Key Points
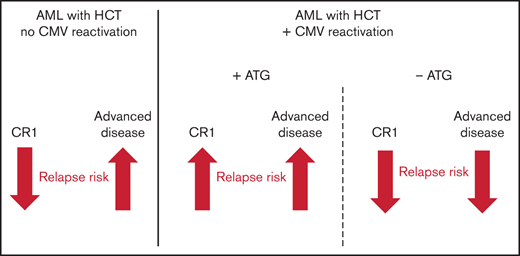
The impact of CMV reactivation on hematologic relapse after HCT is modulated by AML stage (CR1 or advanced) and in vivo T cell depletion.
Following CMV reactivation, NRM was increased in CR1 patients without ATG, but not in patients with ATG or advanced disease stages.
Abstract
Cytomegalovirus (CMV) reactivation is a frequent complication after allogeneic hematopoietic cell transplantation (HCT), whose impact on clinical outcome, in particular on leukemic relapse, is controversial. We retrospectively analyzed 687 HCT recipients with acute myeloid leukemia (AML) and ciclosporin-based immunosuppression to better understand the differential impact of CMV on transplant outcomes depending on AML disease stage and in vivo T cell depletion with antithymocyte globulin (ATG). Without ATG, CMV reactivation associated with significantly reduced relapse, yet its effect was more pronounced for advanced disease AML (P = .0002) than for patients in first complete remission (CR1, P = .0169). Depending on the disease stage, ATG exposure abrogated relapse protection following CMV reactivation in advanced stages (P = .796), while it inverted its effect into increased relapse for CR1 patients (P = .0428). CMV reactivation was associated with significantly increased nonrelapse mortality in CR1 patients without ATG (P = .0187) but not in those with advanced disease and ATG. Following CMV reactivation, only patients with advanced disease had significantly higher event-free survival rates as compared with patients without CMV. Overall, our data suggest that both ATG and disease stage modulate the impact of post-HCT CMV reactivation in opposite directions, revealing a level of complexity that warrants future studies regarding the interplay between antivirus and antitumor immunity.
Introduction
Cytomegalovirus (CMV) reactivation is a very common complication after allogeneic hematopoietic cell transplantation (HCT).1-3 However, its impact on clinical outcome has been controversial: most studies associate CMV viremia and particularly the development of CMV end-organ disease with decreased overall survival (OS)4,5 and with increased nonrelapse mortality (NRM) across different hematologic malignancies.4-8 Conversely, other studies did not find such associations between CMV reactivation and NRM,9,10 or observed comparable OS of patients with and without CMV reactivation.7,11,12 Based on baseline characteristics, recent registry studies from Japan13 and France14 defined CMV risk scores for NRM and CMV reactivation that will need further prospective validation. Currently, the donor (D) and recipient (R) CMV serostatus are the standard risk indicators of CMV reactivation and OS after HCT.15 The D−/R− serostatus was shown to associate with higher OS,4,5 while the R+ serostatus associated with higher rates of both CMV reactivation and NRM.4 As a consequence of these differential outcomes, many HCT centers use the CMV serostatus for donor selection.
A major controversy in ongoing discussions on CMV revolves around its potential protective impact on leukemic relapse. Reduced relapse rates in HCT recipients with CMV replication have been first reported in 1986,16 and the effect of CMV seropositivity on relapse independent of chronic graft-versus-host disease (GVHD) was described in the early 2000s.17 A study from our HCT department associated CMV pp65 antigenemia with reduced relapse, independent from D-R CMV serostatus.9 Other studies confirmed this finding for HCT patients with acute leukemia,7,18 chronic myeloid leukemia,11 myeloproliferative disorders12 and lymphoma19 applying either pp65 detection or CMV-specific, quantitative polymerase chain reaction (qPCR) assays. Intriguingly, it was shown that this association between CMV and relapse was not observed in patients with antithymocyte globulin (ATG) exposure.20 Accordingly, registry studies reported comparable relapse rates for patients with or without CMV reactivation.4,21 Different CMV detection methods and thresholds defining reactivations complicated the comparison of results across different studies and countries. While qPCR has become the current standard for monitoring CMV in most countries, it is not approved for example, in Japan.22 Differences in sensitivity between various assays had also been discussed.23 Given these controversies, we retrospectively analyzed a large, longitudinal cohort of HCT recipients transplanted for acute myeloid leukemia (AML) at our center to better understand the differential impact of CMV on transplant outcomes depending on disease stage, detection technique, and in vivo T cell depletion with ATG.
Patients and methods
Patients
Between October 1997 and October 2017, 687 patients with AML underwent HCT with a uniform calcineurin inhibitor-based GVHD prophylaxis (predominantly ciclosporin plus methotrexate) in the Department of Bone Marrow Transplantation of the West-German Cancer Center at University Hospital Essen. Donors were HLA-matched related donors (MRD, 31%), 10/10 HLA-A-, -B, -C, -DRB1, -DQB1 matched unrelated donors (MUD, 62%), or 9/10 mismatched unrelated donors (MMUD, 7%; Table 1). HLA-DPB1 was not considered for donor-recipient matching. Patients receiving haploidentical HCT with post-transplant cyclophosphamide were not included. Assignment to ATG-prophylaxis was based on standardized clinical treatment protocols (detailed in supplemental Methods) for patients with higher GVHD risk. Patients were followed-up for 60 months after transplantation; surviving patients were censored at maximum follow-up. Early supportive and follow-up care was identical for all patients. The primary study endpoint was relapse, additional endpoints were NRM, acute and chronic GVHD, OS, and event-free survival (EFS). Details on patient treatment, HCT specific assessments, and endpoints are provided in the supplemental Methods section.
Patient baseline characteristics
| . | Overall cohort . | No ATG . | ATG . | P . |
|---|---|---|---|---|
| N (%) . | N (%) . | N (%) . | ||
| Total enrolled and treated, n (%) | 687 (100) | 420 (100) | 267 (100) | |
| Median age at HCT (range) | 50 (16-76) | 48 (16-73) | 55 (18-76) | <.0001 |
| Male gender, n (%) | 346 (51) | 228 (54) | 118 (44) | .0121 |
| Acute myeloid leukemia | 687 (100) | 420 (100) | 267 (100) | |
| First CR,* n (%) | 293 (43) | 164 (39) | 129 (48) | .017 |
| Advanced disease stages,† n (%) | 394 (57) | 256 (61) | 138 (52) | |
| Cytomegalovirus serostatus constellation, n (%) | ||||
| D+/R− | 52 (8) | 29 (7) | 23 (9) | <.0001 |
| D+/R+ | 285 (41) | 172 (41) | 113 (42) | |
| D−/R+ | 147 (21) | 76 (18) | 71 (27) | |
| D−/R− | 203 (30) | 143 (34) | 60 (22) | |
| Donor-recipient constellations | ||||
| MRD | 214 (31) | 214 (51) | 0 (0) | <.0001 |
| MUD | 424 (62) | 198 (47) | 226 (85) | |
| MMUD | 49 (7) | 8 (2) | 41 (15) | |
| D/R gender: f/m | 77 (11) | 58 (14) | 19 (7) | .0063 |
| Other | 610 (89) | 362 (86) | 248 (93) | |
| Donor age, median (95% CI) | 38 (20-64) | 41 (22-64) | 32 (20-52) | <.0001 |
| Graft source | ||||
| PBSC | 617 (90) | 365 (87) | 255 (96) | <.0001 |
| BM | 67 (10) | 55 (13) | 12 (4) | |
| Conditioning | ||||
| MAC | 272 (40) | 226 (54) | 46 (17) | <.0001 |
| RIC | 412 (60) | 191 (46) | 221 (83) | <.0001 |
| . | Overall cohort . | No ATG . | ATG . | P . |
|---|---|---|---|---|
| N (%) . | N (%) . | N (%) . | ||
| Total enrolled and treated, n (%) | 687 (100) | 420 (100) | 267 (100) | |
| Median age at HCT (range) | 50 (16-76) | 48 (16-73) | 55 (18-76) | <.0001 |
| Male gender, n (%) | 346 (51) | 228 (54) | 118 (44) | .0121 |
| Acute myeloid leukemia | 687 (100) | 420 (100) | 267 (100) | |
| First CR,* n (%) | 293 (43) | 164 (39) | 129 (48) | .017 |
| Advanced disease stages,† n (%) | 394 (57) | 256 (61) | 138 (52) | |
| Cytomegalovirus serostatus constellation, n (%) | ||||
| D+/R− | 52 (8) | 29 (7) | 23 (9) | <.0001 |
| D+/R+ | 285 (41) | 172 (41) | 113 (42) | |
| D−/R+ | 147 (21) | 76 (18) | 71 (27) | |
| D−/R− | 203 (30) | 143 (34) | 60 (22) | |
| Donor-recipient constellations | ||||
| MRD | 214 (31) | 214 (51) | 0 (0) | <.0001 |
| MUD | 424 (62) | 198 (47) | 226 (85) | |
| MMUD | 49 (7) | 8 (2) | 41 (15) | |
| D/R gender: f/m | 77 (11) | 58 (14) | 19 (7) | .0063 |
| Other | 610 (89) | 362 (86) | 248 (93) | |
| Donor age, median (95% CI) | 38 (20-64) | 41 (22-64) | 32 (20-52) | <.0001 |
| Graft source | ||||
| PBSC | 617 (90) | 365 (87) | 255 (96) | <.0001 |
| BM | 67 (10) | 55 (13) | 12 (4) | |
| Conditioning | ||||
| MAC | 272 (40) | 226 (54) | 46 (17) | <.0001 |
| RIC | 412 (60) | 191 (46) | 221 (83) | <.0001 |
95% CI, 95% confidence interval; BM, bone marrow; D, donor; HCT, allogeneic hematopoietic stem cell transplantation; MAC, myeloablative conditioning; MRD, matched related donor transplant; MUD, matched unrelated donor transplant; PBSC, peripheral blood stem cells; R, recipient; RIC, reduced intensity conditioning.
De novo AML in first complete remission (CR).
All other disease stages that did not correspond to AML in first CR, such as AML in second remission.
CMV monitoring
Starting with leukocyte reconstitution >500/µL, CMV titers were measured twice weekly at the Institute for Virology using qPCR24 or CMV phosphoprotein pp65 antigenemia assay25 until hospital discharge. Details of both assays are described in the supplemental Methods. Outpatient sampling was done weekly until week 16 after transplantation. Results were expressed as measured CMV copies/mL or as pp65 antigen expressing cells per 5 × 105 leukocytes. CMV reactivation was defined as a replication of >500 CMV copies per mL EDTA blood or as >5 pp65 antigen expressing cells per 5 × 105 white blood cells. In CMV R−/D− patients, CMV de novo replication was detected with the same methods. Only in 5 out of 203 R−/D− patients (2%) a primary CMV infection was detected over this long-term observation period.
Statistical analysis
For discrete variables, we applied the Fisher-Exact 2-tailed test. Continuous variables, described with median and extreme values (min-max), were studied with the Wilcoxon rank sum test. Cumulative incidences of relapse and NRM were calculated as time-dependent endpoints with mutually competing events. The homogeneity of the cumulative incidence functions was tested by the Gray method.26 Corresponding subdistribution hazards and 95% confidence intervals (95% CI) were calculated using the Fine and Gray method.27 OS and EFS were analyzed with the Kaplan-Meier method. The log-rank test compared the heterogeneity of survival distributions. P-values in the log-rank test were calculated for 2-sided 95% CIs, which was also adopted for Cox-regression analyses. For multiple testing, the significances were adjusted according to the method of Šidák,28 and a P-value <.05 was accepted to indicate statistical significance. The multivariate Cox regression models for relapse included the following factors: AML disease stage at HCT, HLA disparities, gender constellation (female donor for male recipient vs others), bone marrow or PBSC, conditioning, CMV+ serostatus, CMV reactivation, and acute and chronic GVHD. Nonbaseline factors, such as CMV reactivation and acute and chronic GVHD, were integrated as variables into the multivariate models. All analyses were performed with Statistical Analysis Software (SAS, Release 9.4, Version 7.11 (7.100.1.2711); SAS Institute Inc., Cary, NC).
Ethics
This study was conducted in accordance with German legislation and the revised Helsinki Declaration. Study design and data acquisition was evaluated by the institutional review board of the University Duisburg-Essen (Protocol No. 18-8496-BO). All patients have given written consent to collection, electronic storage, and scientific analysis of anonymized HCT-specific patient data. We confirm that no patient can be identified by use of anonymized patient data.
Results
A total of 687 consecutive patients with AML underwent HCT with a uniform calcineurin inhibitor-based GVHD prophylaxis. A relevant fraction (N = 267, 39%) additionally received in vivo T cell depletion using ATG. Patient baseline characteristics including disease status at HCT; transplant, donor, and gender constellation; CMV serostatus; and conditioning regimen are detailed in Table 1.
CMV and relapse
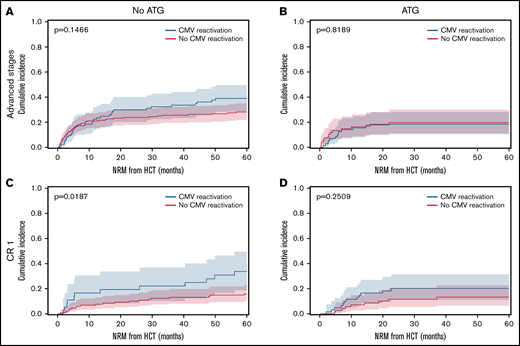
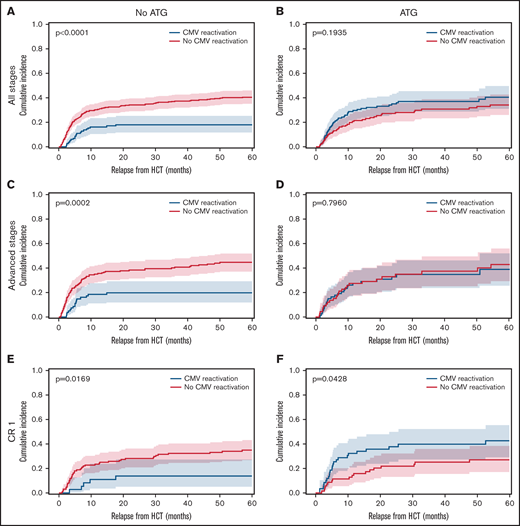
The overall incidence of early CMV reactivation (before d+100) was up to 52% when measured by qPCR. CMV reactivation occurred significantly more frequently in patients with ATG exposure (48.7%) than in patients without ATG (27.8%, P < .0001). No significant differences were detected within ATG dosage subgroups. In absence of ATG prophylaxis, CMV reactivation associated with significantly reduced relapse rates for all AML disease stages. The relapse incidence for patients with CMV reactivation was 0.18 (95% confidence interval [CI], 0.12-0.25) compared with 0.41 (95% CI, 0.35-0.46) for patients without CMV (Figure 1A, P < .0001). However, exposure to ATG abrogated this protective effect on relapse (Figure 1B, P = .1935). That same association and its ATG-dependent loss were confirmed for the subgroup of patients with advanced disease stages (P = .0002, Figure 1C-D). Also, in the no-ATG subgroup of AML patients in CR1 the relapse incidence was significantly lower with CMV reactivation (0.14, 95% CI 0.05-0.27) than without (0.35, 95% CI 0.26-0.43; Figure 1E, P = .0169). In addition to the previously described reduction of early relapse events in the presence of CMV reactivation after HCT, the present data also revealed a reduction in late relapse events (>24 months) for patients with CMV reactivation in the absence of ATG (Figure 1A,C,E). In contrast, the effect on late relapse events was again not detectable in the ATG-receiving cohorts (Figure 1B,D,F), which is indicative of the impact of ATG-susceptible cells, most likely T cells, in the containment of late relapse. Interestingly, the opposite was observed for CMV reactivation in AML patients in CR1 who had received ATG for in vivo T cell depletion (Figure 1F). Here, CMV reactivation associated with an inverse effect of significantly increased relapse (P = .0428). Due to the above-described differences between patients with or without ATG exposure, we separately analyzed the cohorts in a series of multivariate analyses including the above-mentioned covariables. For 420 patients without ATG, multivariate analysis confirmed CMV reactivation as an independent significant factor of relapse after HCT (Figure 2A; hazard ratio [HR] 0.42, 95% CI 0.26-0.68) along with chronic GVHD (HR 0.44, 95% CI 0.30-0.65) and positive donor serostatus (CMV+; HR 0.69, 95%CI 0.48-0.87). In the 267 patients that received ATG, multivariate analysis did not associate these cofactors with significant differences in relapse (Figure 2B). Both CMV+ donor serostatus (HR 0.82, 95% CI 0.54-1.24) and CMV reactivation did not reach significance after ATG exposure (HR 1.35, 95% CI 0.87-2.10). Of notice, the R−/D+ CMV serostatus alone also associated with reduced relapse in patients without ATG (P = .0179, Figure 2C) but had a numerically, nonsignificant, increased relapse with ATG (Figure 2D).
Cumulative relapse incidence depending on CMV reactivation, in vivo T cell depletion and disease stage. (A) Patient cohort without ATG (n = 420). (B) Patient cohort with ATG (n = 267). (C) Advanced disease stages subgroup without ATG (n = 256). (D) Advanced disease stages subgroup with ATG (n = 138). (E) AML in CR1 subgroup without ATG (n = 164). (F) AML in CR1 subgroup with ATG (n = 129). Cumulative incidence function of relapse (60 months censored) depending on CMV reactivation (blue) and absence of CMV reactivation (red). Median (line) with 95% confidence interval (CI) shaded. All P values refer to comparisons of strata with Gray’s test.
Cumulative relapse incidence depending on CMV reactivation, in vivo T cell depletion and disease stage. (A) Patient cohort without ATG (n = 420). (B) Patient cohort with ATG (n = 267). (C) Advanced disease stages subgroup without ATG (n = 256). (D) Advanced disease stages subgroup with ATG (n = 138). (E) AML in CR1 subgroup without ATG (n = 164). (F) AML in CR1 subgroup with ATG (n = 129). Cumulative incidence function of relapse (60 months censored) depending on CMV reactivation (blue) and absence of CMV reactivation (red). Median (line) with 95% confidence interval (CI) shaded. All P values refer to comparisons of strata with Gray’s test.
Multivariate analysis of relapse influencing variables and impact of pre-HCT serostatus constellation. (A) Patient cohort without ATG (n = 420). Multivariate analysis of relapse as time-dependent endpoint. Forest plots showing results from multivariate analysis including all significant covariates from univariate analysis with respect to relapse. (B) Patient cohort with ATG (n = 267). Multivariate analysis of relapse as time-dependent endpoint. Plots as in panel A. (C) Cumulative relapse incidence depending on CMV serostatus without ATG and (D) with ATG. Cumulative incidence function of relapse (60 months censored) compared by CMV serostatus (R+/D+ [blue], R+/D− [red], R−/D+ [green], and R−/D− [brown]. Median [line] with 95% CI shaded). C+D: P values according to Gray’s test. Abbreviations: GVHD, graft-versus-host disease; HLA, human leukocyte antigen; MAC, myeloablative conditioning; PBSC, peripheral blood derived stem cells.
Multivariate analysis of relapse influencing variables and impact of pre-HCT serostatus constellation. (A) Patient cohort without ATG (n = 420). Multivariate analysis of relapse as time-dependent endpoint. Forest plots showing results from multivariate analysis including all significant covariates from univariate analysis with respect to relapse. (B) Patient cohort with ATG (n = 267). Multivariate analysis of relapse as time-dependent endpoint. Plots as in panel A. (C) Cumulative relapse incidence depending on CMV serostatus without ATG and (D) with ATG. Cumulative incidence function of relapse (60 months censored) compared by CMV serostatus (R+/D+ [blue], R+/D− [red], R−/D+ [green], and R−/D− [brown]. Median [line] with 95% CI shaded). C+D: P values according to Gray’s test. Abbreviations: GVHD, graft-versus-host disease; HLA, human leukocyte antigen; MAC, myeloablative conditioning; PBSC, peripheral blood derived stem cells.
OS, NRM, EFS, and GVHD
OS did not significantly differ between the cohort with or without CMV reactivation (P = .833, supplemental Figure 1A), while the NRM was significantly increased in patients with CMV reactivation (P = .0424, supplemental Figure 1B). Patients in CR1 had higher NRM following CMV reactivation, while patients with advanced AML stages had higher NRM than CR1 patients regardless of CMV reactivation (supplemental Figure 1C-D; Figure 3A-B). The increased NRM of patients in CR1 with CMV reactivation, however, only reached significance in the no-ATG subgroup (P = .0187, Figure 3C-D). In both disease constellations, ATG exposure associated with relatively lower NRM (Figure 3B,D) due to reduced GVHD (supplemental Table 1). The observed significant differences in relapse (Figure 1C,E) translated into significantly improved OS for AML patients with CMV reactivation and advanced disease (P = .0485). The 4 CMV serostatus risk categories (D+/R+, D+/R−, D−/R+, D−/R−) had a significant impact on OS only in the absence of ATG (P = .0446, supplemental Figure 2A). Without ATG, positive donor serostatus (D+) also associated with significantly higher OS (P = .0059, supplemental Figure 2C). The EFS of the overall cohort showed no significant differences depending on CMV reactivation. However, when patients were stratified by disease stage and ATG exposure, CMV reactivation associated with significantly higher EFS in patients with advanced disease or without ATG, while the opposite was observed for patients in CR1 or with ATG exposure (supplemental Figure 3). As expected, the incidence of grades III-IV acute GVHD or extensive chronic GVHD was higher in patients without ATG exposure (supplemental Table 1). Both grades II-IV acute GVHD and CMV reactivation reduced the cumulative incidence of relapse (supplemental Figure 4). Interestingly, additive relapse reduction effects were observed for sequential events of acute GVHD and CMV reactivation. In the no-ATG subgroup the sequence of acute GVHD followed by CMV reactivation associated with reduced relapse, while this was not the case for the ATG subgroup with the same sequence. Although being a small subgroup, the sequence of CMV reactivation followed by acute GVHD resulted in the lowest relapse rate both with and without ATG (P = .0148).
Cumulative incidence of NRM depending on CMV reactivation, in vivo T cell depletion and disease stage. Cumulative incidence function of nonrelapse mortality (NRM) with relapse as competing risk (60 months censored). Patients with CMV reactivation (blue) and without CMV reactivation (red). Median (line) with 95% CI shaded. (A) AML advanced disease stages without ATG (n = 256). (B) AML advanced disease stages with ATG (n = 138). (C) AML in CR1 without ATG (n = 164). (D) AML in CR1 with ATG (n = 129). All P values refer to comparisons of strata with Gray’s test.
Cumulative incidence of NRM depending on CMV reactivation, in vivo T cell depletion and disease stage. Cumulative incidence function of nonrelapse mortality (NRM) with relapse as competing risk (60 months censored). Patients with CMV reactivation (blue) and without CMV reactivation (red). Median (line) with 95% CI shaded. (A) AML advanced disease stages without ATG (n = 256). (B) AML advanced disease stages with ATG (n = 138). (C) AML in CR1 without ATG (n = 164). (D) AML in CR1 with ATG (n = 129). All P values refer to comparisons of strata with Gray’s test.
qPCR and pp65 CMV detection methods
In order to analyze the impact of the CMV detection method on the reporting of CMV reactivation rates, we separately analyzed patients monitored by qPCR (n = 294) or pp65 antigen (n = 393) methods within each patient subset. In patients without ATG exposure, both methods led to comparable results. The cumulative incidence of early CMV reactivation detected by pp65 was 27.0% (95% CI 22.2-32.0, P = .39, supplemental Figure 5A) and 30.3% with qPCR (95% CI 21.7-39.5). In patients with ATG, however, the cumulative incidence of early CMV reactivation was significantly higher when measured by qPCR (52.6, 95% CI 45.3-59.4, P = .0176) than by pp65 (38.7, 95% CI 27.6-49.5), and the time to detection of CMV reactivation was shorter.
Discussion
This study adds several new facets to our understanding of the complexity of CMV reactivation after HCT. Our data independently confirms previous studies of reduced relapse risk for AML patients without ATG after CMV reactivation,9,18 but it is also in full agreement with more recent reports,4,20 which have shown that this effect is either abrogated or attenuated if ATG was administered. We show that the protective effect of CMV reactivation observed in patients with advanced disease not receiving ATG is inverted into a predisposing effect in CR1 patients with ATG and that this in turn leads to opposing effects of numerically higher or lower EFS, respectively. Together, our data show that the effect of CMV reactivation is inversely modulated by both the use of ATG and the disease stage at transplant: while CMV reactivation has a positive effect due to lower relapse risks and no impact on NRM in patients with advanced disease not receiving ATG, it is deleterious in CR1 patients with ATG prophylaxis due to higher relapse rates. These findings have important implications for the reevaluation of a number of studies dealing with similar questions. To understand this seemingly paradox constellation, one also needs to integrate both the historical dimension of the discussion as well as novel insights into biological mechanisms. While the first study showing an impact of CMV reactivation on relapse exclusively used pp65 antigenemia monitoring,9 as also did the subsequent confirmatory reports,7,11,18 more recent studies used qPCR to confirm10 or oppose4,20 this finding. In our study, we compared the cumulative incidence of CMV reactivations depending on the detection method, and the number of CMV reactivations was higher in patients receiving ATG measured by qPCR. Beyond differences in the detection method, the definition of CMV reactivation cutoffs have been a matter of debate and heterogeneity between HCT studies.10,29 Also, the proportion of patients who received ATG differed importantly between studies, ranging between 0%,9 17%10 and 100%.20 In order to overcome this bias, we separately analyzed CMV’s association with relapse within the ATG subgroup (39%) and for all other patients. Furthermore, we distinguished AML disease stage subgroups, such as AML in CR1, which previous reports could not distinguish due to its small sample size20 or due to registry data limitations.4
Despite previous reports discussing a biological effect of CMV viremia on leukemic relapse,20,29,30 its exact mechanisms are still insufficiently understood. ATG exposure modulates how CMV replication affects the incidence of relapse after HCT,20 and a recent study31 highlighted the role of CMV kinetics and T cell subpopulations in this interaction. Indeed, the immune reconstitution of T helper cells and naïve T helper cells is impaired after ATG prophylaxis,32 and also lower CD8+ T cell receptor (TCR) repertoire diversity has been reported compared with MUD patients without ATG exposure.33,34 Poor T cell reconstitution may favor relapse.30,35 CMV reactivation after HCT has complex implications on its host T cells, driving CD8+ activation,36 narrowing the TCR repertoire37 and might also influence the presentation of HLA mismatch antigens.38 However, the overwhelming majority of published TCR repertoire data after CMV reactivation were obtained from HCT patients without ATG exposure.33,36,39 Given the limited data involving ATG-exposed HCT patients with CMV,33,38,40 one may speculate if the previously described CMV-induced skewing of the TCR repertoire36 would be consistently detectable in AML patients with ATG or if additive effects would be observed. We observed relatively increased relapse rates for AML patients in CR1 with ATG, which might possibly result from differential cross-reactive TCR profiles. In patients without ATG exposure, multivariate analysis associated CMV+ donor serostatus with reduced relapse. Lower TCR diversity has been described in HCT recipients from CMV+ donors,33 and a previous large analysis of HCT donors revealed TCR repertoires specific to CMV+ donors,41 which may possibly help to explain antirelapse effects as observed in our cohorts. Yet, the clinical impact of CMV+ donor serostatus is under discussion. A relevant European Society for Blood and Marrow Transplantation study focusing on CMV serostatus independent of the hematologic disease at HCT detected an increased relapse risk in MUD patients receiving a graft from a CMV+ donor but not in matched sibling donor HCT.42 In 2 previous studies analyzing the impact of CMV reactivation on HCT patients with T cell depletion, such serostatus-association was not described11 or analyzed.20 The most recently described cytotoxic potential of CMV-induced CD57+/CD27– CD4+ T cells may reflect one mechanism of controlling CMV-infected myeloid cells.43 With or without CMV reactivation, ATG exposed patients had more late relapse events without reaching a plateau. Novel ATG dose optimization strategies32,44,45 may improve relapse incidence and modify CMV-dependent effects. While previous reports associated CMV reactivation both with reduced early (<12 months)7 and later relapse,9,18 our data highlight that a late-relapse (>18 months)-protective impact of CMV is only detected in patients without ATG. This study has limitations due to its retrospective character, the use of 2 different detection assays (qPCR, pp65), and the inclusion of data before the approval of Letermovir for the prophylaxis of CMV reactivation. Patients in the ATG cohort were older and had a higher proportion of MUD recipients and RIC conditioning than in the no-ATG cohort. The potentially resulting increased relapse events could have supported the detection of significant outcome associations. Still, RIC did not significantly impact the relapse rate in univariate and multivariate analysis including CMV reactivation and other cofactors. Despite serostatus proportions comparable to large registry studies,4,5 the relatively small absolute number of patients with D+/R− serostatus might have influenced the OS rates for D+ patients. Both grades II-IV acute GVHD and CMV reactivation can occur sequentially and independently impact leukemic relapse. Sequential analysis of their interactions supported the hypothesis that acute GVHD may be more relevant as trigger of CMV reactivations46 in the absence of ATG than in patients with ATG exposure. Due to the long patient inclusion period, only the first episode of CMV reactivation is documented. The increasing impact of haploidentical HCT is not covered by this study. Its strength is its large AML patient population with homogenous ciclosporin-based immunosuppression, which permitted differential subgroup analyses.
The clinical impact of CMV reactivation after HCT for the treatment of AML significantly depended on both disease stage and ATG, which differentially determined the impact of CMV reactivation on relapse rates and on other HCT outcomes. ATG prophylaxis and disease stage at HCT modulate the impact of post-HCT CMV reactivation in opposite directions, revealing a level of complexity that warrants future studies regarding the interplay between antivirus and antitumor immunity.
Acknowledgments
This study received support in part by Bundesministerium für Bildung und Forschung (BMBF) grant no. 031L0027D (D.W.B.), UDE‐IFORES grant (A.T.T.) and Deutsche Forschungsgemeinschaft-UDE-UMEA grant FU 356/12-1 (A.T.T.). In part, support was received from DKMS grant number DKMS DKMS-SLS-MHG-2018-01 (P.C.). Open Access publication costs were covered by MERCUR-GYF-VI of the Mercator Foundation.
Authorship
Contribution: D.W.B. and A.T.T. designed the study; N.T.-M., T.L., and S.L. performed data collection; F.A. and L.B. participated in data acquisition; D.W.B., N.T.-M., and A.T.T. performed statistical analysis; A.T.T., D.W.B., P.C., K.F., and N.T.-M. interpreted data; M.T., N.B.L., and K.F. participated in data analysis; A.T.T. and D.W.B. wrote the manuscript; N.T.-M., P.C., K.F., and S.L. contributed to writing the manuscript; N.T.-M. contributed importantly to this manuscript with research performed in the framework of his MD thesis; and all authors had access to primary clinical trial data and read and approved the final manuscript.
Conflict-of-interest disclosure: A.T.T., consultancy for MSD, JAZZ Pharmaceuticals, CSL Behring. Travel subsidies from Neovii Biotech. All outside the submitted work. D.W.B. received travel subsidies from Medac, all outside the submitted work. The other authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Correspondence: Dietrich W. Beelen, Department of Hematology and Stem Cell Transplantation, West-German Cancer Center, University Hospital Essen, University of Duisburg-Essen, Hufelandstrasse 55, 45122 Essen, Germany; e-mail: dietrich.beelen@uk-essen.de.
References
Author notes
Requests for data sharing may be submitted to Dietrich W. Beelen (dietrich.beelen@uk-essen.de).
The full-text version of this article contains a data supplement.

![Multivariate analysis of relapse influencing variables and impact of pre-HCT serostatus constellation. (A) Patient cohort without ATG (n = 420). Multivariate analysis of relapse as time-dependent endpoint. Forest plots showing results from multivariate analysis including all significant covariates from univariate analysis with respect to relapse. (B) Patient cohort with ATG (n = 267). Multivariate analysis of relapse as time-dependent endpoint. Plots as in panel A. (C) Cumulative relapse incidence depending on CMV serostatus without ATG and (D) with ATG. Cumulative incidence function of relapse (60 months censored) compared by CMV serostatus (R+/D+ [blue], R+/D− [red], R−/D+ [green], and R−/D− [brown]. Median [line] with 95% CI shaded). C+D: P values according to Gray’s test. Abbreviations: GVHD, graft-versus-host disease; HLA, human leukocyte antigen; MAC, myeloablative conditioning; PBSC, peripheral blood derived stem cells.](https://ash.silverchair-cdn.com/ash/content_public/journal/bloodadvances/6/1/10.1182_bloodadvances.2021005509/3/m_advancesadv2021005509f2.png?Expires=1769095336&Signature=rFS3x65ZhMd9hSZSY68Hzg~3pZjVdeuflbe1BN-TDG~Ft5W40xFOVAoSKYTeM8wkwtuNs-x9nlWlc4qkUsPFab8VFvdAh47PEC8YIddDnceTJlRvtRqGZnFPIDu5HJ-Q0Q99L87S~WXJjJ5He~K8zm3g-aWWRZe8-YdkeTWk7U0ZTkwobfUwbWh2kYEAV52~K92RELen5Stn09OXYfbGShN-xMKJzuylEs51~zFFhr2-ggyk11IUWAoeqitWZgjtUG~sJNnZqXByV7zrMgKuh3hzyjFq9smex8-D9Vl6~APUGeJfTar4tP9wGr4awBnC5bLs3-cV2Cb-Ak7dHMbZFQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)