TO THE EDITOR:
Survival in patients with myelofibrosis (MF), median overall survival (OS) of 6 to 7 years, is shorter than that of the general population.1 MF is also associated with profound negative effects on quality of life. Conventional treatment options for MF have limited efficacy in improving symptomatic manifestation and lack disease-modifying potential; the only curative approach is stem cell transplantation, reserved for a minority of patients. The JAK1/2 inhibitor ruxolitinib (RUXO) acts by reducing splenomegaly and improving constitutional symptoms, with a favorable impact on quality of life. Although post hoc analysis of pooled data from the randomized COMFORT-I and COMFORT-II trials reported improvement of survival,2,3 this finding remains debatable, because the studies were not powered to show effects on OS. In 2013 the ERNEST project, whose acronym defines its purpose, the European Registry for Myeloproliferative Neoplasms: Toward a Better Understanding of Epidemiology, Survival, and Treatment,3 was founded to prospectively enroll patients with MF with the epidemiological goal of assuring reliability, representativeness, and comparability of real-world data across international centers with expertise in the management of MF. The project, promoted by European LeukemiaNet, was coordinated by FROM (Fondazione per la Ricerca Ospedale Maggiore) at Papa Giovanni XXIII hospital in Bergamo, Italy, and supported by Novartis through a research collaboration. From February 2013 through May 2014, the ERNEST registry enrolled 1292 patients with MF from 13 centers in 5 European countries. The current study describes updates (cutoff at 31 December 2018) of those patients who were alive and/or in active surveillance in November 20144 in ERNEST centers in Italy, Spain, and Sweden and who agreed to an update of their respective data; 282 of the original 1292 patients were excluded from the present analysis. The institutional review board and ethics committee of each participating center approved the study, which was conducted in accordance with the Declaration of Helsinki.
We report the results of an analysis of 1010 patients, in which we sought to analyze the impact of RUXO on OS by using prospectively collected real-world data.
Statistical analyses were performed at the biostatistical laboratory of FROM. Continuous variables were summarized by median and interquartile range, and categorical ones were presented as frequencies and percentages. Characteristics of the study population were stratified for survival, and differences between groups were tested with the χ2 test (or Fisher’s exact test, where appropriate) or the rank-sum test for categorical or continuous variables, respectively. OS was estimated by the Kaplan-Meier method and analyzed according to MF diagnosis, prognostic risk category (International Prognostic Scoring System [IPSS],5 Dynamic IPSS [DIPSS],6 myelofibrosis secondary to polycythemia vera and essential thrombocythemia-prognostic model [MYSEC-PM]7 ) and treatment exposure, with the log-rank test. Using a multivariable Cox proportional hazards model, we evaluated association with OS the following variables: age, sex, MF diagnosis, year of diagnosis, prognostic risk category, and cytoreductive therapy. We performed a propensity score (PS) matching analysis8 to balance patients who had been treated or not with RUXO, by forming matched sets of 1 treated and 1 randomly sampled, nontreated, patient (1:1 matching) who shared a similar PS. We estimated The PS by logistic regression of exposure to RUXO on baseline covariates at the beginning of treatment. Matching was performed using the nearest-neighbor method without replacement and with a caliper of width equal to 0.2 of the pooled standard deviation of the PS logit. For all tested hypotheses, a 2-tailed P < .05 was considered significant. Statistical analysis was performed with STATA Software, release 16 (StataCorp LP, College Station, TX).
The updated ERNEST registry cohort comprised 1010 patients with MF: 584 (57.8%) with primary MF (PMF), 207 (20.5%) with postessential thrombocythemia (PET)-MF, and 219 (21.7%) with postpolycythemia vera (PPV)-MF. Overall, 365 patients had died by the end of 2014; clinical data and outcome of the remaining 645 cases were updated to the end of 2018. Patients’ characteristics are shown in supplemental Table 1. The median age was 63.7 years, and 59.9% were men. According to the diagnostic period, 237 (23.5%) patients were diagnosed from 2001 through 2004, 371 (36.7%) from 2005 through 2008, and 402 (39.8%) from 2009 through 2012. In the overall cohort, 598 patients (59.2%) at enrollment had received cytoreduction therapy; 487 (48.2%) had received hydroxyurea (HU) only, and 108 patients received RUXO. Of the latter, 69 (64%) had been treated with HU and 2 (1.9%) with interferon. Among patients treated with cytoreduction during follow-up, at first administration, patients treated with RUXO were significantly younger (64.5 vs 67.0 years; P = .02), had massive splenomegaly (≥20 cm from the left costal margin; 36.6% vs 6.0%; P < .001), and had constitutional symptoms (80% vs 49.1%; P = .03) compared with those treated with HU only. Time to first treatment with HU (median, 0.0 year; range, 0.0-1.2 years) was significantly shorter than that with RUXO (median, 4.5 years; range, 2.2-6.7; P < .001). After a median follow-up of 5.2 years (range, 2.3-8.2), 625 deaths occurred with a mortality rate (per 100 person-years) of 10.9 (95% CI, 10.1-11.8). Median OS was 6.2 years (95% CI, 2.8-12.6), with no significant difference according to diagnostic categories (P = .49). Median OS of the entire study population, according to IPSS category considered at diagnosis, was not reached for the low-risk category; was 7.7 years (95% CI: 3.8-12.9) for intermediate-1, 5.0 years (95% CI: 2.2-9.1) for intermediate-2 risk categories; and was 2.8 years (95% CI: 1.5-5.0) for the high-risk category (P < .0001). According to the MYSEC-PM score, the median OS of secondary MF was not reached for low-risk patients, 6.0 years (95% CI: 2.9-10.5) for patients with intermediate-1 and 3.2 years (95% CI: 1.8-6.0) for intermediate-2 risk, and 1.8 years (95% CI: 0.7-7.1) for those with high risk (P < .001). Median OS was significantly longer in patients treated with RUXO compared with those who received HU (6.7 vs 5.1 years; P = .001). Notably, in the entire study population, the prognostic relevance of RUXO exposure was mostly restricted to patients who, at the beginning of treatment, were in DIPSS higher risk categories (intermediate-2 and high risk; HR, 0.53; 95% CI, 0.35-0.82; P = .004). In a multivariable Cox regression model adjusted for covariates measured at the beginning of treatment, age (linear covariate, HR, 1.03; 95% CI, 1.02-1.04; P < .001), male sex (HR, 1.58; 95% CI, 1.24-2.03; P < .001), and high DIPSS category (HR, 2.96; 95% CI, 1.63-5.38; P < .001) were identified as factors that negatively affected OS. Conversely, protective variables were a more recent diagnosis (2009-2012 vs 2001-2004; HR, 0.47; 95% CI, 0.35-0.65; P < .01) and treatment with RUXO (HR, 0.62; 95% CI, 0.40-0.95; P = .029).
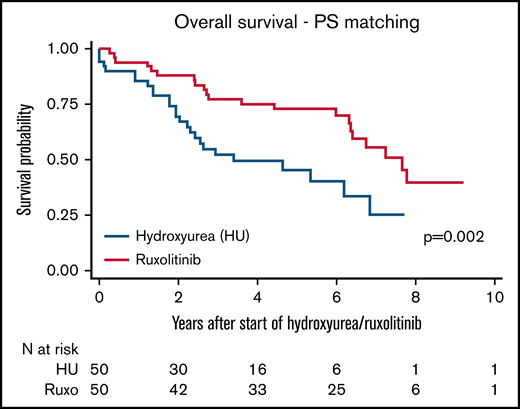
To assure comparability between patients treated with HU and RUXO, we conducted a PS-matching analysis. Characteristics of patients treated with HU only and RUXO (received as either a first- or second-line treatment after HU) before and after PS matching are reported in supplemental Table 2 (n = 50 in each group, regardless of the diagnosis). Median OS was 7.7 years in patients treated with RUXO compared with 3.4 years in patients treated with HU only (P = .002; Figure 1). Furthermore, there was no difference in median OS depending on whether RUXO was used as the first-line treatment (n = 23 or 50; 46%) or after HU (n = 27 of 50; 54%): 6.4 vs 7.8 years, respectively (P = .99).
Ten-year OS after start of HU and RUXO in PS-matched groups. Kaplan-Meier survival curves according to time after diagnosis to first administration of RUXO (red solid line) or HU (blue dotted line). The number of patients at risk is plotted every year. P-values were calculated by log-rank test.
Ten-year OS after start of HU and RUXO in PS-matched groups. Kaplan-Meier survival curves according to time after diagnosis to first administration of RUXO (red solid line) or HU (blue dotted line). The number of patients at risk is plotted every year. P-values were calculated by log-rank test.
In summary, with these long-term patient follow-up registry data, we show that HU remains the most frequently used treatment for patients with MF in European countries, although a steady increase in the use of RUXO has been observed in more recent years. Compared with HU, RUXO treatment was associated with a significant benefit in terms of OS in multivariate analysis; benefit was also seen in PS analysis within the limits of small sample sizes. Our study offers a unique opportunity to provide real-life evidence of the impact of RUXO on survival in patients with primary or secondary MF.
Acknowledgment: The ERNEST registry is supported by Novartis Pharma through a research collaboration.
Contribution: P.G. interpreted the data, and wrote the letter; T.B. conceived and designed the study, supervised the analysis, and critically revised the text; A.G. and A.C. contributed to data set preparation and planned and performed the statistical analyses; A.M. directed the project; A.M.V. interpreted the data and critically revised the letter; P.G., C.M., B.M., E.R., A.T., M.C.F., H.P., C.P., F.M., D.V., A.R., F.P., A.A.-L., and B.A. collected patients’ data for the study; all authors discussed the results and implications and commented on the manuscript; and all authors had full editorial control of the text and provided the final approval of all content.
Conflict-of-interest disclosure: P.G. has received speaker honoraria from Novartis and AbbVie and fees from Novartis and AbbVie for participation on advisory boards. A.M.V. has received personal fees for serving on the advisory board and speaker’s fees from AOP Orphan Pharmaceuticals, Incyte, BMS, and Novartis. A.R. has received personal fees as an advisory board member from Astellas, Amgen, Celgene BMS, Gilead, Italfarmaco, Novartis, Omeros, Roche, and Sanofi. T.B. has received personal fees as an advisory board member from AOP Orphan Pharmaceuticals, Italfarmaco, and Novartis, outside the submitted work. The remaining authors declare no competing financial interests.
Correspondence: Tiziano Barbui, Fondazione per la Ricerca Ospedale Maggiore (FROM) Research Foundation, Papa Giovanni XXIII Hospital, Piazza O.M.S., 1-24127 Bergamo (BG) Italy; e-mail: tbarbui@fondazionefrom.it.
References
Author notes
For data sharing, contact the corresponding author: tbarbui@fondazionefrom.it.
The full-text version of this article contains a data supplement.