Key Points
Marrow remission in newly diagnosed and relapsed post–MPN-AML patients treated with venetoclax-containing regimens was 43% and 0%, respectively.
Venetoclax-based therapy was associated with significant myelosuppression and mortality, with a median survival of 4 months.
Abstract
In patients with acute myeloid leukemia evolving from myeloproliferative neoplasms (post–MPN-AML), the clinical activity of the B-cell lymphoma 2 inhibitor venetoclax remains to be determined. We review our experience with venetoclax-based regimens in 14 newly diagnosed (frontline [FL]) and 17 relapsed/refractory (R/R) post–MPN-AML patients. Venetoclax was used in combination with hypomethylating agents in 58% of cases and in 19% with intensive chemotherapy (treatment including cytarabine ≥1 g/m2 or CPX-351); the remaining patients received cladribine and low-dose cytarabine or isocitrate dehydrogenase 1/2 inhibitors. The median dose of venetoclax during the initial cycle was 100 mg in all patients (range, 50-800 mg) and 200 mg (range, 100-800 mg) for FL patients. The venetoclax dose was adjusted when used concomitantly with azole antifungal agents. In FL patients, complete remission with and without count recovery in 6 patients (median duration of 6.4 months) and partial remission in 1 patient was noted, with a median overall survival of 7 months. In R/R patients, no formal responses were seen, with a median overall survival of 3 months. Hematologic toxicities and adverse events were frequent; 83% of patients developed grade 3 or higher infection during the initial cycle. Severe hemorrhagic complications were observed in 14 patients, including 6 cases of intracranial and subdural hemorrhage. Overall 4-week and 8-week mortality were 10% and 32%, respectively. Given the substantial treatment-associated hematologic toxicity and mortality, and modest short-lived responses only in newly diagnosed patients with venetoclax-based regimens, additional treatment options are urgently needed for these patients.
Introduction
Leukemic or blast transformation of myeloproliferative neoplasms (primary myelofibrosis, polycythemia vera [PV], or essential thrombocytosis [ET]), hereafter referred to as post–MPN-AML, is a rare but devastating complication of these diseases. Post–MPN-AML carries a dismal prognosis, with a median survival of ∼6 months; the only possibility of long-term survival is offered by allogeneic stem cell transplantation (SCT) in the minority of patients who are able to achieve complete remission, or return to chronic phase, with therapy before transplant.1
Given the ineffectiveness of available therapies, there is an urgent need for novel treatment strategies for patients with post–MPN-AML. Despite the recent approval of multiple agents for patients with acute myeloid leukemia (AML), the distinct disease biology of post–MPN-AML might hinder their therapeutic benefit in this entity. One such agent is venetoclax (VEN), an oral, selective, potent BH3-mimetic inhibitor of the B-cell lymphoma 2 (BCL-2) antiapoptotic protein that facilitates survival and chemoresistance of leukemia cells. VEN represents one of the greatest recent breakthroughs for the treatment of AML, significantly improving response rates and survival in older patients unfit for intensive chemotherapy. For instance, in elderly patients newly diagnosed with AML (frontline [FL]), VEN in combination with the hypomethylating agent (HMA) azacitidine (AZA) showed an overall response rate of up to 70%, with a median overall survival (OS) superior to that achieved with AZA alone.2 In the relapsed refractory (R/R) setting, limited data from prospective studies on VEN combinations showed lower but still very promising responses.3
Based on preclinical evidence that patients with post–MPN-AML have increased overexpression of the antiapoptotic family member proteins myeloid cell leukemia 1 (MCL-1) and B-cell lymphoma X long (BCL-XL) known to confer primary resistance to VEN,4-6 these patients were largely excluded from the pivotal trials of VEN. However, VEN regimens have been widely used in post–MPN-AML patients, and limited preliminary results were recently published.7,8 At our institution, we treated 14 FL and 17 R/R patients with VEN-based therapy (further VEN-b therapy). This 31-patient cohort represents the largest analysis to date on the efficacy and safety of VEN-b strategies for post–MPN-AML patients from a single center.
Patients and methods
This study included all adult patients with post–MPN-AML (≥20% blasts) who were treated at the University of Texas MD Anderson Cancer with a regimen including a minimum of 7 days of VEN. Patients received VEN in combination with other therapies at the discretion of their physician; 9 patients were treated on a clinical trial, and the remaining patients were treated off protocol using commercial VEN supply. Previous therapy with HMAs (AZA or decitabine [DAC]) was allowed, except for FL patients treated on clinical protocols with HMA-VEN combination. VEN was initiated in the hospital with a short ramp-up during cycle 1 to a target dose of 400 mg daily (except for 1 patient who received 800 mg) as previously published.9 Patients with leukocytosis required cytoreduction to a white blood cell count <10 × 109 /L before VEN was started.
Responses were evaluated as per standardized criteria.10 Composite remission rate was considered as marrow complete response with or without count recovery (CR and CRi). Overall response included CR, CRi, and partial remission (PR). Molecular testing was performed at the time of VEN-b therapy initiation using our institutional next-generation sequencing myeloid malignancy platform in our Clinical Laboratory Improvement Amendments–certified laboratory (analytical sensitivity, 2.5%-5%). Minimal residual disease (MRD) was assessed in bone marrow aspirates by using a multiparameter flow cytometry assay with a sensitivity of 0.01%.11 Adverse events were summarized according to the National Cancer Institute Common Terminology Criteria for Adverse Events Version 4.0. In the absence of residual morphologic evidence of leukemia and in the presence of myelosuppression at the completion of cycle 1, VEN could be interrupted for up to 14 days to allow for count recovery (absolute neutrophil count ≥0.5 × 109/L). VEN could also be reduced to 14 or 7 days for subsequent cycles in cases of recurrent cytopenias. All potential candidates were evaluated for SCT at their diagnosis or treatment initiation, and it was pursued in all eligible patients once acceptable control of their disease was achieved.
The MD Anderson Cancer Center institutional review board approved the current study, which was performed in accordance with the Declaration of Helsinki.
Patient characteristics and their comparisons were analyzed by using medians or frequencies with ranges, and Fisher’s exact test or Wilcoxon rank sum test as appropriate for nominal and continuous variables, respectively. Median OS (measured from the start of VEN-b therapy to death or last follow-up) was estimated by using the Kaplan-Meier method, and differences between subgroups were evaluated by using the log-rank test. Duration of CR + CRi was analyzed by using the Kaplan-Meier method. Association between clinical variables and survival/response was evaluated by logistic regression. Statistical analyses were performed by using SPSS version 26 (IBM SPSS Statistics, IBM Corporation, Armonk, NY) and GraphPad version 8 (GraphPad Software, La Jolla, CA).
Results
Among all post–MPN-AML patients who received therapy at our institution between the years 2000 and 2019 (n = 241), 31 patients (13%) received VEN-b therapy. Fourteen patients (45%) were newly diagnosed (FL) and 17 patients (55%) had R/R disease (9 primary refractory patients). Patient characteristics at the time of initiation of VEN-b therapy are summarized in Table 1. The median age was 69 years (range, 46-80 years), and FL patients were older (≥65 years, FL vs R/R: 86% vs 53% [P = .03]). Sixteen patients had a history of ET or PV (6 FL), and 5 of them transformed to AML without an obvious myelofibrosis phase.
Demographic and clinical characteristics of all patients (N = 31)
| Characteristic . | All (N = 31) . | FL (n = 14) . | R/R (n = 17) . |
|---|---|---|---|
| Median age (range), y | 69 (46-80) | 74 (46-80) | 66 (47-79) |
| Age ≥65 y, n (%)* | 21 (68) | 12 (86) | 9 (53) |
| Male sex, n (%) | 19 (61) | 9 (64) | 10 (59) |
| Primary MF, n (%) | 13 (42) | 7 (50) | 6 (35) |
| History of ET/PV, n† | 11/5 | 4/2 | 7/3 |
| Performance status ≥2, n (%) | 10 (32) | 5 (36) | 5 (29) |
| WBC, median (range), ×109/L* | 11.7 (0.1-76) | 13.2 (0.1-76) | 4.7 (0.5-47) |
| Platelets, median (range), × 109/L* | 71 (4-445) | 114 (5-445) | 35 (4-376) |
| Hemoglobin, median (range), g/dL | 8.4 (5-15) | 9.8 (7-15) | 8.5 (7.6-12) |
| Karyotype, n (%) | |||
| Diploid | 5 (16) | 3 (21) | 2 (12) |
| Abnormal | 12 (39) | 7 (50) | 5 (29) |
| Complex [3+ Abn]* | 14 (45) | 4 (28) | 10 (59) |
| Driver mutations, n (%) | |||
| JAK2 | 21 (68) | 9 (64) | 12 (71) |
| CALR/MPL‡ | 4 (13)/3 (10) | 1 (7)/2 (14) | 3 (17)/1 (1) |
| TN | 3 (10) | 2 (14) | 1 (1) |
| Additional mutation >10% patients, n (%) | |||
| ASXL1 | 10 (32) | 3 (21) | 7 (41) |
| NRAS/KRAS§ | 9 (29) | 3 (21) | 6 (35) |
| TET2 | 11 (32) | 5 (36) | 6 (35) |
| TP53§ | 8 (26) | 3 (21) | 5 (29) |
| IDH1/2§ | 8 (26) | 6 (43) | 2 (12) |
| RUNX1 | 7 (23) | 4 (28) | 3 (17) |
| U2AF1 | 5 (16) | 3 (21) | 2 (12) |
| SETBP1 | 4 (13) | 2 (14) | 2 (12) |
| Characteristic . | All (N = 31) . | FL (n = 14) . | R/R (n = 17) . |
|---|---|---|---|
| Median age (range), y | 69 (46-80) | 74 (46-80) | 66 (47-79) |
| Age ≥65 y, n (%)* | 21 (68) | 12 (86) | 9 (53) |
| Male sex, n (%) | 19 (61) | 9 (64) | 10 (59) |
| Primary MF, n (%) | 13 (42) | 7 (50) | 6 (35) |
| History of ET/PV, n† | 11/5 | 4/2 | 7/3 |
| Performance status ≥2, n (%) | 10 (32) | 5 (36) | 5 (29) |
| WBC, median (range), ×109/L* | 11.7 (0.1-76) | 13.2 (0.1-76) | 4.7 (0.5-47) |
| Platelets, median (range), × 109/L* | 71 (4-445) | 114 (5-445) | 35 (4-376) |
| Hemoglobin, median (range), g/dL | 8.4 (5-15) | 9.8 (7-15) | 8.5 (7.6-12) |
| Karyotype, n (%) | |||
| Diploid | 5 (16) | 3 (21) | 2 (12) |
| Abnormal | 12 (39) | 7 (50) | 5 (29) |
| Complex [3+ Abn]* | 14 (45) | 4 (28) | 10 (59) |
| Driver mutations, n (%) | |||
| JAK2 | 21 (68) | 9 (64) | 12 (71) |
| CALR/MPL‡ | 4 (13)/3 (10) | 1 (7)/2 (14) | 3 (17)/1 (1) |
| TN | 3 (10) | 2 (14) | 1 (1) |
| Additional mutation >10% patients, n (%) | |||
| ASXL1 | 10 (32) | 3 (21) | 7 (41) |
| NRAS/KRAS§ | 9 (29) | 3 (21) | 6 (35) |
| TET2 | 11 (32) | 5 (36) | 6 (35) |
| TP53§ | 8 (26) | 3 (21) | 5 (29) |
| IDH1/2§ | 8 (26) | 6 (43) | 2 (12) |
| RUNX1 | 7 (23) | 4 (28) | 3 (17) |
| U2AF1 | 5 (16) | 3 (21) | 2 (12) |
| SETBP1 | 4 (13) | 2 (14) | 2 (12) |
Abn, abnormality; MF, myelofibrosis; TN, triple negative; WBC, white blood cell count.
*Statistically significant.
†Five of these 16 patients (3 ET) had no obvious MF phase before transformation to AML.
‡One patient had 2 functional driver mutations, CALR L367fs and MPL R592Q.
§More than 1 mutation of these genes was identified in individual patients (eg, 1 patient with 2 TP53 mutations, 1 patient with both KRAS and NRAS mutation).
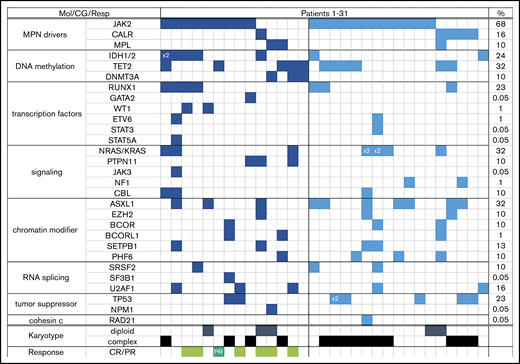
Almost one-half of all patients had complex karyotype (≥3 abnormalities); the majority (69%) had a JAK2 mutation, and every patient had at least one additional molecular abnormality (most common were mutations in ASXL1, NRAS, and TET2) (Table 1). Patients with R/R AML were more likely to be neutropenic, thrombocytopenic, and have complex karyotype (59% vs 28% in FL; P = .02) than FL patients, but the distribution of mutations was comparable between the 2 groups (Table 1; Figure 1).
Each column represents one patient (patient 1 through 31). Detected mutation in respective gene is shown in dark blue (FL) and light blue (R/R) colors; x2 = patient had both mutations (eg, IDH1 and IDH2, or NRAS with KRAS). Last column shows overall frequency of mutated gene in the entire cohort. Bottom rows show karyotype (only depicted diploid vs complex) and response in the FL cohort (light green = complete remission regardless of counts recovery; dark green = partial remission). CG, cytogenetics; Mol, molecular; Resp, response.
Each column represents one patient (patient 1 through 31). Detected mutation in respective gene is shown in dark blue (FL) and light blue (R/R) colors; x2 = patient had both mutations (eg, IDH1 and IDH2, or NRAS with KRAS). Last column shows overall frequency of mutated gene in the entire cohort. Bottom rows show karyotype (only depicted diploid vs complex) and response in the FL cohort (light green = complete remission regardless of counts recovery; dark green = partial remission). CG, cytogenetics; Mol, molecular; Resp, response.
Fifty-two percent of all patients (n = 16) received prior therapy with HMA, 5 in the FL cohort for accelerated phase of MPN (10% to 19% blasts). Four patients had previously undergone allogeneic SCT, including 2 FL patients for their antecedent MPN. Both these patients relapsed post-SCT (after 9.5 years and 3 months, respectively) with only extramedullary disease at the time of VEN-b therapy. In the 2 R/R patients who underwent SCT for post–MPN-AML, VEN-b therapy was used as second salvage after relapse post-SCT, which occurred 4 months’ and 9 years’ post-SCT, respectively. The median number of prior therapies for R/R patients was 1 (range, 1-3). VEN-b therapy was used as first salvage in 65% of R/R patients (n = 11).
Agents used in combination with VEN are detailed in Table 2 and supplemental Figures 1 and 2: HMA was used in 18 patients (58% [AZA in 3; DAC in 15]), cladribine and low-dose cytarabine (LDAC) in 4 patients (13%), chemotherapy with a combination of cladribine, cytarabine, and idarubicin/FLAG ± IDA (fludarabine, cytarabine, granulocyte colony-stimulating factor ± idarubicin), or CPX-351 in a total of 6 patients (19%) and isocitrate dehydrogenase 1/2 (IDH1/2) inhibitors in 2 patients (6%). In addition, the JAK1/2 inhibitor ruxolitinib (RUX) and the CD33 antibody–drug conjugate gemtuzumab ozogamicin were added to VEN-b therapy in 9 and 4 patients, respectively. Six FL patients and three R/R patients were treated on clinical trials (clinical trials.gov identifier); 4 FL patients and 1 R/R patient on DAC-VEN (#NCT03404193); one FL patient each on cladribine-LDAC-VEN (#NCT03586609) and IDH1 inhibitor–VEN (#NCT03471260); and one R/R patient each on CPX351-VEN (#NCT03629171) and FLAG-IDA VEN (#NCT03214562).
Treatment details
| Characteristic . | FL, n (%) (n = 14) . | R/R, n (%) (n = 17) . |
|---|---|---|
| Treatment regimens* | ||
| DAC/AZA + VEN | 8 (57) | 10 (59) |
| Cladribine + LDAC + VEN | 3 (21) | 2 (12) |
| IDH1 inhibitors/IDH2 inhibitors + VEN† | 1 (7) | 1 (6) |
| CPX-351 + VEN | 1 (7) | 2 (12) |
| CLIA + VEN or FLAG (± Ida) + VEN | 1 (7) | 2 (12) |
| No. of VEN cycles | ||
| 1 cycle | 5 (36) | 6 (35) |
| 2 cycles | 3 (21) | 5 (29) |
| ≥3 cycles | 6 (43) | 6 (35) |
| VEN initial dose | ||
| ≥400 mg | 3 (21) | – |
| 200 mg with strong CYP3A4-i | 3 (21) | 4 (26) |
| 200 mg with moderate CYP3A4-i | 3 (21) | 2 (12) |
| 100 mg with strong CYP3A4-i | 5 (36) | 10 (59) |
| 50 mg with strong CYP3A4-i | – | 1 (6) |
| VEN duration reduction | ||
| During first cycle | 2 (14) | 3 (18) |
| During subsequent cycles | 6 (43) | 4 (26) |
| VEN dose reduction: during subsequent cycles‡ | 2 (14) | 2 (12) |
| Characteristic . | FL, n (%) (n = 14) . | R/R, n (%) (n = 17) . |
|---|---|---|
| Treatment regimens* | ||
| DAC/AZA + VEN | 8 (57) | 10 (59) |
| Cladribine + LDAC + VEN | 3 (21) | 2 (12) |
| IDH1 inhibitors/IDH2 inhibitors + VEN† | 1 (7) | 1 (6) |
| CPX-351 + VEN | 1 (7) | 2 (12) |
| CLIA + VEN or FLAG (± Ida) + VEN | 1 (7) | 2 (12) |
| No. of VEN cycles | ||
| 1 cycle | 5 (36) | 6 (35) |
| 2 cycles | 3 (21) | 5 (29) |
| ≥3 cycles | 6 (43) | 6 (35) |
| VEN initial dose | ||
| ≥400 mg | 3 (21) | – |
| 200 mg with strong CYP3A4-i | 3 (21) | 4 (26) |
| 200 mg with moderate CYP3A4-i | 3 (21) | 2 (12) |
| 100 mg with strong CYP3A4-i | 5 (36) | 10 (59) |
| 50 mg with strong CYP3A4-i | – | 1 (6) |
| VEN duration reduction | ||
| During first cycle | 2 (14) | 3 (18) |
| During subsequent cycles | 6 (43) | 4 (26) |
| VEN dose reduction: during subsequent cycles‡ | 2 (14) | 2 (12) |
CLIA, combination of cladribine, cytarabine, and idarubicin; FLAG ± Ida, combination of fludarabine, cytarabine ± idarubicin, and growth factor; CYP3A4-i, cytochrome P450 3A4 inhibitors (strong [eg, posaconazole or voriconazole]; moderate [eg, isavuconazole]).
Ruxolitinib or gemtuzumab ozogamicin were used in some patients, as detailed in the text.
IDH1 inhibitor was used in 1 patient in the FL setting and IDH2 inhibitor in 1 patient in the R/R setting.
These patients had concomitant reduction of duration of VEN therapy.
VEN doses ranged from 50 mg to 400 mg daily in all but 1 patient, who received 800 mg of VEN in combination with an IDH1 inhibitor. VEN was given for a median of 21 days per cycle. The VEN dose was reduced when given with a concomitant moderate or strong cytochrome P450 3A4 inhibitor (mostly azole antifungal agents), but 7 patients received higher than the currently recommended dose12 (Table 2). Median time to VEN-b therapy from post–MPN-AML diagnosis was 1 month (range, 0.4-1.5 months) and 5 months (range, 1.8-106 months) in FL and R/R patients, respectively (P < .01). In R/R patients, median time to VEN-b therapy from the time of first relapse was 3.6 months (range, 1.5-16 months). Overall, patients received a median of 2 cycles of VEN-b therapy (the same for FL and R/R patients), and 20 (65%) patients received >1 cycle. Median time on VEN-b therapy was 68 days (range, 10-672 days), and it was longer in FL patients (102 days; range, 15-658 days) than in R/R patients (48 days; range, 24-141 days; P = .04).
Median follow-up from onset of VEN-b therapy for all 31 patients was 7.8 months (range, 0.3-21 months). Seven patients achieved response: 3 CR, 3 CRi, and 1 PR. Responses were only observed in the FL setting with a CR/CRi rate of 43%. Two patients (one FL and one R/R) obtained stable disease with blasts decreased by <50% for 6 and 12 months, respectively. Details of responders and treatment duration are provided in Table 3 and supplemental Figures 1 and 2. Notably, none of these patients was observed as having any changes in previous marrow fibrosis grading, and all remained in the chronic MPN phase, including presence of initial MPN driver mutation.
Characteristics of responders (CR, CRi, PR)
| Patient . | Antec MPN . | Karyotype . | Molecular type (VAF) . | VEN regimen . | VEN dose/induction . | VEN cycles . | Best response . | Cycles to best response . | Response: DoR/outcome . |
|---|---|---|---|---|---|---|---|---|---|
| 69 y/F | PV 3 y (HU), MF 1 y (RUX) | add(21)(p11.2) | JAK2 V617F (89%), IDH2 R140Q (31%), RUNX1 D198G (23%), SRSF2 P95L (10%) | Cladribine-LDAC-VEN | 100 mg [VORI] | 4 | CR, MRD(–) | 1 | 4 mo → SCT, ongoing |
| 46 y/M | ET 19 y, MF 1 y (RUX) | Diploid | CALR L367fs (41%), ASXL1 G646 (35%), EZH2 D185H (39%), SETBP1 D868N (28%), PTPN11 R265Q (<10%), IDH1 R132 (20%) | IDH1i-VEN | 800 mg | 3 | CR, MRD(+) | 1 | 3 mo → SCT, ongoing |
| 74 y/F | MPN-u 4 y (HU) | Monosomy 7 | JAK2 V617F (27%), TP53 G302E (<10%), U2AF1 Q157R (<3%) | DAC-VEN | 200 mg [ISA] | 14 | CR, MRD(–) | 2 | 18 mo, ongoing on RX |
| 73 y/F | MF 1 y (supp) | Diploid | MPL W288 (unk), NPM1 W288 (31%), DNMT3A R882H (<2%) | DAC-VEN | 100 mg [POSA] | 5 | CRp, MRD(–) | 3 | 5 cycles/R (died, infection) |
| 76 y/M | ET 6 y (HU) | +8, +20 | NRAS G12D (12%), U2AF1 Q157P (<3%), TET2 N1890S (42%), PTPN11 R265Q (28%), DNMT3A R882H (<10%) | DAC-VEN | 200 mg [ISA] | 6 | CRp, MRD(+) | 2 | 4 cycles/R (next RX) |
| 65 y/M | PV 22 y (HU) | +1,der(1;7)(q10;p10) | JAK2 V671F (42%), IDH1 R132H (18%), RUNX1 T196I (10%), WT1 E384 (<5%) | DAC-VEN | 200 mg [ISA] | 2 | CRn, MRD(+) | 1 | 1 cycle/R (next RX) |
| 74 y/F | MF 3 y (RUX) | +Y, t(8;21) | JAK2 V617F (55%), TET2 L590¥F (<3%) | DAC-VEN-GO | 100 mg (POSA) | 1 | PR [7% bl] MRD(+) | 1 | 3 wks/died with SDH (palliative) |
| Patient . | Antec MPN . | Karyotype . | Molecular type (VAF) . | VEN regimen . | VEN dose/induction . | VEN cycles . | Best response . | Cycles to best response . | Response: DoR/outcome . |
|---|---|---|---|---|---|---|---|---|---|
| 69 y/F | PV 3 y (HU), MF 1 y (RUX) | add(21)(p11.2) | JAK2 V617F (89%), IDH2 R140Q (31%), RUNX1 D198G (23%), SRSF2 P95L (10%) | Cladribine-LDAC-VEN | 100 mg [VORI] | 4 | CR, MRD(–) | 1 | 4 mo → SCT, ongoing |
| 46 y/M | ET 19 y, MF 1 y (RUX) | Diploid | CALR L367fs (41%), ASXL1 G646 (35%), EZH2 D185H (39%), SETBP1 D868N (28%), PTPN11 R265Q (<10%), IDH1 R132 (20%) | IDH1i-VEN | 800 mg | 3 | CR, MRD(+) | 1 | 3 mo → SCT, ongoing |
| 74 y/F | MPN-u 4 y (HU) | Monosomy 7 | JAK2 V617F (27%), TP53 G302E (<10%), U2AF1 Q157R (<3%) | DAC-VEN | 200 mg [ISA] | 14 | CR, MRD(–) | 2 | 18 mo, ongoing on RX |
| 73 y/F | MF 1 y (supp) | Diploid | MPL W288 (unk), NPM1 W288 (31%), DNMT3A R882H (<2%) | DAC-VEN | 100 mg [POSA] | 5 | CRp, MRD(–) | 3 | 5 cycles/R (died, infection) |
| 76 y/M | ET 6 y (HU) | +8, +20 | NRAS G12D (12%), U2AF1 Q157P (<3%), TET2 N1890S (42%), PTPN11 R265Q (28%), DNMT3A R882H (<10%) | DAC-VEN | 200 mg [ISA] | 6 | CRp, MRD(+) | 2 | 4 cycles/R (next RX) |
| 65 y/M | PV 22 y (HU) | +1,der(1;7)(q10;p10) | JAK2 V671F (42%), IDH1 R132H (18%), RUNX1 T196I (10%), WT1 E384 (<5%) | DAC-VEN | 200 mg [ISA] | 2 | CRn, MRD(+) | 1 | 1 cycle/R (next RX) |
| 74 y/F | MF 3 y (RUX) | +Y, t(8;21) | JAK2 V617F (55%), TET2 L590¥F (<3%) | DAC-VEN-GO | 100 mg (POSA) | 1 | PR [7% bl] MRD(+) | 1 | 3 wks/died with SDH (palliative) |
Antec., antecedent; CRn, complete remission without neutrophil recovery; CRp, complete remission without platelet recovery; DoR, duration of response; F, female; GO, gemtuzumab; HU, hydroxyurea; IDH1-i, IDH1 inhibitor; ISA, isavuconazole; M, male; MF, myelofibrosis; POSA, posaconazole; R, relapsed; RX, therapy; SDH, subdural hematoma; supp; supportive therapy (included steroids and danazol); VAF, variant allele frequency; VORI, voriconazole.
Three of the 6 patients with a bone marrow response (CR and CRi) achieved MRD negativity. Among all 7 responders, 5 were treated with DAC-VEN, one received cladribine-LDAC-VEN, and one was treated with an IDH1 inhibitor + VEN. Four of 6 and 2 of 7 patients with antecedent ET/PV or post-ET/post-PV–myelofibrosis and primary myelofibrosis had a response, respectively. Responses were achieved mostly in patients who did not have an unfavorable karyotype13 (6 of 7 [86%]). Three of 8 patients with an IDH1/2 mutation had a response (38%); additional responses were also observed in patients with RUNX1, TP53, U2AF1, and RAS mutations.
The median times to initial and best response were 1 and 2 months, respectively. The median follow-up for responders was 12 months (range, 2.5-21 months). The median duration of response was 6.4 months (range, 0.7-20 months). Two responders successfully underwent SCT with matched unrelated donors and are currently alive in remission 9 and 16 months after SCT, respectively. One patient achieved CR after 1 cycle of cladribine-LDAC-VEN and underwent SCT after 4 total cycles (in MRD-negative CR); the second patient achieved CR after 1 cycle of IDH1 inhibitor + VEN and underwent SCT after 3 cycles (with 0.18% positive MRD) (no. 1 and no. 2 in Table 3 and supplemental Figure 1). Three responders relapsed after 1, 4, and 5 cycles of VEN-b therapy and died with disease, and one patient who was in a PR for 3 weeks died in hospice after developing bilateral subdural hematomas. Only 2 additional patients are still alive without SCT. One is a 74-year old woman who achieved CR on DAC-VEN and is receiving therapy for the past 20 months (ongoing MRD-negative CR; no. 3 in Table 3 and supplemental Figure 1). The second patient did not have any response to 2 cycles of DAC-VEN but achieved a CR on their current therapy of DAC-RUX (no. 10 in supplemental Figure 1). The remaining 27 patients (87%) have died.
Reason for therapy discontinuation in the entire cohort included SCT in 2 patients, death by day 60 after VEN-b therapy in 10, no response or disease progression in 15, and patient’s choice in 3 patients.
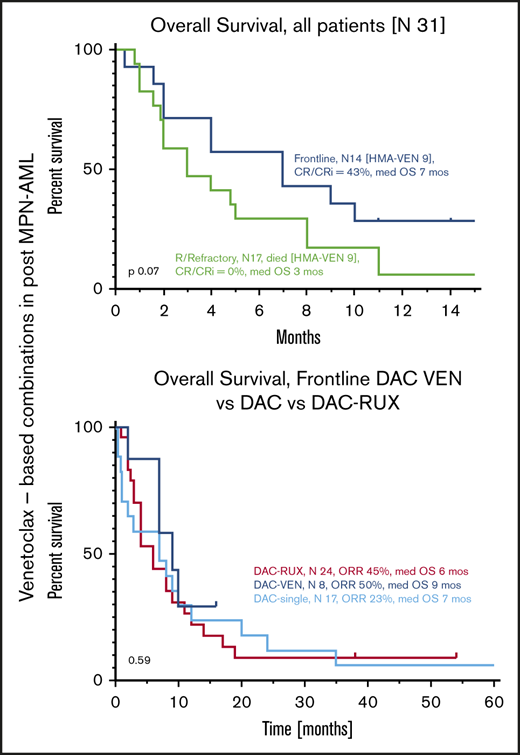
Median OS of all patients from the time of initiation of VEN-b therapy was 4 months (95% confidence interval [CI], 2-8) (Figure 2A). Estimated 6- and 12- month survival rates were 42% and 16%, respectively. Median OS of FL and R/R patients was 7 and 3 months (P = .07) (Figure 2B). Among all patients, OS of responders (CR, CRi, PR) was superior to that of nonresponders, with respective medians of 9 and 3 months (hazard ratio, 0.37; 95% CI, 0.15-0.77; P = .02) (Figure 2C). Comparison of responders vs nonresponders among FL patients in terms of survival was not statistically different, but median OS times were 9 and 2 months, respectively (P = .13) (Figure 2D).
OS from VEN-b therapy. Data given for all patients (A), in FL vs R/R patients (B), in all responders vs nonresponders (C), and in FL responders vs nonresponders (D).
OS from VEN-b therapy. Data given for all patients (A), in FL vs R/R patients (B), in all responders vs nonresponders (C), and in FL responders vs nonresponders (D).
The most frequently used agent together with VEN in FL patients was DAC (n = 8). Median OS of these patients was 9 months, which seems comparable to that of patients treated with single-agent DAC or DAC-RUX in our own institution as we previously reported (median of 7 months).14,15 None of our patients treated with DAC-VEN were able to undergo SCT, whereas we had 2 and 4 patients who received SCT after therapy with DAC and DAC-RUX, respectively.
Clinical factors associated with survival at 6 months and overall response among FL patients are given in supplemental Table 1. Achievement of response, absence of thrombocytopenia (platelet levels <100 × 109/L), and absence of RAS mutation were all associated with being alive at 6 months. The only factor associated with response was the absence of thrombocytopenia (platelet levels <100 × 109 /L).
The main adverse events observed during VEN-b therapy were prolonged cytopenias and infections. Count recovery was only observed in patients with responses and in 1 patient with stable disease who had platelet recovery (n = 8). Among these patients, the median time to neutrophil recovery (absolute neutrophil count >1) was 32 days (range, 23-46 days), and the median time to platelet recovery (platelet levels >100 × 109 /L) was 47 days (range, 28-86 days). Grade 3 or higher neutropenia was observed in 30 patients (97%).
The most notable adverse events are outlined in Table 4. Eighty-four percent of patients (n = 26) experienced grade 3 or higher infection during the initial cycle, including 15 patients with pneumonia and 6 with bacteremia and/or sepsis. Among 20 patients who received subsequent cycle(s) of VEN-b therapy, 85% (n = 17) experienced grade 3 or higher infection. In total, 8 patients (26%) had probable invasive fungal infection during VEN-b therapy, 5 patients after the initial cycle. Grade 5 infections included 1 case of pneumonia, 2 cases each of fungal pneumonia and bacteremia (Pseudomonas in one and Staphylococcus in the other), and 3 cases of septic shock with multi-organ failure, resulting in 8 deaths due to infection. Severe hemorrhagic complications were observed in 14 patients (45%), including 6 cases of intracranial and subdural hemorrhage.
The most notable adverse events
| Treatment/infectious adverse events . | FL (n = 14) . | R/R (n = 17) . |
|---|---|---|
| Admission during first cycle, n (%) | 12 (86) | 17 (100) |
| Days in hospital during first cycle, median (range) | 25 (7-60) | 31 (7-64) |
| Neutropenic (ANC <1) before VEN initiation, n (%) | 9 (64) | 14 (82) |
| Infection requiring IV antibiotic before VEN initiation, n (%) | 2 (14) | 13 (76) |
| Pneumonia | 2 (14) | 8 (47) |
| Others* | – | 5 (29) |
| Grade 3 or higher infection during first cycle requiring IV antibiotic, n (%) | 12 (86) | 14 (93) |
| Pneumonia | 6 | 9 |
| Bacteremia† | 3: 2 GN, 1 GP | 3: 1 GN, 2 GP |
| Others‡ | 3 | 2 |
| Grade 3 or higher infection during subsequent cycles, n/N treated (%) | 7/9 (78) | 10/11 (91) |
| Pneumonia | 3 | 7 |
| Bacteremia† | 2: 2 GN | 1: 1 GP |
| Others§ | 2 | 2 |
| New probable/proven IFD while on VEN therapy, n (%) | 3 (21) | 5 (29) |
| Patients with >1 infection/>1 organ involvement, n (%) | 7 (50) | 9 (53) |
| Severe hemorrhage (requiring inpatient intervention), n (%) | ||
| CNS (subdural, intracranial) hemorrhage | 2 (14) | 4 (24) |
| Gastrointestinal | 1 (7) | 2 (12) |
| Lung (diffuse alveolar hemorrhage) | 2 (14) | 3 (18) |
| Non-hematologic grade 3 or higher in >2 patients, n (%) | ||
| Fatigue | 4 (29) | 5 (29) |
| Gastrointestinal symptoms | 3 (21) | 4 (24) |
| Mortality <30 d | 1 (7) | 2 (12) |
| Total mortality <60 d | 3 (21) | 7 (42) |
| Treatment/infectious adverse events . | FL (n = 14) . | R/R (n = 17) . |
|---|---|---|
| Admission during first cycle, n (%) | 12 (86) | 17 (100) |
| Days in hospital during first cycle, median (range) | 25 (7-60) | 31 (7-64) |
| Neutropenic (ANC <1) before VEN initiation, n (%) | 9 (64) | 14 (82) |
| Infection requiring IV antibiotic before VEN initiation, n (%) | 2 (14) | 13 (76) |
| Pneumonia | 2 (14) | 8 (47) |
| Others* | – | 5 (29) |
| Grade 3 or higher infection during first cycle requiring IV antibiotic, n (%) | 12 (86) | 14 (93) |
| Pneumonia | 6 | 9 |
| Bacteremia† | 3: 2 GN, 1 GP | 3: 1 GN, 2 GP |
| Others‡ | 3 | 2 |
| Grade 3 or higher infection during subsequent cycles, n/N treated (%) | 7/9 (78) | 10/11 (91) |
| Pneumonia | 3 | 7 |
| Bacteremia† | 2: 2 GN | 1: 1 GP |
| Others§ | 2 | 2 |
| New probable/proven IFD while on VEN therapy, n (%) | 3 (21) | 5 (29) |
| Patients with >1 infection/>1 organ involvement, n (%) | 7 (50) | 9 (53) |
| Severe hemorrhage (requiring inpatient intervention), n (%) | ||
| CNS (subdural, intracranial) hemorrhage | 2 (14) | 4 (24) |
| Gastrointestinal | 1 (7) | 2 (12) |
| Lung (diffuse alveolar hemorrhage) | 2 (14) | 3 (18) |
| Non-hematologic grade 3 or higher in >2 patients, n (%) | ||
| Fatigue | 4 (29) | 5 (29) |
| Gastrointestinal symptoms | 3 (21) | 4 (24) |
| Mortality <30 d | 1 (7) | 2 (12) |
| Total mortality <60 d | 3 (21) | 7 (42) |
ANC, absolute neutrophil count; CNS, central nervous system; IFD, invasive fungal disease.
Others: 3 colitis or abdominal infection, and 2 cellulitis in R/R.
Gram-negative bacteremia (GN); gram-positive bacteremia (GP).
Others: 1 mastoiditis and 2 cellulitis for FL, and 1 abdominal infection and 1 cellulitis in R/R.
Others: 2 colitis for FL, 1 colitis and 1 cellulitis for R/R.
Nonhematologic grade 3 or higher adverse events were uncommon and mostly included fatigue and gastrointestinal symptoms (diarrhea, nausea/vomiting) in 9 and 7 patients, respectively. With a short ramp-up of the VEN,16 no clinically significant tumor lysis syndrome was observed. Two patients were treated only as outpatients, 11 patients remained in the hospital for the first 7 to 10 days during VEN ramp-up, and the remaining patients required hospitalization for >21 days.
Ten patients (32%) died within 60 days from initiation of VEN-b therapy; 3 patients (21%) were on FL therapy. Three patients (1 FL, 2 R/R) died within the first 30 days (4-week mortality, 10%), 2 due to infection (Pseudomonas sepsis and fungal pneumonia), and 1 due to intracranial hemorrhage. The remaining 7 deaths within the first 60 days included intracranial hemorrhage in 1 patient and infectious complications in 6 patients. None of these 10 patients was in remission or had recovered blood counts.
Discussion
Post–MPN-AML is a relatively rare but a serious consequence of MPNs that is associated with a dismal prognosis. These individuals represent the most difficult to treat subgroup of AML patients, with very limited treatment options. Notwithstanding many recently approved therapies for patients with AML, including the BCL-2 inhibitor VEN, their promise in patients with post–MPN-AML remains unproven.
In this retrospective analysis, we saw modest clinical responses with no evidence of improvement in OS in patients newly diagnosed with post–MPN-AML and lack of clinical efficacy in those with relapsed disease treated with regimens containing VEN. These therapies were associated with considerable toxicity and treatment-associated mortality stemming from prolonged myelosuppression, especially in relapsed patients. Eighty-one percent of agents administered in combination with VEN were of lower intensity, 58% of which were HMAs (AZA or DAC, in 18 total).
Among FL patients treated with all regimens (n = 14) and those treated with HMA-VEN (n = 8), 6 (43%) and 4 (50%) achieved marrow responses (CR/CRi), respectively. These results are comparable to DAC single-agent or DAC-RUX responses reported by our own group and others (CR/CRi, ∼40%-50%)14,15,17 and compare favorably with the 25% CR (2 patients) rate observed among 8 newly diagnosed post–MPN-AML patients treated with HMA-VEN at the Mayo Clinic.8 These responses have not translated into improved survival of these patients; median OS of all patients and of the FL patients was 7 and 9 months, respectively. This is similar to the median of 6 months reported by others.8 We anticipated that these patients would have inferior OS compared with those with non–MPN-transformed AML treated with FL HMA-VEN (median, 14.7 months with AZA-VEN)2 ; however, the addition of VEN did not improve OS over that reported with HMA regimens used without VEN by our own group (single-agent DAC and DAC-RUX produced a median OS of 7 months each).14,15
None of our 17 relapsed patients (10 treated with HMA-VEN) exhibited any clinical response, and only 1 patient had disease stabilization for >6 months. These patients were younger than our FL cohort (nearly 50% were aged <65 years), and 65% were treated with VEN-b therapy as first salvage. Median OS from initiation of VEN-b therapy was 3 months, and 42% of patients died within 8 weeks from VEN-b therapy initiation without count recovery or subsequent treatment. Although a recent report from another group7 showed 2 CRs of 7 R/R patients with post–MPN-AML treated with HMA-VEN, their median OS was similarly poor (only 4.2 months). Other reports on relapsed patients with non–MPN-transformed AML treated with VEN-b regimens found variable response rates between 21% and 64%, with a median OS of up to 11 months.18-21
In line with other reports, we confirmed the importance of SCT for long-term survival. In this cohort, 2 FL patients were able to proceed to SCT after therapy with VEN–IDH1 inhibitor and VEN-cladribine-LDAC. None of our 18 patients who were treated with HMA-VEN (FL or R/R) were able to undergo SCT, which was different from the experience of others,7,8 in which 4 total patients (2 FL and 2 R/R) with post–MPN-AML were able to undergo SCT after VEN-b therapy. In our series, both FL responders undergoing subsequent SCT acquired an IDH1/2 mutation upon progression from the chronic MPN phase, indicating a role of the mutant IDH clone in disease transformation, as is well known.22 These mutations are known to be sensitive to VEN14,23 and might explain the favorable outcome. Indeed, 1 patient received therapy with VEN in combination with an IDH1 inhibitor only and achieved CR after 1 cycle. In the era of IDH inhibitors, these agents should be preferred for IDH1/2–mutated post–MPN-AML patients. Recently, we reported 43% CR/CRi achieved with therapy based on IDH inhibitors in FL patients with a post–MPN-AML harboring IHD1/2 mutation who had an impressive OS of 19 months and ongoing.24
In contrast to a few reports of VEN in combination with intensive chemotherapy for non–MPN-transformed AML patients exhibiting CR/CRi rates in excess of 60% for both FL and R/R patients, median OS not yet reached with 60% of patients surviving >6 months,25,26 this approach did not yield any responses in our post–MPN-AML patients. Among the 6 patients with a median age of 60 years (range, 52-71 years) in our series treated with VEN and high-dose chemotherapy (2 FL, 3 first salvage, 1 second salvage), none achieved any response.
Overall, infections in the setting of myelosuppression were the most common complications of VEN-b therapy. New grade 3 or higher infections during the initial cycle were observed in 86% of FL patients and 93% of R/R patients. Although many factors could have contributed to this prolonged myelosuppression, such as poor-risk disease features, pretreatment cytopenias (grade 3 neutropenia and thrombocytopenia were present at baseline in 64% and 29% of FL patients and 82% and 67% of R/R patients, respectively), previous therapies or VEN dosing (7 patients received higher than the currently recommended dose with a concomitant cytochrome P450 3A4 inhibitor12 ), a similar rate of serious infections (87%) was observed in R/R post–MPN-AML patients treated with HMA-VEN in other studies.7
Reduction of VEN dose or therapy duration at any point occurred in 8 (57%) FL patients and 7 (42%) R/R patients, respectively. Although these treatment modifications could have affected the response, they were primarily done for myelosuppression during subsequent cycles in patients without disease progression. The question of whether higher VEN exposure in subsequent cycles could have induced more delayed responses remains unanswered. Likewise, previous exposure to HMA observed in 36% and 59% of FL and R/R patients, respectively, could have had an impact on the responses. Among 9 FL patients who were not given HMA for their antecedent MPN, 6 belonged to the responder group, making this a viable hypothesis that is worth further investigation.
Preliminary reports in the literature have speculated that VEN might represent a valuable treatment of patients with post–MPN-AML, especially for patients intended for SCT. Although we showed that VEN in combination with low-intensity therapy was capable of inducing overall marrow responses in 43% of newly diagnosed patients, this therapy failed to induce responses in the relapsed disease, including first salvage, and responses were not durable. Observed responses in FL patients were comparable to those described with already available regimens (∼40% with DAC-RUX in our experience15 ) and, more importantly, did not translate into improved survival or greater ability to undergo SCT, the ultimate goal for all eligible patients. Lastly, severe infectious complications in the majority of patients and treatment-related mortality in about one-third make this approach challenging for clinical practice.
Although our report is limited by its retrospective nature, sample size, and the heterogeneity of regimens used, it represents the largest series of patients with post–MPN-AML treated with VEN-b therapy reported from one institution. A currently ongoing clinical trial (#NCT03874052) evaluating RUX-VEN in R/R AML shall provide prospective data for clinical use of these agents even in this patient subset. Preclinical data yielded debatable results on the role of VEN in this subtype of AML. It is known that a crucial survival pathway for MPN cells is the hyperactive JAK2/STAT5/ BCL-XL signaling axis, causing increased expression of BCL-XL and/or MCL-1 and decreased levels of BCL2, suggesting primary resistance to BCL-2 inhibitors. Acquired resistance might occur through chronic exposure to VEN and according to some reports even RUX, although others suggest the exact opposite.4,5,27-30 It remains to be determined what is the precise role of these medications in patients with AML evolving from MPN who have been chronically pretreated with RUX and might have experienced RUX failure (via re-establishment of JAK2/STAT5 signaling31 ), and what were their major mechanisms leading to AML transformation.
Alternative treatment strategies, such as DAC-RUX, cladribine-based regimens (without addition of VEN), or targeted IDH inhibitors for patients carrying these mutations, should remain priorities for patients with post–MPN-AML. Novel approaches, ideally targeting key mechanisms involved in disease pathogenesis or progression, are urgently needed. Identification of such targets is of utmost importance, as it would help us design strategies with the potential to elicit disease responses while maintaining acceptable safety and allow eligible patients to undergo potentially curative SCT. Some promising compounds with preclinical rationale include bromodomain inhibitors or the BCL-2/BCL-2-XL inhibitor navitoclax.27 As treatment options continue to expand for patients with AML, including the most vulnerable elderly population, we hope that the therapeutic repertoire for patients with post–MPN-AML will follow suit.
Send data sharing requests via e-mail to the corresponding author, Srdan Verstovsek (sverstov@mdanderson.org).
Acknowledgment
This work was supported in part by a Cancer Center Support Grant to MD Anderson Cancer Center (P30 CA016672) from the National Cancer Institute, National Institutes of Health.
Authorship
Contribution: L.M. and S.V. designed the study, analyzed the data, and wrote the manuscript; L.M. and L.Z. reviewed patients’ charts and collected the data; and L.Z. provided administrative support. All authors treated patients and provided study material, and all authors had access to data and reviewed and approved the final version of the manuscript.
Conflict-of-interest disclosure: P.B. reports honoraria and research funding from Incyte, Celgene, CTI BioPharma, Blueprint, and Kartos Therapeutics; and research funding from Constellation Pharmaceuticals, Promedior, NS Pharma, Astellas, and Pfizer. N.G.D. reports an advisory role for Daiichi Sankyo, BMS, Astellas, AbbVie, Genentech, Immunogen, Pfizer, Amgen, Forty-Seven, and Novartis; and research funds from BMS, Pfizer, Forty-Seven, Genentech, AbbVie, Astellas, Daiichi Sankyo, Incyte, Novimmune, and Immunogen. N.P. reports consulting/honorarium from Pacylex, Celgene, Stemline, Incyte, Novartis, Mustang Bio, Roche Diagnostics, and LFB; and research funding from Stemline, Novartis, AbbVie, Samus, Cellectis, Plexxikon, Daiichi Sankyo, Affymetrix, and the SagerStrong Foundation. T.M.K. reports consulting for Agios, Genentech, Jazz, Novartis, Pfizer, and AbbVie; and research support from Bristol Myers Squibb, Pfizer, Amgen, Jazz, Genentech, Celgene, and AbbVie. S.V. reports research funding from Incyte, Roche, NS Pharma, Celgene, Gilead, Promedior, CTI Biopharma, AbbVie, Blueprint Medicines Corp., Novartis, Sierra Oncology, PharmaEssentia, Constellation, Ital Pharma, Protagonist, and Kartos. M.K. reports research funding and grants from AbbVie, Genentech, F. Hoffmann–La Roche, Stemline Therapeutics, Amgen, Forty-Seven, Kisoji, Eli Lilly, Cellectis, Calithera, Ablynx, Agios, Ascentage, Astra Zeneca, Reata Pharmaceuticals, Rafael Pharmaceuticals, and Sanofi; and Patent US 7 795 305 B2 CDDO-compounds and combination therapies, with royalties paid to Reata Pharmaceuticals, a patent combination therapy with a mutant IDH1 inhibitor, a BCL-2 licensed to Eli Lilly, and a patent 62/993 166 combination of MCL-1 inhibitor and midostaurin, uses and pharmaceutical compositions thereof pending to Novartis. C.D.D. reports research support from AbbVie, Agios, Calithera, Cleave, BMS/Celgene, Daiichi Sankyo, ImmuneOnc, Loxo; and consultant/Advisory Board roles for AbbVie, Agios, Aprea, Celgene/BMS, Foghorn, ImmuneOnc, Novartis, Takeda, and Notable Labs. The remaining authors declare no competing financial interests.
Correspondence: Srdan Verstovsek, Department of Leukemia, The University of Texas MD Anderson Cancer Center, 1515 Holcombe Blvd, Unit 428, Houston, TX 77030; e-mail: sverstov@mdanderson.org.
References
Author notes
The full-text version of this article contains a data supplement.