .007 |
| First allo-SCT | 166 | Ref | Ref. | ||||
| Second allo-SCT | 15 | 0.93 | 0.45-1.90 | .83 | 1.08 | 0.52-2.24 | .84 |
| Myeloablative conditioning | 149 | Ref | Ref | ||||
| Reduced intensity conditioning | 32 | 1.0 | 0.58-1.75 | .99 | 1.0 | 0.56-1.76 | .99 |
| Hematopoietic stem cell source | |||||||
| PBSC | 115 | Ref | Ref | ||||
| Cord blood unit | 3 | 3.72 | 0.95-14.62 | .06 | 7.59 | 1.81-31.8 | .006 |
| Bone marrow | 63 | 1.53 | 0.95-2.48 | .08 | 1.26 | 0.76-2.09 | .37 |
| HCT-CI <4 | 124 | Ref | Ref | ||||
| HCT-CI >3 | 57 | 1.37 | 0.9-2.09 | .14 | 1.44 | 0.93-2.24 | .10 |
| Variable . | n . | RFS . | OS . | ||||
|---|---|---|---|---|---|---|---|
| HR . | 95% CI . | P . | HR . | 95% CI . | P . | ||
| Observation | 94 | Ref | |||||
| AZA maintenance | 87 | 0.73 | 0.49-1.10 | .14 | 0.84 | 0.55-1.29 | .43 |
| AML | 134 | Ref | Ref | ||||
| MDS | 47 | 0.38 | 0.22-0.67 | <.001 | 0.39 | 0.22-0.71 | .002 |
| Cytogenetic risk groups | |||||||
| Good | 23 | Ref. | Ref | ||||
| Intermediate | 75 | 1.97 | 0.84-4.66 | .12 | 1.5 | 0.63-3.57 | .36 |
| Poor | 83 | 2.43 | 1.06-5.61 | .04 | 2.13 | 0.92-4.9 | .08 |
| Active disease | 115 | Ref | Ref | ||||
| Complete remission* | 66 | 0.48 | 0.3-0.75 | .001 | 0.53 | 0.33-0.84 | .007 |
| First allo-SCT | 166 | Ref | Ref. | ||||
| Second allo-SCT | 15 | 0.93 | 0.45-1.90 | .83 | 1.08 | 0.52-2.24 | .84 |
| Myeloablative conditioning | 149 | Ref | Ref | ||||
| Reduced intensity conditioning | 32 | 1.0 | 0.58-1.75 | .99 | 1.0 | 0.56-1.76 | .99 |
| Hematopoietic stem cell source | |||||||
| PBSC | 115 | Ref | Ref | ||||
| Cord blood unit | 3 | 3.72 | 0.95-14.62 | .06 | 7.59 | 1.81-31.8 | .006 |
| Bone marrow | 63 | 1.53 | 0.95-2.48 | .08 | 1.26 | 0.76-2.09 | .37 |
| HCT-CI <4 | 124 | Ref | Ref | ||||
| HCT-CI >3 | 57 | 1.37 | 0.9-2.09 | .14 | 1.44 | 0.93-2.24 | .10 |
Only patients in CR with count recovery were included in this group.
The risk of relapse did not improve with azacitidine posttransplant maintenance
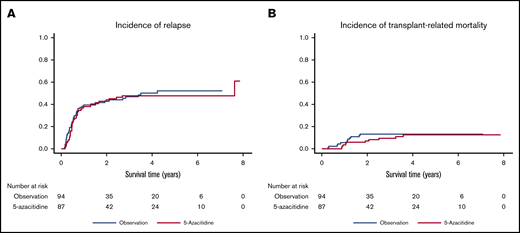
The azacitidine maintenance and control arms had a very similar cumulative incidence of relapse, with a 1-year incidence of 41% (95% CI, 31-51) vs 39% (95% CI, 29-49), respectively (Figure 3A). Transplant-related mortality incidence was low in both groups, with 1-year transplant-related mortality of 4.3% (95% CI, 1.3-9.9) in the azacitidine group compared with 5.3% (95% CI, 2-11.2) in the control group (Figure 3B).
Relapse and transplant-related mortality incidences. In the randomized, prospective trial, subcutaneous 5-azacitidine did not lead to decreased risk of relapse after transplant in AML/MDS patients (A) but also did not increase transplant-related mortality (B) compared with control group.
Relapse and transplant-related mortality incidences. In the randomized, prospective trial, subcutaneous 5-azacitidine did not lead to decreased risk of relapse after transplant in AML/MDS patients (A) but also did not increase transplant-related mortality (B) compared with control group.
GVHD incidences were comparable between comparison groups
The incidence of aGVHD, grades 2 to 4 and grades 3 to 4, was also similar between study arms. At day 100, the cumulative incidence of grade 2 to 4 aGVHD was 25.5% with azacitidine (95% CI, 16.7-34.4) and 28.7% with observation (95% CI, 19.2-38.3) (P = .73). The incidence of grade 3 to 4 aGVHD was low in both groups: 4.3% (95% CI, 1.4-9.9) with azacitidine and 2.1% (95% CI, 0.4-6.8) with observation. The incidence of chronic GVHD did not exhibit any difference, with a 1-year incidence of 25.8% (95% CI, 17.4-35.1) with azacitidine and 30.8% (95% CI, 21.8-40) with observation.
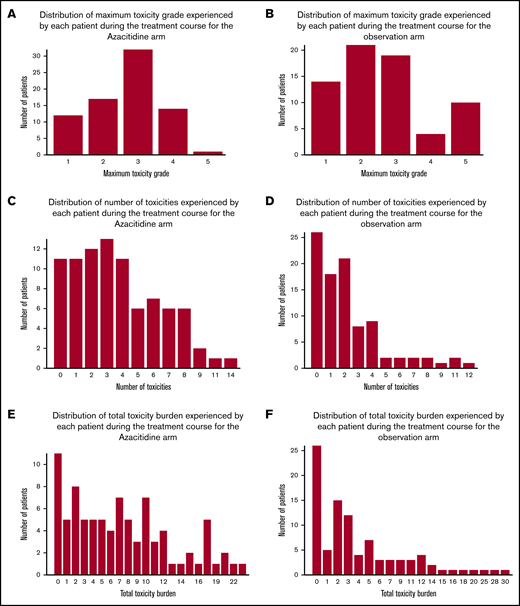
Toxicity
No unexpected adverse events (AEs) were observed; AEs that are typically associated with hematopoietic stem cell transplantation and with chemotherapy administration were noted. Of 87 patients in the azacitidine arm, 76 (87.4%) experienced at least 1 AE during the study period, and the median number of AEs was 3. Of 94 control patients, 68 (72.3%) had at least 1 AE, and the median number of events per patient was 2 (Figure 4A-B). The total number of all grades of AEs is presented in Figure 4C-D. Defining total toxicity burden as the sum of the all grades of all toxicities experienced by a patient, the mean total toxicity burden was 7.3 in the azacitidine arm compared with 4.8 in the control arm (Figure 4E-F).
Distribution of AEs. The distribution of maximum grade of AEs by each patient on the azacitidine arm (A) and the control arm (B). The distribution of number of AEs by each patient on the azacitidine arm (C) and the control arm (D). The distribution of total toxicity burden by each patient on the azacitidine arm (E) and the control arm (F).
Distribution of AEs. The distribution of maximum grade of AEs by each patient on the azacitidine arm (A) and the control arm (B). The distribution of number of AEs by each patient on the azacitidine arm (C) and the control arm (D). The distribution of total toxicity burden by each patient on the azacitidine arm (E) and the control arm (F).
In the control arm, 26 (27.7%) of 94 patients experienced no toxicities compared with 11(12.6%) of 87 patients in the azacitidine arm. There were 91 grade 3 or higher AEs observed in the azacitidine arm; 79 (87%) of these were attributed to the study drug. Of the 91 grade 3 or higher AEs, 58 were related to bone marrow suppression (64%), 13 to infection (16%), and 9 to liver toxicity (10%) (Table 4). There was only one grade 5 toxicity in the azacitidine arm, which was pulmonary and associated with aspiration pneumonia. No graft failures were observed in the azacitidine arm. It is notable that a substantial number of AEs occurred in the control arm (ie, no maintenance treatment was received) and were related to typical posttransplant complications. Myelosuppression was the only toxicity clearly related to the azacitidine treatment.
Summary of grade 3, 4, and 5 AEs
| AE . | Azacitidine arm* . | Observation arm . | ||
|---|---|---|---|---|
| Any grade (no. of events), n = 302 . | Grade 3-5 (no. of events)† . | Any grade (no. of events, n = 215 . | Grade 3-5 (no. of events) . | |
| Hematologic | 143 | 58 | 5 | 5 |
| Thrombocytopenia | 119 | 29 | 1 | 1 |
| Poor graft function | 33 | 29 | 2 | 2 |
| Nonhematologic | 159 | 33 | 210 | 56 |
| Infection | 42 | 13 | 52 | 19 |
| Gastrointestinal | 41 | 0 | 44 | 12 |
| Hepatic | 17 | 9 | 19 | 5 |
| Pulmonary | 7 | 4 | 8 | 6 |
| Skin | 27 | 2 | 32 | 5 |
| AE . | Azacitidine arm* . | Observation arm . | ||
|---|---|---|---|---|
| Any grade (no. of events), n = 302 . | Grade 3-5 (no. of events)† . | Any grade (no. of events, n = 215 . | Grade 3-5 (no. of events) . | |
| Hematologic | 143 | 58 | 5 | 5 |
| Thrombocytopenia | 119 | 29 | 1 | 1 |
| Poor graft function | 33 | 29 | 2 | 2 |
| Nonhematologic | 159 | 33 | 210 | 56 |
| Infection | 42 | 13 | 52 | 19 |
| Gastrointestinal | 41 | 0 | 44 | 12 |
| Hepatic | 17 | 9 | 19 | 5 |
| Pulmonary | 7 | 4 | 8 | 6 |
| Skin | 27 | 2 | 32 | 5 |
There were 325 any grade AEs in the azacitidine arm, but 302 were related to the azacitidine intervention.
There was no grade 5 hematologic toxicity in the azacitidine arm. There was only one grade 5 toxicity, which was pulmonary and associated with aspiration pneumonia.
Discussion
This phase 3, open-label, randomized trial is, to the best of our knowledge, the first of its kind in the posttransplant setting. Azacitidine maintenance, in the dose and schedule chosen, failed to significantly improve RFS in high-risk adult AML and MDS patients who underwent HSCT compared with control subjects. There was also no improvement in secondary endpoints, including OS, risk of relapse, or incidence of GVHD.
The study found no improvement in RFS despite the encouraging results of phase 2 studies of hypomethylating agents (HMAs) as a maintenance strategy after HSCT. We and others had previously reported 57% to 58% event-free survival with subcutaneous azacitidine maintenance in high-risk patients with AML/MDS.16 Other prospective phase 2 dose-finding studies with oral azacitidine17 or subcutaneous decitabine18 also suggested improved transplant outcomes. These observations were the basis for the current study to be conducted with azacitidine.
We designed the study to improve median RFS. The goal was to improve median RFS from 6 months to 9 months with the pharmacologic intervention. However, the median RFS was substantially longer in this study than predicted at design. The control arm had a median RFS of 1.28 years, and the median RFS with azacitidine was 2.07 years. These observations are noteworthy that the observed absolute improvement with azacitidine compared with control subjects (2.07 years vs 1.28 years) was higher than the 3 months’ improvement we predicted at design. However, the power to detect a statistically significant difference was lost due to less-than-expected events observed in the control arm. We need to compare our results in the control arm vs those of other large trials on high-risk AML or MDS posttransplant patients to be able to comprehend the observations. Retrospective studies of AML patients with a complex karyotype or other adverse risk cytogenetics showed a cumulative incidence of relapse as high as 48% to 55% with a median RFS of <1 year.19-21 High-risk patients with MDS reportedly have poor transplant outcomes, with 1-year RFS <30%.22,23 A possible explanation for the unexpectedly better outcomes observed in our control arm is the eligibility criteria used for enrollment. We used classification schemas available at the onset of the study in 2008, which lacked many of the recently documented prognostic factors such as pretransplant minimal residual disease assessment and mutations detected by using next-generation sequencing.
This study has several limitations, the most important being the slow accrual and failure of many azacitidine patients to complete 12 cycles of planned treatment: 7.5 years were needed to enroll 187 high-risk AML/MDS patients, and the study was closed due to slow accrual. Approximately one-half of the screened patients did not enroll due to concerns regarding the commitment for 1 year of additional chemotherapy posttransplant. With greater interest by the field in maintenance treatment strategies, patients may be more willing to participate in future studies. Failing eligibility criteria was another major reason of screen failure (41%), highlighting the difficulty of enrollment into clinical trials after HSCT due to multiple posttransplant complications. After enrollment and initiation of azacitidine, only 24 (27.6%) of 87 patients received the 12 cycles of maintenance treatment as planned, and the median number of treatment cycles was 4. The major reason for early discontinuation was relapse in nearly one-half of the patients, and 40% of patients withdrew from the study due to toxicity, infections, patient’s preference, emotional fatigue, or logistical reasons, highlighting the challenges in conducting prolonged studies of posttransplant therapies in patients with multiple coexisting medical and psychosocial issues. Most of the grade 3 or higher AEs were related to reversible bone marrow suppression without graft failure. As the initial randomized study of maintenance therapy, patients and physicians may have been overly cautious regarding continuing treatment.
The dose and schedule of azacitidine must be considered when interpreting these results and the potential role of posttransplant maintenance therapies for AML/MDS. Monotherapy with HMAs has been reported to induce CR plus CR with incomplete count recovery (CRi) of ∼15% to 30%, with a median OS of <12 months in the older AML population.24,25 Similarly, in high-risk MDS, azacitidine was associated with a CR rate of 7% to 17%. The dose of azacitidine used in this study (32 mg/m2 subcutaneously daily for 5 days) is substantially lower than the standard dose used outside the transplant setting, owing to the limited hematologic reserve of patients after hematopoietic transplantation. It is possible that a higher dose or different schedule might provide improved results.
In the nontransplant setting, a randomized phase 3 study (HOVON97)26 of older patients (aged ≥60 years) with AML or MDS with refractory anemia with excess of blasts was recently reported; patients were administered azacitidine given subcutaneously as 50 mg/m2 daily after achieving CR, and significant improvements in RFS were reported. Oral azacitidine, which has been developed to simplify azacitidine administration and dose adaptation, has been investigated as a maintenance study (QUAZAR) in patients with AML after first CR or CRi following intensive chemotherapy.27 The primary results were also suggestive of improved RFS and OS. Those results indicate that maintenance therapy remains a promising strategy for patients with AML. Combination regimens may also prove superior to single agents for maintenance after HST. The combination of azacitidine or decitabine with venetoclax showed significantly better activity in the up-front treatment setting of AML, compared with an HMA alone, with a CR/CRi rate of 71% to 74%.28,29 Indeed, we are investigating the safety and efficacy of venetoclax with low-dose subcutaneous azacitidine in an ongoing maintenance trial at our institution (ClinicalTrials.gov identifier #NCT04128501).
In conclusion, this study provides the first prospective evidence for the feasibility and safety of azacitidine maintenance after HSCT in patients with AML and MDS. Although we failed to confirm the efficacy of azacitidine with the applied dose and schedule, HMAs have the potential to be an important part of the armamentarium for myeloid malignancies to decrease risk of relapse after HSCT. More importantly, despite encouraging previous phase 2 studies, our study showed that monthly courses of single-agent azacitidine, 32 mg/m2 daily for 5 days, might not be enough to improve transplant outcomes. This finding indicates the importance of effectively conducting randomized trials of posttransplant maintenance strategies to establish therapeutic benefit. These studies need to incorporate consideration of all factors influencing the risk of relapse, including recent cytogenetic, genomic, and measurable residual disease information. Only then can we improve our capability to deliver practice‐changing, outcome-improving posttransplant therapies.
Presented in oral form at the 60th annual meeting of the American Society of Hematology, San Diego, CA, 1-4 December 2018.
Requests for data sharing may be submitted to the corresponding author (Betül Oran; e-mail: boran@mdanderson.org).
Acknowledgment
This study was funded by Celgene Pharmaceuticals.
Authorship
Contribution: B.O., M.d.L., and R.E.C. designed the research, performed the research, analyzed the data, and wrote the paper; P.F.T. and R.L. designed the research, contributed analytical tools, analyzed the data, and wrote the paper; and G.G.-M., U.P., A.M.A., C.H., S.G., G.R., and G.W. performed the research, analyzed the data, and wrote the paper.
Conflict-of-interest disclosure: B.O. reports research funding from AROG Pharmaceuticals and Astex Pharmaceuticals (all on maintenance trials) and a consulting role at Celgene. The remaining authors declare no competing financial interests.
Correspondence: Betül Oran, Department of Stem Cell Transplantation and Cellular Therapy, University of Texas MD Anderson Cancer Center, 1515 Holcombe Blvd, Unit 423, Houston, TX 77005; e-mail: boran@mdanderson.org.