TO THE EDITOR:
Maintenance therapy in acute myeloid leukemia (AML) after induction and consolidation chemotherapy has been studied for more than 2 decades.1 No randomized trial had shown an overall survival (OS) benefit compared with observation until the recent QUAZAR AML-001 trial of oral azacitidine.2
The QUAZAR AML-001 trial randomized 472 patients with transplant-ineligible AML ≥ 55 years of age, who were in complete remission (CR) or CR with incomplete count recovery after induction and/or consolidation therapy, to maintenance therapy with oral azacitidine or placebo.2 Oral azacitidine demonstrated improvements in both OS and relapse-free survival (RFS) compared with placebo, leading to its approval in the United States.2 Quality of life was similar in the oral azacitidine and placebo arms.2
With a current average wholesale price (AWP) of $25 390 for a 1-month supply using the standard 14-day schedule or $1813.55 per tablet, oral azacitidine could add a substantial financial burden on the US health care system.3 We therefore conducted a cost-effectiveness analysis of maintenance therapy with oral azacitidine compared with observation in patients with AML from a US health care system perspective.
We used a partitioned survival analysis similar to previous publications from our group, which was based on the QUAZAR AML-001 study.2,4 Additional information on the model development is provided in the supplemental Materials. Briefly, patients entered the model as patients with AML in remission and received oral azacitidine using dosing schedules outlined in the QUAZAR AML-001 study or no treatment (ie, observation).2 Patients who experienced disease progression entered a postprogression disease state and received salvage therapies that included intensive chemotherapy, allogeneic hematopoietic cell transplant (allo-HCT), lower-intensity therapies, or best supportive care using the distributions outlined in the QUAZAR AML-001 study.2 Among patients treated with lower-intensity therapies, we assumed that patients with targetable mutations in FLT3, IDH1, or IDH2 would be treated with gilteritinib, ivosidenib, or enasidenib, respectively.
Costs and utilities were modeled over a lifetime horizon. Utilities were measured in quality-adjusted life years (QALYs). Model outputs were used to calculate the incremental cost-effectiveness ratio (ICER) for oral azacitidine, which represents the cost in 2020 US dollars (USD) of each additional QALY gained compared with observation. Costs and utilities were discounted by 3% annually as recommended by the second panel on cost-effectiveness in health and medicine.5
We used Kaplan-Meier curves and at-risk tables for RFS and OS for both study arms of the QUAZAR AML-001 trial to recreate individual patient-level data, which was then fit to various parametric survival distributions.4,6 Log-logistic regression distributions were chosen based on Akaike information criterion and visual inspection (supplemental Figure 1).
Clinical parameters used in this model were derived from the QUAZAR AML-001 study or post hoc analyses of the trial (Table 1).2,7 Costs for subsequent lines of therapy,8-10 management of complications,7 terminal care,11 and supportive care12,13 were derived from the literature and assumed to be equal between both treatment arms. Costs for oral medications (gilteritinib, ivosidenib, enasidenib) were derived using a methodology previously reported by the Memorial Sloan-Kettering Drug Pricing Laboratory that is based on the Medicare plan finder tool.14,15 Because the price of oral azacitidine has not been included in Medicare Part B price files as of May 2021, we estimated the cost using the published AWP.3 The AWP was discounted in our base case analysis by 28%, which was varied between 18% and 38% during sensitivity analyses.16,17 All costs were adjusted for inflation using the personal consumption expenditure health index to 2020 USD.18 Utilities were derived from the literature (Table 1).19-21 Finally, we conducted 1-way and probabilistic sensitivity analyses.
Costs and clinical variables included in the model
| Model variable . | Oral azacitidine . | Observation . | Reference . | ||
|---|---|---|---|---|---|
| Base-case scenario . | Range . | Base-case scenario . | Range . | ||
| Clinical variables | |||||
| Rate of dose escalation to 21-day course | 0.21 | 0.105-0.315 | NA | NA | 2 |
| Median number of cycles with escalated dose | 2 | 1-3 | NA | NA | 2 |
| Median duration of treatment | 11.4 mo | NA | 6.1 mo | NA | 2 |
| Salvage therapies used Intensive chemotherapy Allogeneic HCT Lower-intensity therapy FLT3 inhibitor* IDH1/2 inhibitor* Other lower-intensity therapy Best supportive care only | 0.290 0.060 0.4 0.112 (= 0.4 × 0.28) 0.080 (= 0.4 × 0.20) 0.208 0.420 | 0.145-0.435 0.030-0.090 0.056-0.168 0.040-0.120 0.104-0.312 0.210-0.630 | 0.380 0.140 0.47 0.132 (= 0.4 × 0.28) 0.094 (= 0.4 × 0.20) 0.244 0.270 | 0.190-0.570 0.070-0.210 0.066-0.198 0.047-0.141 0.122-0.366 0.135-0.405 | 2 2 2 2 2 2 2 |
| Monthly treatment discontinuation rate because of adverse events | 0.012 | 0.006-0.018 | NA | NA | 2 |
| Cumulative probability of dose interruption rate due to adverse events | 0.43 | 0.215-0.645 | NA | NA | 2 |
| Duration of dose interruption | 7 d | 4-10 d | NA | NA | Expert opinion |
| Treatment costs | |||||
| Average wholesale price of oral azacitidine (monthly) | $25 389.73 | NA | NA | NA | 15 |
| Discount for average sales price of oral azacitidine | 0.28 | 0.18-0.38 | NA | NA | 16,17 |
| Cost of various salvage regimens Intensive chemotherapy Allogeneic HCT FLT3 inhibitor (gilteritinib) IDH1/2 inhibitor (ivosidenib/enasidenib) Other lower-intensity therapy Best supportive care only (monthly) | $153 737.26 $145 891.52 $26 037.62/cycle $29 782.20/cycle $10 521.87/cycle $5094.56 | $76 869-$230 606 $72 946-$218 837 NA NA $5261-$15 783 $2548-$7643 | $153 737.26 $145 891.52 $26 037.62 $29 782.20 $10 521.87 $5094.56 | $76 869-$230 606 $72 946-$218 837 NA NA $5261-$15 783 $2548-$7643 | 10 9 27 28 8 13 |
| Number of FLT3 inhibitor salvage therapy cycles | 5 | 3-8 cycles | 5 | 3-8 cycles | 27 |
| Number of IDH inhibitor salvage therapy cycles | 5 | 3-8 cycles | 5 | 3-8 cycles | 28 |
| Number of other lower-intensity salvage therapy cycles | 6 | 3-9 cycles | 6 | 3-9 cycles | 8 |
| Cost of office visit (monthly) | $682.57 | $342-$1025 | $682.57 | $342-$1025 | 12 |
| Cost of AML-related hospitalization (per month) | $4840.08 | $2420-$7260 | $6921.5 | $2420-$7260 | 7 |
| Cost of terminal care | $188 677.66 | $94 339-$283 017 | $188 677.66 | $94 339-$283 017 | 11 |
| Utility | |||||
| Utility of active/relapsed AML | 0.53 | 0.48-0.58 | 0.53 | 0.48-0.58 | 21 |
| Utility of AML in early remission (<7 mo) | 0.66 | 0.60-0.72 | 0.66 | 0.60-0.72 | 19 |
| Utility of AML in prolonged remission (≥7 mo) | 0.82 | 0.74-0.90 | 0.82 | 0.74-0.90 | 20 |
| Model variable . | Oral azacitidine . | Observation . | Reference . | ||
|---|---|---|---|---|---|
| Base-case scenario . | Range . | Base-case scenario . | Range . | ||
| Clinical variables | |||||
| Rate of dose escalation to 21-day course | 0.21 | 0.105-0.315 | NA | NA | 2 |
| Median number of cycles with escalated dose | 2 | 1-3 | NA | NA | 2 |
| Median duration of treatment | 11.4 mo | NA | 6.1 mo | NA | 2 |
| Salvage therapies used Intensive chemotherapy Allogeneic HCT Lower-intensity therapy FLT3 inhibitor* IDH1/2 inhibitor* Other lower-intensity therapy Best supportive care only | 0.290 0.060 0.4 0.112 (= 0.4 × 0.28) 0.080 (= 0.4 × 0.20) 0.208 0.420 | 0.145-0.435 0.030-0.090 0.056-0.168 0.040-0.120 0.104-0.312 0.210-0.630 | 0.380 0.140 0.47 0.132 (= 0.4 × 0.28) 0.094 (= 0.4 × 0.20) 0.244 0.270 | 0.190-0.570 0.070-0.210 0.066-0.198 0.047-0.141 0.122-0.366 0.135-0.405 | 2 2 2 2 2 2 2 |
| Monthly treatment discontinuation rate because of adverse events | 0.012 | 0.006-0.018 | NA | NA | 2 |
| Cumulative probability of dose interruption rate due to adverse events | 0.43 | 0.215-0.645 | NA | NA | 2 |
| Duration of dose interruption | 7 d | 4-10 d | NA | NA | Expert opinion |
| Treatment costs | |||||
| Average wholesale price of oral azacitidine (monthly) | $25 389.73 | NA | NA | NA | 15 |
| Discount for average sales price of oral azacitidine | 0.28 | 0.18-0.38 | NA | NA | 16,17 |
| Cost of various salvage regimens Intensive chemotherapy Allogeneic HCT FLT3 inhibitor (gilteritinib) IDH1/2 inhibitor (ivosidenib/enasidenib) Other lower-intensity therapy Best supportive care only (monthly) | $153 737.26 $145 891.52 $26 037.62/cycle $29 782.20/cycle $10 521.87/cycle $5094.56 | $76 869-$230 606 $72 946-$218 837 NA NA $5261-$15 783 $2548-$7643 | $153 737.26 $145 891.52 $26 037.62 $29 782.20 $10 521.87 $5094.56 | $76 869-$230 606 $72 946-$218 837 NA NA $5261-$15 783 $2548-$7643 | 10 9 27 28 8 13 |
| Number of FLT3 inhibitor salvage therapy cycles | 5 | 3-8 cycles | 5 | 3-8 cycles | 27 |
| Number of IDH inhibitor salvage therapy cycles | 5 | 3-8 cycles | 5 | 3-8 cycles | 28 |
| Number of other lower-intensity salvage therapy cycles | 6 | 3-9 cycles | 6 | 3-9 cycles | 8 |
| Cost of office visit (monthly) | $682.57 | $342-$1025 | $682.57 | $342-$1025 | 12 |
| Cost of AML-related hospitalization (per month) | $4840.08 | $2420-$7260 | $6921.5 | $2420-$7260 | 7 |
| Cost of terminal care | $188 677.66 | $94 339-$283 017 | $188 677.66 | $94 339-$283 017 | 11 |
| Utility | |||||
| Utility of active/relapsed AML | 0.53 | 0.48-0.58 | 0.53 | 0.48-0.58 | 21 |
| Utility of AML in early remission (<7 mo) | 0.66 | 0.60-0.72 | 0.66 | 0.60-0.72 | 19 |
| Utility of AML in prolonged remission (≥7 mo) | 0.82 | 0.74-0.90 | 0.82 | 0.74-0.90 | 20 |
Model assumptions were derived preferentially from the QUAZAR AML-001 trial and its post hoc analyses.2,29 If not available, costs and utilities were derived from the literature reporting cost and treatment patterns from a US perspective. If data from both a Medicare and commercial insurance perspective were available, Medicare data were used given the primarily older population in the QUAZAR AML-001 study. All costs were adjusted for inflation to 2020 USD. Costs and utilities were varied by 50% and 10%, respectively, during sensitivity analyses.
Among patients treated with lower-intensity therapies, we assumed that patients with targetable mutations in FLT3, IDH1, or IDH2 would be treated with gilteritinib, ivosidenib, or enasidenib, respectively. As the percentage of patients with these mutations was not specified in the QUAZAR AML-001 study, we assumed a prevalence of those mutations similar to AML patients included in the Cancer Genome Analysis.30 The duration of treatment with subsequent lower-intensity therapies was derived from the literature for the respective agents.8,27,28
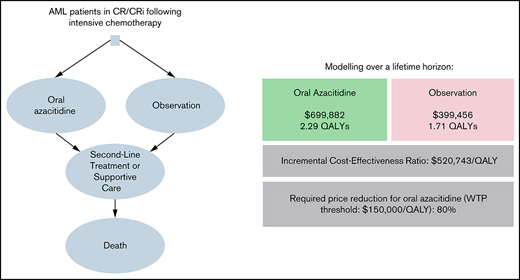
Our parametric survival curves estimated a median RFS/OS of 10.8/25.0 months for oral azacitidine and 5.7/16.1 months for observation, respectively, which was comparable to the results of the QUAZAR AML-001 trial.2 Maintenance treatment with oral azacitidine compared with observation was associated with lifetime costs of $699 882 and $399 456, respectively, resulting in an incremental cost of $300 425. Oral azacitidine demonstrated an incremental gain of 0.57 QALYs over observation (2.29 vs 1.71 QALYs) for an ICER of $520 743/QALY gained.
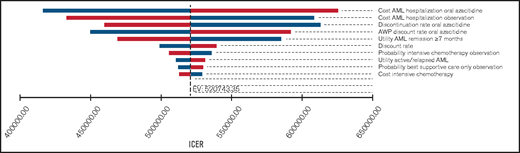
One-way sensitivity analyses showed that our model was most sensitive to the costs of adverse events requiring hospitalization in either group, the monthly probability of discontinuing oral azacitidine, and the discount rate off the AWP (Figure 1). In threshold analyses, we found that an 80.2% decrease in the AWP of oral azacitidine (from $25 389/mo to $5027/mo) would be required to reduce the ICER below a willingness-to-pay (WTP) threshold of $150 000/QALY.
One-way sensitivity analysis of the most influential variables on the ICER. Tornado diagram of the 10 most influential model variables and their influence on the ICER. Our model was most sensitive to the costs of adverse event-related hospitalizations in either treatment group. However, only the monthly medication costs of oral azacitidine were able to reduce the ICER below a willingness-to-pay threshold of $150 000. Variables were varied by 50% for costs and probabilities and by 10% for utilities as outlined in Table 1. Bars shown in blue and red represent lower and higher costs, respectively.
One-way sensitivity analysis of the most influential variables on the ICER. Tornado diagram of the 10 most influential model variables and their influence on the ICER. Our model was most sensitive to the costs of adverse event-related hospitalizations in either treatment group. However, only the monthly medication costs of oral azacitidine were able to reduce the ICER below a willingness-to-pay threshold of $150 000. Variables were varied by 50% for costs and probabilities and by 10% for utilities as outlined in Table 1. Bars shown in blue and red represent lower and higher costs, respectively.
In probabilistic sensitivity analysis, 99.99% of 10 000 Monte Carlo simulations yielded an ICER that exceeded the WTP threshold of $150 000/QALY (mean: $486 523/QALY; 95% confidence interval: $313 354/QALY-$674 537/QALY; supplemental Figure 2).
Although the QUAZAR AML-001 trial was the first to show a mortality benefit with postinduction chemotherapy maintenance and could herald a new treatment paradigm, longer follow-up is necessary to evaluate whether maintenance therapy with oral azacitidine leads to durable survival benefits or is rather delaying an inevitable disease relapse. Although extrapolations are limited, previous studies did not find a statistically significant difference in long-term survival rates compared with no maintenance treatment.1
From a patient perspective, both the objective financial burden and the subjective psychosocial stress inflicted by high medication costs can be significant and has been associated with higher levels of depression, symptom burden, and lower quality of life.22 Our study provides additional support for efforts aimed at curtailing the steady increase in drug costs in oncology. This is especially important for maintenance therapies that can be used for extended periods of time. Data on adjuvant breast cancer therapy suggest that higher prescription copayments are associated with higher rates of discontinuation of adjuvant therapy, which may translate into adverse long-term outcomes.23 Because the copayment for oral AML therapies can be substantial, it is possible that a similar relationship between out-of-pocket costs and treatment persistence exists in AML as well, but additional studies are warranted.24
In addition to oral azacitidine, the multikinase inhibitor sorafenib has been shown to provide an OS benefit in patients with AML with FLT3 mutations after allo-HCT in recent randomized trials.25 Several other maintenance strategies in AML are currently undergoing randomized phase 3 trials, and our study could serve as a reference for the economic evaluation of those trials.
Our partitioned survival analysis model has several strengths because it took advantage of the direct comparison of oral azacitidine maintenance therapy and placebo in the QUAZAR AML-001 trial, which was used to model the RFS and OS curves underlying our model.2 Additionally, we based our model on the patient population, subsequent treatments, and health care utilization reported by the QUAZAR AML-001 study, as well as previously reported costs and variable distributions in similar AML populations. Finally, several sensitivity analyses confirmed the robustness of our results.
Potential limitations include differences between clinical trials and real-world practice, limited data on health care resource utilization with oral azacitidine,7 and use of AWP instead of average sales price and Medicare reimbursement for oral azacitidine. However, as our threshold analyses required an 80.2% price reduction to reach the WTP threshold of $150 000/QALY, it is unlikely that routine discounts will be as substantial to meet this threshold. Our study was conducted from a US perspective, which limits generalizability to other settings in terms of health care expenses, practice patterns, and medication availability. As such, differences in supportive care and management of adverse events across countries in an international clinical trial may lead to differences in associated costs, and using a US perspective can only provide an approximation of the costs in other settings. Finally, because our results were based on a randomized clinical trial and differences in patient populations, treatment patterns, and potentially efficacy between clinical trials and real-world practice have been documented for AML patients receiving injectable azacitidine,26 future studies are warranted to evaluate the cost-effectiveness of oral azacitidine in the real-world setting. However, in 1-way sensitivity analyses, neither the rate of treatment discontinuation with oral azacitidine nor the costs of hospitalization related to adverse events led to an ICER that would make oral azacitidine cost-effective at the conventional willingness-to-pay threshold of $150 000/QALY under the current its pricing.
In summary, we conducted a partitioned survival analysis to evaluate the cost-effectiveness of maintenance therapy with oral azacitidine compared with observation based on the QUAZAR AML-001 trial and found an ICER of $520 743/QALY gained for oral azacitidine maintenance therapy, with 99.99% of simulations in a probabilistic sensitivity analysis yielding an ICER > $150 000/QALY. Under our model assumptions, a price reduction by 80.2% to a monthly price of $5027 per month would be needed to make oral azacitidine maintenance cost-effective compared with observation.
Acknowledgments: The Frederick A. DeLuca Foundation supported this work. K.K.P. is funded by the American Society of Hematology Physician-Scientist Career Development Award. A.M.Z. is a Leukemia and Lymphoma Society Scholar in Clinical Research and was also supported by a National Cancer Institute (NCI) Cancer Clinical Investigator Team Leadership Award. Research reported in this publication was in part supported by the NCI, National Institutes of Health (under award P30 CA016359). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Contribution: J.P.B. and K.K.P. performed statistical analysis; K.K.P., S.F.H., and A.M.Z. obtained funding; A.M.Z. and S.F.H. supervised the study; all authors had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis; and all authors were responsible for conception and design; acquired, analyzed, and interpreted the data; and drafted the manuscript.
Conflict-of-interest disclosure: A.M.Z. received research funding (institutional) from Celgene/Bristol-Myers Squibb, AbbVie, Astex, Pfizer, Medimmune/AstraZeneca, Boehringer-Ingelheim, Trovagene/Cardiff Oncology, Incyte, Takeda, Novartis, Aprea, and ADC Therapeutics; participated in advisory boards and/or had a consultancy with and received honoraria from AbbVie, Otsuka, Pfizer, Celgene/Bristol-Myers Squibb, Jazz Pharmaceuticals, Incyte, Agios, Boehringer-Ingelheim, Novartis, Acceleron, Astellas, Daiichi Sankyo, Cardinal Health, Taiho, Seattle Genetics, BeyondSpring, Trovagene/Cardiff Oncology, Takeda, Ionis, Amgen, Janssen, Epizyme, Syndax, and Tyme; served on clinical trial committees for Novartis, AbbVie, Geron, and Celgene/Bristol-Myers Squibb; and received travel support for meetings from Pfizer, Novartis, and Trovagene/Cardiff Oncology. S.F.H. was a consultant for Celgene, Bayer, Genentech, Pharmacyclics, and AbbVie and received research funding from DTRM Biopharm, Celgene, and TG Therapeutics. The remaining authors declare no competing financial interests.
Correspondence: Amer M. Zeidan, Section of Hematology, Department of Internal Medicine, Yale University, 333 Cedar St, PO Box 208028, New Haven, CT 06520-8028; e-mail: amer.zeidan@yale.edu.
References
Author notes
The original data are available by e-mail request to Amer Zeidan (amer.zeidan@yale.edu).
The full-text version of this article contains a data supplement.