Key Points
CMV gastroenteritis after acute GVHD is associated with an inferior overall survival because of a higher risk of NRM.
Letermovir significantly reduces the risk of CMV gastroenteritis and NRM in patients with G24GVHD.
Abstract
A preemptive strategy has successfully decreased cytomegalovirus (CMV) disease after allogeneic hematopoietic cell transplantation (HCT). However, some recipients still develop CMV gastroenteritis, especially after acute graft-versus-host disease (aGVHD), and its incidence, risk factors, and prognostic impact remain to be elucidated. We retrospectively analyzed 3759 consecutive adult patients who developed grade II-IV aGVHD using a Japanese registry database. The cumulative incidence of CMV gastroenteritis was 5.7% by day 365 from the development of grade II-IV aGVHD. Advanced age (hazard ratio [HR], 1.60; 95% confidence interval [CI], 1.16-2.22; P = .004), GVHD prophylaxis with mycophenolate mofetil and calcineurin inhibitor (HR, 1.73; 95% CI, 1.08-2.77; P = .024), lower-gut aGVHD (HR, 2.17; 95% CI, 1.58-2.98; P < .001), and the use of systemic steroids (HR, 1.78; 95% CI, 1.16-2.74; P = .008) were independent risk factors for CMV gastroenteritis. Development of CMV gastroenteritis was associated with an increased risk of nonrelapse mortality (HR, 1.89; 95% CI, 1.50-2.39; P < .001). Moreover, letermovir prophylaxis significantly reduced both the incidence of CMV gastroenteritis (HR, 0.50; 95% CI, 0.25-0.99; P = .047) and the risk of nonrelapse mortality (HR, 0.72; 95% CI, 0.52-0.99; P = .043). In summary, CMV gastroenteritis is a life-threatening complication that sets the need for preventive strategies with letermovir and targeted surveillance.
Introduction
Cytomegalovirus (CMV) reactivation and disease after allogeneic hematopoietic cell transplantation (HCT) are important contributors to morbidity and mortality.1-5 Over the past few decades, the incidence of CMV disease such as pneumonia, hepatitis, and retinitis has dramatically decreased with the advance of preemptive strategies.6-9 However, gastroenteritis is the predominant CMV disease that accounts for approximately 80% of all cases despite adoption of a preemptive strategy.10 A preemptive strategy might not significantly reduce the incidence of CMV gastroenteritis because a routine monitoring test with antigenemia or polymerase chain reaction (PCR) assay often shows negative results before the development of CMV gastroenteritis, which is initially considered to be a localized infection.10-14 Acute graft-versus-host disease (aGVHD) and use of systemic steroids are well-known risk factors for CMV disease,8,15,16 and subsequent CMV gastroenteritis, especially after lower-gut aGVHD, is occasionally identified in clinical practice.14,17 The prognosis of aGVHD concurrent with CMV gastroenteritis might be poor because immunosuppression for aGVHD can make CMV gastroenteritis resistant to treatment,18 and rapid tapering of immunosuppressive agents could result in the recurrence or persistence of aGVHD.19 Furthermore, other lethal infections would be increased according to delayed immune reconstitution20-22 or bone marrow suppression caused by antiviral agents.23 However, little information has been reported regarding the incidence, risk factors, and impact of CMV gastroenteritis in patients with aGVHD.
Many trials for prophylactic strategies against CMV have been conducted over the past few decades.24 Notably, a phase 3 trial and post hoc analyses of novel anti-CMV prophylaxis with letermovir demonstrated a significant reduction of CMV reactivation and superior survival outcomes with an acceptable safety profile.25,26 Meanwhile, the incidence of CMV disease in the letermovir and placebo groups was 1.5% and 1.8%, respectively, and all cases were gastroenteritis.25 Although letermovir prophylaxis could theoretically reduce the incidence of CMV gastroenteritis, unfortunately, the previous phase 3 study was not designed to detect a significant difference in the incidence of CMV disease or gastroenteritis.
In the current study, we used a large Japanese nationwide dataset to address the incidence and risk factors for CMV gastroenteritis and its associated nonrelapse mortality (NRM) after HCT, with particular focus on patients with grade II-IV aGVHD (G24GVHD) who are considered at high risk of CMV reactivation and disease.15,23 In addition, we evaluated the role of letermovir prophylaxis on the incidences of CMV gastroenteritis and NRM in this population.
Methods
Data source and patient selection
Clinical data were obtained from the Transplant Registry Unified Management Program (TRUMP), which is the registry database of the Japan Society for Transplantation and Cellular Therapy (JSTCT).27 Informed consent was obtained locally at the time of HCT. This retrospective cohort study included adult patients aged ≥16 years with acute myeloid leukemia (AML) or acute lymphoblastic leukemia (ALL) in first or second complete remission (CR), myelodysplastic syndrome (MDS), chronic myeloid leukemia (CML), adult T-cell leukemia/lymphoma (ATL) in CR, non-Hodgkin lymphoma (NHL) in CR, or myeloproliferative neoplasms (MPN) who underwent their first allogeneic HCT from an HLA matched related donor (MRD), an HLA 1-antigen-mismatched related donor (1MMRD), an HLA matched unrelated donor (MUD), an HLA 1-locus mismatched unrelated donor (1MMUD), umbilical cord blood (UCB) or a haploidentical donor (Haplo) between 2008 and 2019. Only patients with a CMV-seropositive donor or recipient and development of G24GVHD by day 100 were included. We excluded patients who relapsed before development of G24GVHD. Donor CMV serological status with UCB was determined to be negative. We excluded 45 patients who received prophylactic anti-CMV agents with ganciclovir, valganciclovir, or foscarnet. We also excluded patients without information on the donor’s sex (n = 10), Karnofsky performance status (KPS) (n = 9), HCT-specific comorbidity index (HCT-CI) (n = 27), or GVHD prophylaxis (n = 62). In addition, patients who developed CMV gastroenteritis before G24GVHD (n = 36) were excluded because we were interested in CMV gastroenteritis after aGVHD. Letermovir was available in Japan beginning in May 2018.
This study was approved by the data management committee of JSTCT and the Institutional Review Board of Jichi Medical University Saitama Medical Center.
CMV monitoring and definitions
Weekly CMV monitoring by pp65 antigenemia assay was performed from the time of engraftment. CMV reactivation was defined as the start of CMV preemptive therapy.23,28,29 In most centers, preemptive therapy was initiated once the number of antigenemia-positive cells reached 3 per 2 slides, which was comparable to real-time PCR with a threshold of 300 CMV DNA copies per milliliter.13 A diagnosis of CMV gastroenteritis required gastrointestinal symptoms with histological proof of CMV on biopsy samples by endoscopy, and other CMV diseases were diagnosed as previously described.30 Disease risk index (DRI), HCT-CI scores, and the intensity of the conditioning regimen were classified as previously reported.31-33 We defined ATL as a high risk in DRI. Related donors with 6/6 and 5/6 antigen matches of HLA-A, -B, and -DR were considered MRD and 1MMRD, respectively. MUD and 1MMUD were considered an unrelated donor with an 8/8 and 7/8 allelic match of HLA-A, -B, -C, and -DRB1, respectively. In vivo T-cell depletion was defined as patients who received anti-thymocyte globulin or alemtuzumab as GVHD prophylaxis.
Statistical analysis
The day when patients initially developed G24GVHD was considered to be the start day for the subsequent clinical events in all analyses. The primary end point was NRM by day 365 in previous studies4,23 because the proportional hazard assumption for the main effect was violated and the observation period of patients with letermovir prophylaxis was relatively short. The secondary end points were overall survival and relapse by day 365. The probability of overall survival was determined by the Kaplan-Meier method. A cumulative incidence estimation was used to evaluate the incidence of NRM and relapse. The cumulative incidence of CMV reactivation and gastroenteritis was estimated by treating death as a competing event. In all multivariate analyses, Cox proportional hazards regression models were used. The adjusted survival curves and cumulative incidence of NRM and relapse are illustrated as previously described.34,35 The impact of CMV reactivation and gastroenteritis as a time-dependent covariate was graphically illustrated using Simon-Makuch plots.36
The following potential covariates were included in the multivariate analyses: recipient’s age at HCT (<50 vs ≥50 years), sex mismatch (female recipient or male to male vs female to male), CMV serological status (R−/D+ vs R+/D− vs R+/D+), letermovir prophylaxis (no vs yes), disease (AML vs ALL vs MDS vs CML vs ATL vs NHL vs MPN), DRI (low vs intermediate vs high risk), KPS (≤80% vs >80%), HCT-CI (<2 vs ≥2), donor source (MRD vs 1MMRD vs MUD vs 1MMUD vs UCB vs Haplo), conditioning intensity (myeloablative vs reduced intensity), GVHD prophylaxis (methotrexate and calcineurin inhibitor [CNI] vs mycophenolate mofetil [MMF] and CNI vs other), in vivo T-cell depletion (no vs yes), year of HCT (2008-2013 vs 2014-2019), sites of organ involvement by aGVHD (skin, lower gut, or liver), use of systemic steroids (no vs yes), and CMV gastroenteritis (time dependent).
Statistical significance was defined as a two-tailed P value < .05. All statistical analyses were performed with SAS version 9.4 and EZR version 1.53 (Saitama Medical Center, Jichi Medical University), which is a graphic user interface for R (version 3.2.2; The R Foundation for Statistical Computing, Vienna, Austria).37
Results
Patient characteristics
We identified 3759 patients who fulfilled the eligibility criteria (Table 1). The median age at HCT was 50 years (range, 16-74). The median onset of G24GVHD from HCT was day 29 (range, 5-99). Of the 3759 patients with G24GVHD, most received systemic steroids (80.0%), 586 (15.9%) received second-line treatment, and 1120 (29.8%) developed grade III-IV aGVHD. Letermovir prophylaxis was administered in 275 patients (7.3%), and the median start timing was 1 day after HCT (range, −8 to 36). The median total duration of letermovir administration was 91 days (range, 2-332). Patient characteristics with and without letermovir prophylaxis were summarized in supplemental Table 1. The median observation period of survivors who received letermovir prophylaxis was 320 days from the development of G24GVHD. Of 212 patients who received HCT from a haploidentical donor, 89 (42.0%) received posttransplant cyclophosphamide. The overall survival rate at day 365 was 70.9% (95% confidence interval [CI], 69.4% to 72.4%). The cumulative incidences of NRM and relapse at day 365 were 19.5% (95% CI, 18.2% to 20.8%) and 10.3% (95% CI, 9.4% to 11.3%), respectively. By day 365, CMV pneumonia, hepatitis, and retinitis were identified in 26 (0.7%), 11 (0.3%), and 25 (0.7%) patients, respectively.
Patient characteristics
| . | n = 3759 . |
|---|---|
| Median age at HCT, y (range) | 50 (16-74) |
| Age, category | |
| <50 | 1807 (48.1) |
| ≥50 | 1952 (51.9) |
| Sex match between recipient and donor | |
| Female recipient or male to male | 3004 (79.9) |
| Female to male | 755 (20.1) |
| Recipient/donor CMV serostatus | |
| Negative/positive | 352 (9.4) |
| Positive/negative | 1406 (37.4) |
| Positive/positive | 2001 (53.2) |
| Letermovir prophylaxis | |
| No | 3484 (92.7) |
| Yes | 275 (7.3) |
| Disease | |
| AML | 1400 (37.2) |
| ALL | 917 (24.4) |
| MDS | 784 (20.9) |
| CML | 158 (4.2) |
| ATL | 179 (4.8) |
| NHL | 197 (5.2) |
| MPN | 124 (3.3) |
| DRI | |
| Low | 290 (7.7) |
| Intermediate | 2779 (73.9) |
| High | 690 (18.4) |
| KPS | |
| >80% | 3257 (86.7) |
| ≤80% | 502 (13.4) |
| HCT-CI | |
| <2 | 2838 (75.5) |
| ≥2 | 921 (24.5) |
| Donor source | |
| HLA matched related | 797 (21.2) |
| HLA 1-antigen-mismatched related | 108 (2.9) |
| HLA matched unrelated | 1049 (27.9) |
| HLA 1-locus-mismatched unrelated | 1147 (30.5) |
| Umbilical cord blood | 446 (11.9) |
| Haploidentical | 212 (5.6) |
| Conditioning intensity | |
| Myeloablative | 2624 (69.8) |
| Reduced intensity | 1135 (30.2) |
| GVHD prophylaxis | |
| MTX and CNI | 3190 (84.9) |
| MMF and CNI | 405 (10.8) |
| Other | 164 (4.4) |
| In vivo T-cell depletion | |
| No | 3430 (91.3) |
| Yes | 329 (8.8) |
| Year of HCT | |
| 2008-2013 | 1818 (48.4) |
| 2014-2019 | 1941 (51.6) |
| Organ involvement at the development of grade II-IV acute GVHD | |
| Skin GVHD | |
| No | 929 (24.7) |
| Yes | 2830 (75.3) |
| Lower-gut GVHD | |
| No | 1980 (52.7) |
| Yes | 1779 (47.3) |
| Liver GVHD | |
| No | 3504 (93.2) |
| Yes | 255 (6.8) |
| Use of systemic steroids | |
| No | 753 (20.0) |
| Yes | 3006 (80.0) |
| . | n = 3759 . |
|---|---|
| Median age at HCT, y (range) | 50 (16-74) |
| Age, category | |
| <50 | 1807 (48.1) |
| ≥50 | 1952 (51.9) |
| Sex match between recipient and donor | |
| Female recipient or male to male | 3004 (79.9) |
| Female to male | 755 (20.1) |
| Recipient/donor CMV serostatus | |
| Negative/positive | 352 (9.4) |
| Positive/negative | 1406 (37.4) |
| Positive/positive | 2001 (53.2) |
| Letermovir prophylaxis | |
| No | 3484 (92.7) |
| Yes | 275 (7.3) |
| Disease | |
| AML | 1400 (37.2) |
| ALL | 917 (24.4) |
| MDS | 784 (20.9) |
| CML | 158 (4.2) |
| ATL | 179 (4.8) |
| NHL | 197 (5.2) |
| MPN | 124 (3.3) |
| DRI | |
| Low | 290 (7.7) |
| Intermediate | 2779 (73.9) |
| High | 690 (18.4) |
| KPS | |
| >80% | 3257 (86.7) |
| ≤80% | 502 (13.4) |
| HCT-CI | |
| <2 | 2838 (75.5) |
| ≥2 | 921 (24.5) |
| Donor source | |
| HLA matched related | 797 (21.2) |
| HLA 1-antigen-mismatched related | 108 (2.9) |
| HLA matched unrelated | 1049 (27.9) |
| HLA 1-locus-mismatched unrelated | 1147 (30.5) |
| Umbilical cord blood | 446 (11.9) |
| Haploidentical | 212 (5.6) |
| Conditioning intensity | |
| Myeloablative | 2624 (69.8) |
| Reduced intensity | 1135 (30.2) |
| GVHD prophylaxis | |
| MTX and CNI | 3190 (84.9) |
| MMF and CNI | 405 (10.8) |
| Other | 164 (4.4) |
| In vivo T-cell depletion | |
| No | 3430 (91.3) |
| Yes | 329 (8.8) |
| Year of HCT | |
| 2008-2013 | 1818 (48.4) |
| 2014-2019 | 1941 (51.6) |
| Organ involvement at the development of grade II-IV acute GVHD | |
| Skin GVHD | |
| No | 929 (24.7) |
| Yes | 2830 (75.3) |
| Lower-gut GVHD | |
| No | 1980 (52.7) |
| Yes | 1779 (47.3) |
| Liver GVHD | |
| No | 3504 (93.2) |
| Yes | 255 (6.8) |
| Use of systemic steroids | |
| No | 753 (20.0) |
| Yes | 3006 (80.0) |
CNI, calcineurin inhibitor; MTX, methotrexate.
CMV reactivation
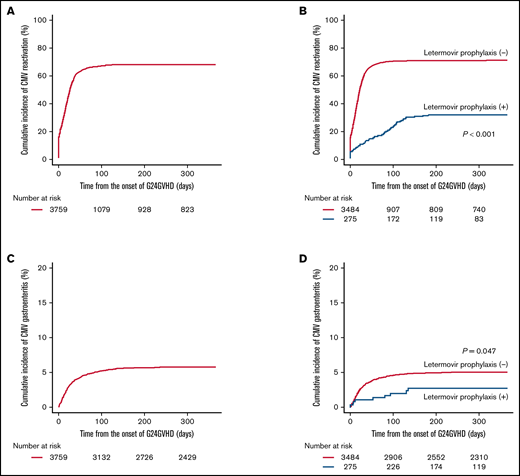
The median onset of CMV reactivation from HCT was day 41 (range, 1-399). Before the development of G24GVHD, 567 patients (15.1%) had already developed CMV reactivation. By day 365 from the onset of G24GVHD, 2529 patients developed CMV reactivation, and the cumulative incidence was 68.3% (95% CI, 66.7-69.8) (Figure 1A). The multivariate analysis demonstrated that CMV prophylaxis with letermovir was significantly associated with a decreased risk of CMV reactivation by day 365 (hazard ratio [HR], 0.25; 95% CI, 0.20-0.32; P < .001) (supplemental Table 2; Figure 1B).
Cumulative incidence of CMV reactivation and gastroenteritis. The cumulative incidence of CMV reactivation (A), the adjusted cumulative incidence of CMV reactivation in patients with and without letermovir prophylaxis (B), the cumulative incidence of CMV gastroenteritis (C), and the adjusted cumulative incidence of CMV gastroenteritis in patients with and without letermovir prophylaxis (D). Adjusted curves were plotted with the following covariates: recipient’s age at HCT, sex mismatch, CMV serological status, disease, DRI, KPS, HCT-CI, donor source, conditioning intensity, GVHD prophylaxis, in vivo T-cell depletion, year of HCT, organ involvement sites of aGVHD, and use of systemic steroids.
Cumulative incidence of CMV reactivation and gastroenteritis. The cumulative incidence of CMV reactivation (A), the adjusted cumulative incidence of CMV reactivation in patients with and without letermovir prophylaxis (B), the cumulative incidence of CMV gastroenteritis (C), and the adjusted cumulative incidence of CMV gastroenteritis in patients with and without letermovir prophylaxis (D). Adjusted curves were plotted with the following covariates: recipient’s age at HCT, sex mismatch, CMV serological status, disease, DRI, KPS, HCT-CI, donor source, conditioning intensity, GVHD prophylaxis, in vivo T-cell depletion, year of HCT, organ involvement sites of aGVHD, and use of systemic steroids.
Risk factors for CMV gastroenteritis
The median onset of CMV gastroenteritis from HCT was day 56 (range, 16-310). By day 365 from the onset of G24GVHD, CMV gastroenteritis was identified in 207 patients, and the cumulative incidence was 5.7% (95% CI, 5.0% to 6.5%) (Figure 1C). The median duration between the onset of G24GVHD and CMV gastroenteritis was 22 days (range, 1-235), and 36 cases (17.4%) were diagnosed with CMV gastroenteritis within 7 days after G24GVHD. Because the frequency and date of endoscopy were not available in our database, early onset of CMV gastroenteritis after G24GVHD might be identified by the same biopsy specimen in which immunohistochemistry staining delayed the diagnosis of CMV infection. In patients with gut GVHD at the development of G24GVHD, the median duration between the diagnosis of gut GVHD and CMV gastroenteritis was 19 days (range, 1-223). Among 207 patients, 70 (33.8%) received second-line treatment of aGVHD, and 37 (17.9%) did not develop CMV reactivation before the onset of CMV gastroenteritis. In the multivariate analysis, the risk of CMV gastroenteritis by day 365 was significantly reduced with letermovir prophylaxis (HR, 0.50; 95% CI, 0.25-0.99; P = .047) (Table 2;,Figure 1D). Advanced age (HR, 1.60; 95% CI, 1.16-2.22; P = .004), GVHD prophylaxis with MMF and CNI (HR, 1.73; 95% CI, 1.08-2.77; P = .024), lower-gut aGVHD at the development of G24GVHD (HR, 2.17; 95% CI, 1.58-2.98; P < .001), and use of systemic steroids (HR, 1.78; 95% CI, 1.16-2.74; P = .008) were significantly associated with an increased risk of CMV gastroenteritis (Table 2; supplemental Figure 1A-D).
Risk factors for developing CMV gastroenteritis in the multivariate analysis
| . | Hazard ratio (95% CI) . | P value . |
|---|---|---|
| Age, category | ||
| <50 | 1 | Reference |
| ≥50 | 1.60 (1.16-2.22) | .004 |
| Sex match between recipient and donor | ||
| Female recipient or male to male | 1 | Reference |
| Female to male | 0.98 (0.69-1.39) | .913 |
| Recipient/donor CMV serostatus | ||
| Negative/positive | 1 | Reference |
| Positive/negative | 1.80 (0.91-3.56) | .091 |
| Positive/positive | 1.85 (0.97-3.56) | .064 |
| Letermovir prophylaxis | ||
| No | 1 | Reference |
| Yes | 0.50 (0.25-0.99) | .047 |
| Disease | ||
| AML | 1 | Reference |
| ALL | 1.15 (0.78-1.71) | .474 |
| MDS | 1.35 (0.90-2.03) | .144 |
| CML | 1.40 (0.66-2.97) | .385 |
| ATL | 1.20 (0.58-2.48) | .620 |
| NHL | 1.20 (0.63-2.30) | .585 |
| MPN | 1.15 (0.52-2.53) | .733 |
| DRI | ||
| Low | 1 | Reference |
| Intermediate | 1.47 (0.73-2.94) | .277 |
| High | 1.65 (0.74-3.68) | .217 |
| KPS | ||
| >80% | 1 | Reference |
| ≤80% | 0.75 (0.49-1.16) | .195 |
| HCT-CI | ||
| <2 | 1 | Reference |
| ≥2 | 0.93 (0.67-1.28) | .646 |
| Donor source | ||
| HLA matched related | 1 | Reference |
| HLA 1-antigen-mismatched related | 1.20 (0.53-2.71) | .660 |
| HLA matched unrelated | 0.84 (0.54-1.31) | .449 |
| HLA 1-locus-mismatched unrelated | 1.36 (0.91-2.03) | .130 |
| Umbilical cord blood | 0.85 (0.45-1.58) | .597 |
| Haploidentical | 0.72 (0.34-1.52) | .390 |
| Conditioning intensity | ||
| Myeloablative | 1 | Reference |
| Reduced intensity | 1.10 (0.80-1.50) | .559 |
| GVHD prophylaxis | ||
| MTX and CNI | 1 | Reference |
| MMF and CNI | 1.73 (1.08-2.77) | .024 |
| Other | 1.59 (0.86-2.93) | .139 |
| In vivo T-cell depletion | ||
| No | 1 | Reference |
| Yes | 1.11 (0.68-1.80) | .684 |
| Year of HCT | ||
| 2008-2013 | 1 | Reference |
| 2014-2019 | 1.00 (0.75-1.34) | .986 |
| Organ involvement at the development of grade II-IV acute GVHD | ||
| Skin GVHD | ||
| No | 1 | Reference |
| Yes | 1.26 (0.90-1.76) | .187 |
| Lower-gut GVHD | ||
| No | 1 | Reference |
| Yes | 2.17 (1.58-2.98) | <.001 |
| Liver GVHD | ||
| No | 1 | Reference |
| Yes | 1.32 (0.78-2.26) | .304 |
| Use of systemic steroids | ||
| No | 1 | Reference |
| Yes | 1.78 (1.16-2.74) | .008 |
| . | Hazard ratio (95% CI) . | P value . |
|---|---|---|
| Age, category | ||
| <50 | 1 | Reference |
| ≥50 | 1.60 (1.16-2.22) | .004 |
| Sex match between recipient and donor | ||
| Female recipient or male to male | 1 | Reference |
| Female to male | 0.98 (0.69-1.39) | .913 |
| Recipient/donor CMV serostatus | ||
| Negative/positive | 1 | Reference |
| Positive/negative | 1.80 (0.91-3.56) | .091 |
| Positive/positive | 1.85 (0.97-3.56) | .064 |
| Letermovir prophylaxis | ||
| No | 1 | Reference |
| Yes | 0.50 (0.25-0.99) | .047 |
| Disease | ||
| AML | 1 | Reference |
| ALL | 1.15 (0.78-1.71) | .474 |
| MDS | 1.35 (0.90-2.03) | .144 |
| CML | 1.40 (0.66-2.97) | .385 |
| ATL | 1.20 (0.58-2.48) | .620 |
| NHL | 1.20 (0.63-2.30) | .585 |
| MPN | 1.15 (0.52-2.53) | .733 |
| DRI | ||
| Low | 1 | Reference |
| Intermediate | 1.47 (0.73-2.94) | .277 |
| High | 1.65 (0.74-3.68) | .217 |
| KPS | ||
| >80% | 1 | Reference |
| ≤80% | 0.75 (0.49-1.16) | .195 |
| HCT-CI | ||
| <2 | 1 | Reference |
| ≥2 | 0.93 (0.67-1.28) | .646 |
| Donor source | ||
| HLA matched related | 1 | Reference |
| HLA 1-antigen-mismatched related | 1.20 (0.53-2.71) | .660 |
| HLA matched unrelated | 0.84 (0.54-1.31) | .449 |
| HLA 1-locus-mismatched unrelated | 1.36 (0.91-2.03) | .130 |
| Umbilical cord blood | 0.85 (0.45-1.58) | .597 |
| Haploidentical | 0.72 (0.34-1.52) | .390 |
| Conditioning intensity | ||
| Myeloablative | 1 | Reference |
| Reduced intensity | 1.10 (0.80-1.50) | .559 |
| GVHD prophylaxis | ||
| MTX and CNI | 1 | Reference |
| MMF and CNI | 1.73 (1.08-2.77) | .024 |
| Other | 1.59 (0.86-2.93) | .139 |
| In vivo T-cell depletion | ||
| No | 1 | Reference |
| Yes | 1.11 (0.68-1.80) | .684 |
| Year of HCT | ||
| 2008-2013 | 1 | Reference |
| 2014-2019 | 1.00 (0.75-1.34) | .986 |
| Organ involvement at the development of grade II-IV acute GVHD | ||
| Skin GVHD | ||
| No | 1 | Reference |
| Yes | 1.26 (0.90-1.76) | .187 |
| Lower-gut GVHD | ||
| No | 1 | Reference |
| Yes | 2.17 (1.58-2.98) | <.001 |
| Liver GVHD | ||
| No | 1 | Reference |
| Yes | 1.32 (0.78-2.26) | .304 |
| Use of systemic steroids | ||
| No | 1 | Reference |
| Yes | 1.78 (1.16-2.74) | .008 |
Bold indicates statistical significance.
Transplant outcomes
We visualized the impact of CMV reactivation and gastroenteritis treated as time-dependent covariates on NRM by day 365 using a Simon-Makuch method (Figure 2A). In multivariate analyses, CMV gastroenteritis as a time-dependent covariate was significantly associated with an increased risk of NRM by day 365 (HR, 1.89; 95% CI, 1.50-2.39; P < .001) (Table 3). This result was also confirmed in the multivariate analysis limited to patients with lower-gut aGVHD at the development of G24GVHD (HR, 1.80; 95% CI, 1.36-2.38; P < .001). On the other hand, CMV prophylaxis with letermovir was significantly associated with a decreased risk of NRM (HR, 0.72; 95% CI, 0.52-0.99; P = .043) (Table 3; Figure 2B). The impact of letermovir prophylaxis was also confirmed by a multivariate analysis in which we did not include the development of CMV gastroenteritis as a confounder (HR, 0.70; 95% CI, 0.50-0.96; P = .028).
Impact of CMV gastroenteritis and letermovir on NRM. Simon-Makuch plots for the effect of CMV reactivation and gastroenteritis on NRM (A) and the adjusted cumulative incidence of NRM in patients with and without letermovir prophylaxis (B). Adjusted curves were plotted with the following covariates: recipient’s age at HCT, sex mismatch, CMV serological status, disease, DRI, KPS, HCT-CI, donor source, conditioning intensity, GVHD prophylaxis, in vivo T-cell depletion, year of HCT, organ involvement sites of aGVHD, and use of systemic steroids.
Impact of CMV gastroenteritis and letermovir on NRM. Simon-Makuch plots for the effect of CMV reactivation and gastroenteritis on NRM (A) and the adjusted cumulative incidence of NRM in patients with and without letermovir prophylaxis (B). Adjusted curves were plotted with the following covariates: recipient’s age at HCT, sex mismatch, CMV serological status, disease, DRI, KPS, HCT-CI, donor source, conditioning intensity, GVHD prophylaxis, in vivo T-cell depletion, year of HCT, organ involvement sites of aGVHD, and use of systemic steroids.
Risk factors for nonrelapse mortality in the multivariate analysis
| . | Hazard ratio (95% CI) . | P value . |
|---|---|---|
| Age, category | ||
| <50 | 1 | Reference |
| ≥50 | 1.93 (1.64-2.28) | <.001 |
| Sex match between recipient and donor | ||
| Female recipient or male to male | 1 | Reference |
| Female to male | 1.01 (0.85-1.20) | .929 |
| Recipient/donor CMV serostatus | ||
| Negative/positive | 1 | Reference |
| Positive/negative | 0.87 (0.66-1.15) | .332 |
| Positive/positive | 1.00 (0.77-1.29) | .984 |
| Letermovir prophylaxis | ||
| No | 1 | Reference |
| Yes | 0.72 (0.52-0.99) | .043 |
| Disease | ||
| AML | 1 | Reference |
| ALL | 0.93 (0.75-1.14) | .467 |
| MDS | 1.30 (1.06-1.60) | .011 |
| CML | 0.84 (0.54-1.31) | .445 |
| ATL | 1.42 (1.01-2.01) | .043 |
| NHL | 1.06 (0.74-1.50) | .755 |
| MPN | 1.28 (0.90-1.81) | .171 |
| DRI | ||
| Low | 1 | Reference |
| Intermediate | 1.12 (0.83-1.61) | .393 |
| High | 1.47 (0.99-2.16) | .051 |
| KPS | ||
| >80% | 1 | Reference |
| ≤80% | 1.35 (1.12-1.62) | .001 |
| HCT-CI | ||
| <2 | 1 | Reference |
| ≥2 | 1.21 (1.04-1.42) | .016 |
| Donor source | ||
| HLA matched related | 1 | Reference |
| HLA 1-antigen-mismatched related | 1.42 (0.91-2.23) | .127 |
| HLA matched unrelated | 1.21 (0.96-1.53) | .107 |
| HLA 1-locus-mismatched unrelated | 1.78 (1.44-2.22) | <.001 |
| Umbilical cord blood | 1.39 (1.00-1.93) | .049 |
| Haploidentical | 1.98 (1.39-2.81) | <.001 |
| Conditioning intensity | ||
| Myeloablative | 1 | Reference |
| Reduced intensity | 0.90 (0.76-1.05) | .180 |
| GVHD prophylaxis | ||
| MTX and CNI | 1 | Reference |
| MMF and CNI | 1.17 (0.90-1.52) | .248 |
| Other | 1.49 (1.10-2.02) | .011 |
| In vivo T-cell depletion | ||
| No | 1 | Reference |
| Yes | 0.96 (0.75-1.24) | .758 |
| Year of HCT | ||
| 2008-2013 | 1 | Reference |
| 2014-2019 | 0.85 (0.73-0.99) | .032 |
| Organ involvement at the development of grade II-IV acute GVHD | ||
| Skin GVHD | ||
| No | 1 | Reference |
| Yes | 1.02 (0.86-1.22) | .812 |
| Lower-gut GVHD | ||
| No | 1 | Reference |
| Yes | 1.37 (1.17-1.61) | <.001 |
| Liver GVHD | ||
| No | 1 | Reference |
| Yes | 2.64 (2.14-3.26) | <.001 |
| Use of systemic steroids | ||
| No | 1 | Reference |
| Yes | 2.51 (1.96-3.20) | <.001 |
| CMV gastroenteritis* | ||
| No | 1 | Reference |
| Yes | 1.89 (1.50-2.39) | <.001 |
| . | Hazard ratio (95% CI) . | P value . |
|---|---|---|
| Age, category | ||
| <50 | 1 | Reference |
| ≥50 | 1.93 (1.64-2.28) | <.001 |
| Sex match between recipient and donor | ||
| Female recipient or male to male | 1 | Reference |
| Female to male | 1.01 (0.85-1.20) | .929 |
| Recipient/donor CMV serostatus | ||
| Negative/positive | 1 | Reference |
| Positive/negative | 0.87 (0.66-1.15) | .332 |
| Positive/positive | 1.00 (0.77-1.29) | .984 |
| Letermovir prophylaxis | ||
| No | 1 | Reference |
| Yes | 0.72 (0.52-0.99) | .043 |
| Disease | ||
| AML | 1 | Reference |
| ALL | 0.93 (0.75-1.14) | .467 |
| MDS | 1.30 (1.06-1.60) | .011 |
| CML | 0.84 (0.54-1.31) | .445 |
| ATL | 1.42 (1.01-2.01) | .043 |
| NHL | 1.06 (0.74-1.50) | .755 |
| MPN | 1.28 (0.90-1.81) | .171 |
| DRI | ||
| Low | 1 | Reference |
| Intermediate | 1.12 (0.83-1.61) | .393 |
| High | 1.47 (0.99-2.16) | .051 |
| KPS | ||
| >80% | 1 | Reference |
| ≤80% | 1.35 (1.12-1.62) | .001 |
| HCT-CI | ||
| <2 | 1 | Reference |
| ≥2 | 1.21 (1.04-1.42) | .016 |
| Donor source | ||
| HLA matched related | 1 | Reference |
| HLA 1-antigen-mismatched related | 1.42 (0.91-2.23) | .127 |
| HLA matched unrelated | 1.21 (0.96-1.53) | .107 |
| HLA 1-locus-mismatched unrelated | 1.78 (1.44-2.22) | <.001 |
| Umbilical cord blood | 1.39 (1.00-1.93) | .049 |
| Haploidentical | 1.98 (1.39-2.81) | <.001 |
| Conditioning intensity | ||
| Myeloablative | 1 | Reference |
| Reduced intensity | 0.90 (0.76-1.05) | .180 |
| GVHD prophylaxis | ||
| MTX and CNI | 1 | Reference |
| MMF and CNI | 1.17 (0.90-1.52) | .248 |
| Other | 1.49 (1.10-2.02) | .011 |
| In vivo T-cell depletion | ||
| No | 1 | Reference |
| Yes | 0.96 (0.75-1.24) | .758 |
| Year of HCT | ||
| 2008-2013 | 1 | Reference |
| 2014-2019 | 0.85 (0.73-0.99) | .032 |
| Organ involvement at the development of grade II-IV acute GVHD | ||
| Skin GVHD | ||
| No | 1 | Reference |
| Yes | 1.02 (0.86-1.22) | .812 |
| Lower-gut GVHD | ||
| No | 1 | Reference |
| Yes | 1.37 (1.17-1.61) | <.001 |
| Liver GVHD | ||
| No | 1 | Reference |
| Yes | 2.64 (2.14-3.26) | <.001 |
| Use of systemic steroids | ||
| No | 1 | Reference |
| Yes | 2.51 (1.96-3.20) | <.001 |
| CMV gastroenteritis* | ||
| No | 1 | Reference |
| Yes | 1.89 (1.50-2.39) | <.001 |
Bold indicates statistical significance.
Time-dependent covariate.
The impact of CMV gastroenteritis on overall survival and relapse by day 365 was also illustrated using a Simon-Makuch method (supplemental Figure 2A-B). In the multivariate analyses, CMV gastroenteritis was significantly associated with inferior overall survival (HR, 1.66; 95% CI, 1.33-2.06; P < .001), but there was no significant difference in relapse (HR, 0.84; 95% CI, 0.51-1.39; P = .492) (supplemental Table 3). Next, the impact of letermovir prophylaxis on overall survival and relapse by day 365 was plotted (supplemental Figure 3A-B). In the multivariate analyses, the survival benefit of letermovir prophylaxis was not statistically significant (HR, 0.79; 95% CI, 0.60-1.04; P = .095), whereas letermovir prophylaxis was significantly associated with an increased risk of relapse (HR, 1.47; 95% CI, 1.03-2.10; P = .032) (supplemental Table 3).
Finally, we assessed the cause of death by day 365. There was a significant difference in the profile of the cause of death between patients with and without CMV gastroenteritis (P < .001) (Table 4). In particular, the proportions of death caused by infection, GVHD, and organ failure were increased in patients who developed CMV gastroenteritis. In contrast, the profile of the cause of death was similar between patients with and without letermovir prophylaxis (P = .628).
Cause of death by day 365
| Cause . | CMV-GE (−) . | CMV-GE (+) . | P value . | Letermovir (−) . | Letermovir (+) . | P value . |
|---|---|---|---|---|---|---|
| Relapse | 271 (28.1) | 11 (12.0) | <.001 | 263 (26.4) | 19 (31.7) | .628 |
| Infection | 203 (21.1) | 24 (26.1) | 215 (21.6) | 12 (20.0) | ||
| GVHD | 171 (17.8) | 27 (29.3) | 184 (18.5) | 14 (23.3) | ||
| Organ failure | 156 (16.2) | 25 (27.2) | 173 (17.4) | 8 (13.3) | ||
| Other | 162 (16.8) | 5 (5.4) | 160 (16.1) | 7 (11.7) | ||
| Total death/total number | 963/3552 | 92/207 | 995/3484 | 60/275 |
| Cause . | CMV-GE (−) . | CMV-GE (+) . | P value . | Letermovir (−) . | Letermovir (+) . | P value . |
|---|---|---|---|---|---|---|
| Relapse | 271 (28.1) | 11 (12.0) | <.001 | 263 (26.4) | 19 (31.7) | .628 |
| Infection | 203 (21.1) | 24 (26.1) | 215 (21.6) | 12 (20.0) | ||
| GVHD | 171 (17.8) | 27 (29.3) | 184 (18.5) | 14 (23.3) | ||
| Organ failure | 156 (16.2) | 25 (27.2) | 173 (17.4) | 8 (13.3) | ||
| Other | 162 (16.8) | 5 (5.4) | 160 (16.1) | 7 (11.7) | ||
| Total death/total number | 963/3552 | 92/207 | 995/3484 | 60/275 |
Data are n (%).
Bold indicates statistical significance.
Χ2 tests were conducted to determine the distribution of the cause of death.
CMV-GE, cytomegalovirus gastroenteritis.
Discussion
We recently addressed the impact of CMV reactivation with or without G24GVHD on transplant outcomes,23 whereas little was known about CMV gastroenteritis after aGVHD. In this study that focused on patients who developed G24GVHD, CMV gastroenteritis developed in 5.7% of cases. We found that advanced age, GVHD prophylaxis with MMF and CNI, lower-gut aGVHD, and the use of systemic steroids were independent risk factors for subsequent CMV gastroenteritis after G24GVHD, whereas letermovir prophylaxis decreased the risk of CMV gastroenteritis. We also demonstrated that the risk of overall mortality in patients who developed CMV gastroenteritis was high because of the high risk of NRM. Importantly, CMV prophylaxis with letermovir was associated with a decreased risk of NRM.
To the best of our knowledge, 2 single-center studies, which found 26 and 31 patients with CMV gastroenteritis after lower-gut aGVHD, reported that it is associated with poor prognosis.14,17 These studies analyzed patients who underwent HCT more than a decade ago. However, considerable progress has been made in the selection of donor sources, GVHD prophylaxis, and supportive care over the past decade, which affect immune reconstitution and CMV reactivation. Moreover, the introduction of letermovir in prophylaxis has changed the management of CMV reactivation after HCT.7,25,38 An important strength of the current study is that we identified risk factors and clarified the prognosis of CMV gastroenteritis in a large multicenter registry cohort that reflects real-world experience under recent advances in HCT, including the introduction of letermovir.
We identified independent and significant risk factors for CMV gastroenteritis after G24GVHD. The use of systemic steroids is a well-recognized factor that influences CMV disease.15 Interestingly, the presence of lower-gut aGVHD at the development of G24GVHD had a more than 2-fold higher risk of CMV gastroenteritis. Wikstrom et al demonstrated that GVHD itself prevents the induction of an adaptive immune response against CMV due to a severe dendritic cell defect and leads to severe CMV disease in mouse models.39 Therefore, preceding lower-gut aGVHD might promote the local activation of CMV because of the restricted antiviral T-cell responses. Advanced age and GVHD prophylaxis with MMF and CNI might contribute to a higher risk of CMV gastroenteritis because they were associated with a poor response of initial therapy for aGVHD40,41 and the development of severe aGVHD.42,43 Several studies have shown that GVHD prophylaxis with MMF and CNI was associated with an increased risk of CMV reactivation,44,45 indicating that lymphocyte suppression related to an immune response against CMV might be driven under continuous exposure to MMF. In addition to a higher risk of CMV gastroenteritis and NRM in patients with advanced age, because our previous study showed a higher NRM in elderly patients who developed both G24GVHD and CMV reactivation compared with younger patients,23 our findings suggest the need for CMV prophylaxis with letermovir in elderly patients.
The development of CMV gastroenteritis was associated with dismal outcomes after G24GVHD. The risk of NRM was ∼2-fold higher in patients who developed CMV gastroenteritis, which was attributable to death caused by infection, GVHD, and organ failure. It is difficult to determine precisely the impact of CMV gastroenteritis itself on outcomes because patients with a higher risk of NRM might be likely to develop CMV gastroenteritis. However, these findings might indicate that the treatment of simultaneous aGVHD and CMV gastroenteritis remains a challenge despite recent advances in supportive care. Hence, it stresses the importance of comprehensive risk assessment for CMV gastroenteritis, allowing for the implementation of early detection and prevention strategies to mitigate adverse outcomes in patients with G24GVHD.
Letermovir has recently been available in clinical practice according to a previous phase 3 trial.25,26 After approval of letermovir, several real-world experiences have been reported on its use in primary46,47 and secondary prophylaxis,48,49 and they have demonstrated a significant reduction in CMV reactivation. Recently, Johnsrud et al reported a single-center experience in which 114 patients received letermovir prophylaxis. In their study, none of the patients with letermovir prophylaxis developed CMV disease, but the limited sample size did not allow a detailed statistical assessment.50 To the best of our knowledge, this is the largest study to address the significant reduction in CMV gastroenteritis with the use of letermovir prophylaxis among patients with G24GVHD. Furthermore, letermovir prophylaxis was also associated with a decreased risk of NRM in a multivariate analysis that included CMV gastroenteritis as a confounder, suggesting that letermovir prophylaxis has a direct effect on NRM independent of the reduction of CMV gastroenteritis. Interestingly, letermovir prophylaxis was related to an increased risk of relapse. Additional studies that are stratified by disease and disease risk are needed to confirm this finding. One possible explanation of the increased risk of relapse is that delayed CMV-specific T-cell reconstitution caused by letermovir51 results in a reduced graft-versus-leukemia effect against CMV-infected malignant cells. Further studies are required to determine the underlying mechanism.
The methods used for monitoring CMV reactivation should be discussed. The pp65 antigenemia method, but not PCR assay, has been approved for monitoring CMV reactivation in Japan. There was no difference in CMV disease between ganciclovir prophylaxis and preemptive strategy groups using antigenemia,6 indicating that an antigenemia assay-based preemptive strategy is highly sensitive for preventing CMV disease. In addition, the preventive effect against CMV disease with monitoring of antigenemia was equivalent to that of PCR assay,13 and the current study actually showed that the incidences of CMV pneumonia, hepatitis, and retinitis were each <1% despite the development of G24GVHD. In contrast, although PCR assay might show positive slightly earlier than antigenemia, preemptive strategies with PCR or antigenemia assay are not sufficient to prevent CMV gastroenteritis after HCT.10,11,13,14,25,52,53 Moreover, PCR and/or antigenemia assay is also considered to have no diagnostic value for CMV gastroenteritis in inflammatory bowel disease because of the lack of sensitivity,54 suggesting that CMV gastroenteritis is a localized infection that does not often accompany CMV viremia initially. Therefore, our results need to be verified in other studies using PCR assay, but the lack of sensitivity with blood-based testing emphasizes the need for performing endoscopy repeatedly in refractory lower-gut aGVHD regardless of the monitoring method.
This study has several limitations, including its retrospective nature. First, although Japanese guidelines recommend a repeated endoscopy in patients with persistent or worsening gastrointestinal symptoms because of a requirement for histological proof of CMV gastroenteritis, the timing of endoscopy was at the discretion of each center. Unfortunately, the frequency of endoscopy was not available in our registry. Thus, it is difficult to exclude a diagnostic bias, and the incidence of CMV gastroenteritis in our study might be underestimated, especially in mild cases. Second, although our analyses included patients who underwent HCT with in vivo T-cell depletion or haploidentical HCT with posttransplant cyclophosphamide, the sample size of these subgroups was relatively small, which was partially explained by the lower risk of G24GVHD.
In conclusion, we revealed the independent risk factors for CMV gastroenteritis and its poor prognosis in patients with G24GVHD, which highlight the need to pay attention to subsequent CMV gastroenteritis after aGVHD. Moreover, the incidence of CMV gastroenteritis and NRM could be reduced by letermovir prophylaxis. Our findings can serve as a basis for developing intensified surveillance and prevention strategies for patients who develop G24GVHD.
Acknowledgments
The authors greatly appreciate the contributions of many physicians and data managers throughout the Japan Society for Transplantation and Cellular Therapy (JSTCT), the Japan Marrow Donor Program (JMDP), and the Japan Cord Blood Bank Network (JCBBN), who made this analysis possible. The authors would also like to thank the members of the Transplant Registry Unified Management committees at JSTCT, JMDP, and JCBBN for their dedicated management of data.
Y. Akahoshi is a Research Fellow of Japan Society of the Promotion of Science (JSPS), and this work was supported by JSPS KAKENHI (grant JP20J10298).
Authorship
Contribution: Y. Akahoshi designed the study, analyzed the data, and wrote the manuscript; S.-i.K., Y.T., T.M., M. Tamaki, M.M., and S.T. reviewed and revised the manuscript; N.D., N.U., M. Tanaka, H. Nakamae, T. Kuriyama, K.-i.M., T.I., and T. Kimura provided important clinical data; T.F., Y.K., and Y. Atsuta collected the patient data; H. Nakasone designed the study and reviewed and revised the manuscript; and all authors contributed to the writing of the report and approved the final version of the article.
Conflict-of-interest disclosure: Y. Akahoshi reported a grant from JSPS during the study. S.-i.K. reported personal fees from MSD, Sumitomo Dainippon Pharma, Astellas, Pfizer, Kyowa Kirin, Chugai Pharmaceutical, Bristol-Myers Squibb, Ono Pharmaceutical, Eisai, Nippon Kayaku, Takeda Pharmaceutical, and SymBio Pharmaceutical outside the submitted work. Y.K. reported honoraria from MSD outside the submitted work. Y. Atsuta reported honoraria from Kyowa Kirin, AbbVie GK, Mochida Pharmaceutical, Meiji Seika Pharmaceutical, and Chugai Pharmaceutical outside the submitted work. M.M. reported personal fees from MSD, Kyowa Kirin, Sumitomo Dainippon Pharma, FUJIFILM, Toyama Chemical, Novartis, JCR Pharmaceutical, Astellas, Miyarisan Pharmaceutical, Asahi Kasei, GlaxoSmithKline, Celgene, and Otsuka Pharmaceutical outside the submitted work. H. Nakasone reported personal fees from Takeda Pharmaceutical, Otsuka Pharmaceutical, Bristol-Myers Squibb, Celgene, Pfizer, Novartis, Janssen Pharmaceutical K.K., Eisai, Chugai Pharmaceutical, and Nippon Shinyaku outside the submitted work. The remaining authors declare no competing financial interests.
Correspondence: Yu Akahoshi, Division of Hematology, Jichi Medical University Saitama Medical Center, 1-847 Amanuma-cho, Omiya-ku, Saitama City, Saitama 330-8503, Japan; e-mail: akahoshiu@gmail.com; and Hideki Nakasone, Division of Hematology, Jichi Medical University Saitama Medical Center, 1-847 Amanuma-cho, Omiya-ku, Saitama City, Saitama 330-8503, Japan; e-mail: nakasone-tky@umin.ac.jp.
References
Author notes
Requests for data sharing may be submitted to Y. Akahoshi (akahoshiu@gmail.com).
The full-text version of this article contains a data supplement.