Abstract
Despite recent advances in the treatment of hematologic malignancies, relapse still remains a consistent issue. One of the primary contributors to relapse is the bone marrow microenvironment providing a sanctuary to malignant cells. These cells interact with bone marrow components such as osteoblasts and stromal cells, extracellular matrix proteins, and soluble factors. These interactions, mediated by the cell surface proteins like cellular adhesion molecules (CAMs), induce intracellular signaling that leads to the development of bone marrow microenvironment–induced chemoprotection (BMC). Although extensive study has gone into these CAMs, including the development of targeted therapies, very little focus in hematologic malignancies has been put on a family of cell surface proteins that are just as important for mediating bone marrow interactions: the transmembrane 4 superfamily (tetraspanins; TSPANs). TSPANs are known to be important mediators of microenvironmental interactions and metastasis based on numerous studies in solid tumors. Recently, evidence of their possible role in hematologic malignancies, specifically in the regulation of cellular adhesion, bone marrow homing, intracellular signaling, and stem cell dynamics in malignant hematologic cells has come to light. Many of these effects are facilitated by associations with CAMs and other receptors on the cell surface in TSPAN-enriched microdomains. This could suggest that TSPANs play an important role in mediating BMC in hematologic malignancies and could be used as therapeutic targets. In this review, we discuss TSPAN structure and function in hematologic cells, their interactions with different cell surface and signaling proteins, and possible ways to target/inhibit their effects.
Bone marrow microenvironment–induced chemoprotection
The bone marrow microenvironment (BMM) is the site of normal differentiation of hematopoietic stem cells (HSCs) and the development of healthy blood cells. Located within this microenvironment are also other cells such as osteoblasts, endothelial cells, stromal cells, and mesenchymal stem cells.1 During normal hematopoiesis, HSCs can interact with these cells or their secreted extracellular matrix proteins and soluble factors to foster proper development and differentiation.2
Interaction of malignant cells with these cells and proteins can activate intracellular signaling pathways that drive cells into a chemoresistant state.3 Thus, association of cancer cells with BMM elements can be problematic in hematologic malignancies. This phenomenon is known as BMM–induced chemoprotection (BMC). Often, these effects rely on the expression of cellular adhesion molecules (CAMs) and their binding to cognate ligands.4 Therefore, the study of interactions between malignant cells and the BMM has been crucial to the better understanding of the development of hematologic malignancies and their resistance to chemotherapy.
TSPAN structure and function
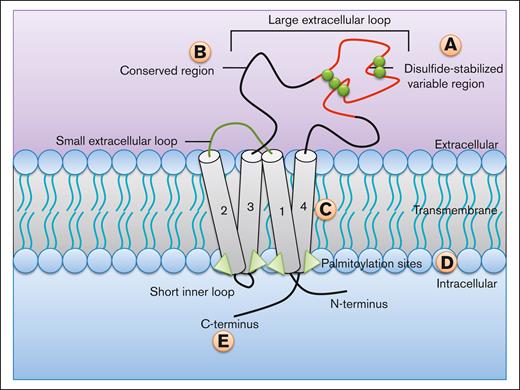
Tetraspanins (TSPANs) are characterized by 4 transmembrane domains with intracellular N- and C-termini5 (Figure 1) and 2 extracellular loops, a short 13 to 30 amino acid loop (EC1) and a large 200 to 300 amino acid loop (EC2). TSPANs have not been shown to act like typical adhesion or ligand receptors; however, they can interact with other cell surface receptors, signaling molecules, and each other.6-8 There are 4 main domains, which have functional consequences within TSPANs9: (1) the EC2 loop contains a disulfide-stabilized hypervariable region and a highly conserved region,10 the variable region mediates the specific interactions unique to each TSPAN, whereas the conserved region is involved in homodimerization.11 (2) The transmembrane regions are important for the formation of the “TSPAN web” or “TSPAN-enriched microdomains” (TEMs),9 which will be discussed in more depth in further sections. These regions show a plurality of hydrophobic interactions, as well as strong hydrogen bonding between polarized amino acids of adjacent TSPAN neighbors, all of which allow for the tight packing and formation of TEMs.9 (3) Intracellularly, the C-terminus plays a role in intracellular signaling by interacting with different signaling molecules and cytoskeletal proteins.6,12-14 (4) TSPANs contain palmitoylation sites (typically cysteine residues modified by attachment of fatty acids), which are important for both signaling and the formation of new TEMs.15-18
Structure and domain functions of TSPANs. TSPANs are characterized by the presence of 4 transmembrane domains (1-4). There are multiple regions within the protein that mediate key effects. (A) Disulphide-stabilized (green spheres) variable region of the large extracellular loop (EC2) responsible for the unique interactions specific to each TSPAN. (B) The conserved region of the EC2 loop mediates homodimerization. (C) Transmembrane regions are important for the formation of TEMs. (D) Palmitoylation sites are crucial to the proper formation of new TEMs and play a role in signaling. (E) The C-terminus is responsible for interacting with signaling and cytoskeletal proteins.
Structure and domain functions of TSPANs. TSPANs are characterized by the presence of 4 transmembrane domains (1-4). There are multiple regions within the protein that mediate key effects. (A) Disulphide-stabilized (green spheres) variable region of the large extracellular loop (EC2) responsible for the unique interactions specific to each TSPAN. (B) The conserved region of the EC2 loop mediates homodimerization. (C) Transmembrane regions are important for the formation of TEMs. (D) Palmitoylation sites are crucial to the proper formation of new TEMs and play a role in signaling. (E) The C-terminus is responsible for interacting with signaling and cytoskeletal proteins.
TSPAN-enriched microdomains (TEMs)
TEMs, also called the TSPAN web, are dynamic membrane entities that act as signaling platforms.19 They are heterogeneous compositions of interconnected proteins that are involved in functionally related processes (Figure 2). TEMs consist of different TSPANs, adhesion molecules like integrins and immunoglobulin superfamily members, and other signaling receptors such as G-proteins and tyrosine kinases.20 TSPANs act like scaffolding proteins within these microdomains, by facilitating the assembly of these different proteins to potentiate the activation of associated intracellular signaling pathways.21-23
TEMs play a key role in TSPAN function. TEMs are dynamic membrane entities that play a key role in mediating interactions with the BM. TSPANs act as scaffolding proteins to bring together many proteins with similar functions such as CAMs (like integrins and immunoglobulin superfamily [IgSF] members) and signaling receptors (G protein–coupled receptors [GPCRs] and receptor tyrosine kinases [RTKs]). The crosslinking of TSPANs creates a large secondary signaling network, which can effectively transduce extracellular stimuli inside to intracellular signaling pathways.
TEMs play a key role in TSPAN function. TEMs are dynamic membrane entities that play a key role in mediating interactions with the BM. TSPANs act as scaffolding proteins to bring together many proteins with similar functions such as CAMs (like integrins and immunoglobulin superfamily [IgSF] members) and signaling receptors (G protein–coupled receptors [GPCRs] and receptor tyrosine kinases [RTKs]). The crosslinking of TSPANs creates a large secondary signaling network, which can effectively transduce extracellular stimuli inside to intracellular signaling pathways.
Within TEMs, TSPANs can have strong interactions with one another. Because of this, the specific interactions of each individual TSPAN can crosslink into large secondary signaling networks.19 This crosslinkage is stabilized by multiple regions within the TSPAN protein, such as the EC2 loop and the transmembrane domain but relies most heavily on the palmitoylation of intracellular cysteines.9 Despite their reliance on palmitoylation, TEMs reportedly stay intact after disruption of these palmitoylation sites; however, new TEMs were unable to form, suggesting that the palmitoylation is required for the initial formation.19 In addition to palmitoylation, TEMs also rely on the functional redundancy of other TSPAN domains such as the transmembrane regions and EC2 loops. These domains can interact with those on adjacent TSPAN proteins to allow for efficient formation and functional activity of TEMs.9
The structure of TEMs is maintained by binding to cholesterol and sphingolipids, similar to that in lipid rafts, which are specialized microdomains that facilitate signal transduction.24 Although TEMs appear to be similar to lipid rafts, they are distinguished by extractability in strong detergents such as Triton X-100, and lack of glycosylphosphatidylinositol-anchored proteins.25 Recent studies have identified functional interactions between TEMs and lipid rafts mediated by TSPANs such as CD82.26
TSPANs and their role in BMC in hematologic malignancies
TSPAN interactions with other cell surface proteins and the formation of TEMs are important for the function of a variety of cellular processes such as cellular adhesion, BM homing, survival signaling, and stem cell dynamics. Many of these functions have been discovered in solid tumors. However, evidence of TSPAN effects in hematologic cells and malignancies is growing. The effects of TSPANs on HSCs have been described earlier.27 In further sections, we provide specific examples of the aforementioned functions of TSPANs in hematologic cells, with an in-depth summary provided in Table 1.
Interactions and effects of TSPANs on malignant hematologic cells
| Protein . | Cancer . | Function/interaction . | Ref . |
|---|---|---|---|
| CD9 | B-ALL | Modulates CXCR-4–mediated cell migration through RAC1 signaling | 28 |
| Regulates LSC activity | 29 | ||
| Poor prognostic marker and blockade with anti-CD9 antibodies suppressed disease progression and enhanced chemosensitivity in vivo | 30 | ||
| Activates PI3K/Akt signaling via direct interaction with PI3K/p85 | 31 | ||
| Mediates cellular adhesion and inhibits cellular death via a p53-dependent mechanism | 32 | ||
| B-CLL | Abnormal expression associated with disease infiltration patterns | 33 | |
| AML | Relevant marker for MRD and targeting of LSCs | 34 | |
| AML, CML, lymphoma | Mediates self-renewal of LSCs via STAT5B signaling | 35 | |
| Multiple myeloma | Enhances susceptibility to cell-mediated cytolysis | 36 | |
| CD37 | CLL | Mediates prosurvival signaling via PI3K through ITAM-like motif in C-terminus, induces SHP1-dependent apoptotic signaling through ITIM-like motif in N-terminus | 37 |
| AML | Poor prognostic marker | 38 | |
| CD53 | Lymphoma | Increases Bcl-XL and decreases bax expression via activation of Akt | 39 |
| CD63 | AML | Expression on exosomes induces interleukin-8 production in bone marrow stromal cells to induce chemoprotection | 40 |
| CD81 | B-ALL | Loss of expression associated with mature B-cells being ready to migrate and leave the BM | 41 |
| Mediates BMC and in vivo BM homing and engraftment, controls BTK signaling through CD19 expression, thereby inhibiting p53-mediated cell death | 42 | ||
| AML | Controls cellular adhesion, migration, BM homing, and drug resistance | 43 | |
| B-CLL | Abnormal expression associated with disease infiltration patterns | 33 | |
| CD82 | AML | BM homing via N-cadherin organization | 44 |
| LSC adhesion and survival through STAT5/interleukin-10 | 45 | ||
| Akt regulation of BCL2L12 | 46 | ||
| Increases expression of EZH2 by inhibiting p38 activity | 47 | ||
| Activates β1 integrin and PKCα signaling | 48 | ||
| Promotes survival via activation of Wnt/β-catenin signaling | 49 | ||
| B-ALL | Higher expression in malignant cells compared with nonmalignant cells, prognosis marker | 50 |
| Protein . | Cancer . | Function/interaction . | Ref . |
|---|---|---|---|
| CD9 | B-ALL | Modulates CXCR-4–mediated cell migration through RAC1 signaling | 28 |
| Regulates LSC activity | 29 | ||
| Poor prognostic marker and blockade with anti-CD9 antibodies suppressed disease progression and enhanced chemosensitivity in vivo | 30 | ||
| Activates PI3K/Akt signaling via direct interaction with PI3K/p85 | 31 | ||
| Mediates cellular adhesion and inhibits cellular death via a p53-dependent mechanism | 32 | ||
| B-CLL | Abnormal expression associated with disease infiltration patterns | 33 | |
| AML | Relevant marker for MRD and targeting of LSCs | 34 | |
| AML, CML, lymphoma | Mediates self-renewal of LSCs via STAT5B signaling | 35 | |
| Multiple myeloma | Enhances susceptibility to cell-mediated cytolysis | 36 | |
| CD37 | CLL | Mediates prosurvival signaling via PI3K through ITAM-like motif in C-terminus, induces SHP1-dependent apoptotic signaling through ITIM-like motif in N-terminus | 37 |
| AML | Poor prognostic marker | 38 | |
| CD53 | Lymphoma | Increases Bcl-XL and decreases bax expression via activation of Akt | 39 |
| CD63 | AML | Expression on exosomes induces interleukin-8 production in bone marrow stromal cells to induce chemoprotection | 40 |
| CD81 | B-ALL | Loss of expression associated with mature B-cells being ready to migrate and leave the BM | 41 |
| Mediates BMC and in vivo BM homing and engraftment, controls BTK signaling through CD19 expression, thereby inhibiting p53-mediated cell death | 42 | ||
| AML | Controls cellular adhesion, migration, BM homing, and drug resistance | 43 | |
| B-CLL | Abnormal expression associated with disease infiltration patterns | 33 | |
| CD82 | AML | BM homing via N-cadherin organization | 44 |
| LSC adhesion and survival through STAT5/interleukin-10 | 45 | ||
| Akt regulation of BCL2L12 | 46 | ||
| Increases expression of EZH2 by inhibiting p38 activity | 47 | ||
| Activates β1 integrin and PKCα signaling | 48 | ||
| Promotes survival via activation of Wnt/β-catenin signaling | 49 | ||
| B-ALL | Higher expression in malignant cells compared with nonmalignant cells, prognosis marker | 50 |
TSPANs have a large variety of interacting partners and can mediate many different cellular processes.
B-CLL, B-cell chronic lymphocytic leukemia; BTK, Bruton tyrosine kinase; CLL, chronic lymphocytic leukemia; CML, chronic myeloid leukemia; ITAM, immunoreceptor tyrosine-based activation motif; ITIM, immunoreceptor tyrosine-based inhibitory motif; LSC, leukemic stem cell; MRD, measurable residual disease; PKC, protein kinase Cα.
TSPANs are known for their ability to mediate interactions with the local microenvironment through their lateral associations with CAMs on the cell surface. One of the most common CAMs to interact with TSPANs is integrin α4β1 (VLA-4). When interacting with the TSPAN proteins CD9, CD81, or CD82, adhesion of VLA-4 to its target (vascular cell adhesion molecule-1) is increased.51-53 Often, this augmentation will occur via clustering of VLA-4 proteins on the cell surface. TSPAN-mediated adhesion can also be induced via activation of downstream signaling pathways associated with specific TSPAN proteins. Upon CD53 activation via monoclonal antibodies (mAbs) in lymphoma, phosphoinositide 3-kinase (PI3K) and protein kinase C signaling are activated intracellularly and lead to homotypic cellular adhesion.54
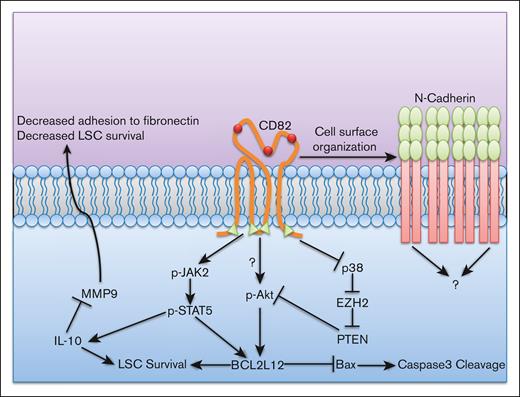
CD82 is also known to mediate BM homing in acute myeloid leukemia (AML) via cell surface clustering of the CAM N-cadherin.44 This clustering is reliant upon the proper palmitoylation and N-glycosylation of CD82. CD82 palmitoylation mutants lack N-cadherin assembly into clusters whereas N-glycosylation mutants lack adequate nanoscale packing. Increased cellular adhesion in the BM in hematologic malignancies can lead to the activation of chemoprotective signaling. Therefore, TSPAN functions can be indirectly responsible for mediating BMC. In AML, CD82-mediated adhesion enhances the activation of STAT5/Akt signaling, culminating in the increased expression of the antiapoptotic protein BCL2L12 (Figure 3).45,46 Considering the role of CD82 in mediating cellular adhesion to the BM via cell surface clustering of N-cadherin, it is possible that these signaling events are downstream of N-cadherin. However, this still requires further study.
CD82 mediates leukemic stem cell survival and interactions with the microenvironment. CD82 mediates leukemic stem cell (LSC) interaction with the BM in multiple ways. It increases microenvironment interaction by organizing N-cadherin at the cell surface in high densities via CD82 palmitoylation (green triangles) and glycosylation (red spheres). However, the connection of N-cadherin to CD82-associated intracellular effects is still unknown. CD82-associated STAT5 signaling can combine with increased p-Akt (via inhibition of PTEN by DNA methylation from EZH2 due to downregulation of p38 caused by CD82-associated signaling) to increase the expression of antiapoptotic protein BCL2L12. The combination of these effects leads to increased LSC survival.
CD82 mediates leukemic stem cell survival and interactions with the microenvironment. CD82 mediates leukemic stem cell (LSC) interaction with the BM in multiple ways. It increases microenvironment interaction by organizing N-cadherin at the cell surface in high densities via CD82 palmitoylation (green triangles) and glycosylation (red spheres). However, the connection of N-cadherin to CD82-associated intracellular effects is still unknown. CD82-associated STAT5 signaling can combine with increased p-Akt (via inhibition of PTEN by DNA methylation from EZH2 due to downregulation of p38 caused by CD82-associated signaling) to increase the expression of antiapoptotic protein BCL2L12. The combination of these effects leads to increased LSC survival.
TSPANs also play a role in mediating the expression of key surface receptor components such as CD19, which is part of the B-cell coreceptor. CD19 relies on the expression of CD81 for proper trafficking to the cell membrane.41 CD81 downregulation was observed in B-cell acute lymphoblastic (B-ALL) cells resistant to blinatumomab, which targets CD19, indicating that cancer cells disrupt this association as a mechanism to overcome blinatumomab sensitivity.55 Likewise, in B-ALL CD81-knockout cells, CD19 expression was completely lost on the cell surface. This resulted in the depletion of Bruton tyrosine kinase signaling and a sensitization to chemotherapy.42 This highlights the secondary effect that TSPANs can have on intracellular signaling by controlling the expression of specific cell surface proteins.
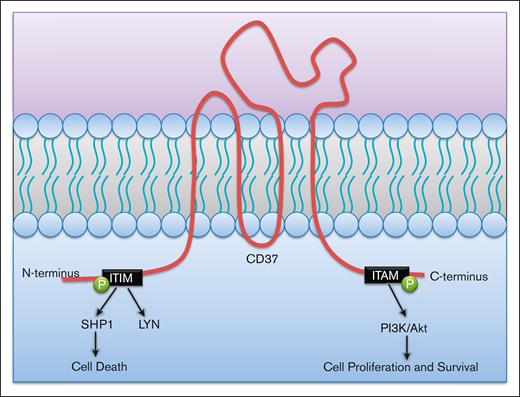
TSPANs can also directly initiate signaling pathways via their intracellular N- and C-termini. Antibody ligation of CD37 using a mAb in chronic lymphocytic leukemia can activate 2 separate, contradictory signaling pathways (Figure 4). After ligation, an N-terminus immunoreceptor tyrosine-based inhibitory motif–like motif becomes phosphorylated and forms a SHP1 phosphatase–containing complex that inhibits Akt and induces cell death. However, ligation also phosphorylates a C-terminus immunoreceptor tyrosine-based activation motif–like motif, which mediates prosurvival signaling through PI3Kδ activity.37 Therefore, it is possible that activation of the immunoreceptor tyrosine-based activation motif–like motif via lateral surface associations and interactions within the BMM could play a critical role in inducing malignant cell survival. However, because of the cell death properties associated with the immunoreceptor tyrosine-based inhibitory motif–like motif, combination therapy treatment of anti-CD37 mAb with PI3Kδ inhibitors could provide a synergistic mechanism for inducing cancer cell death.
CD37 mediates both prosurvival and prodeath effects from its N- and C-termini. CD37 initiates cell death via phosphorylation of an immune tyrosine-based inhibitory motif (ITIM)-like motif in its N-terminus tail. This leads to a SHP1-dependent cell death. IT can also initiate prosurvival signaling through phosphorylation of its immune tyrosine-based activation motif (ITAM) on the C-terminus tail. This occurs through PI3K/Akt signaling.
CD37 mediates both prosurvival and prodeath effects from its N- and C-termini. CD37 initiates cell death via phosphorylation of an immune tyrosine-based inhibitory motif (ITIM)-like motif in its N-terminus tail. This leads to a SHP1-dependent cell death. IT can also initiate prosurvival signaling through phosphorylation of its immune tyrosine-based activation motif (ITAM) on the C-terminus tail. This occurs through PI3K/Akt signaling.
The presence of stem cell properties is a major hallmark of malignant cells, and TSPANs play an important role in regulating these processes. CD9 is known to mediate the stem cell properties of B-ALL cells via modulation of the expression of Src family proteins and the upregulation of the histone deubiquitinase USP22.29 It is also a modulator of STAT5B signaling, which is responsible for HSC and leukemic stem cell self-renewal.35 Although the study of TSPANs and their regulation of stem cell dynamics in hematologic malignancies still requires further investigation, their role in HSCs has been better defined. Expression of CD81 is crucial for the maintenance of long-term HSCs via its deactivation of the Akt pathway after proliferative stress.56 This allows for a return to a quiescent state, a state that has therapeutic impacts in malignant cells. CD82 can also maintain this quiescent state in HSCs when it binds to its partner CD234 (DARC) on BM macrophages.57 This interaction stabilizes CD82 on the cell surface, thus activating protein kinase Cα–dependent TGF-β1/Smad3 signaling, resulting in cell cycle inhibition.
The role of TSPANs in exosome formation and their effects on the BM and immunosuppression
It is evident how TSPANs can control the cell surface dynamics and microenvironmental interactions of hematologic malignant cells. In addition to these phenomena, TSPANs also participate in intercellular communication as mediators of extracellular vesicle formation (exosomes) and are considered as markers for exosomes.58 TSPANs such as CD9, CD63, and CD81 play a critical role in cargo and target selection of exosomes, which can have significant effects on cancer progression.59 Cross talk between AML cells and BM mesenchymal stem cells mediated by AML-derived exosomes induce interleukin 8 secretion from mesenchymal stem cells. This results in a decreased sensitivity of AML cells to etoposide treatment.40 Although it was reported that the exosomes secreted by AML cells expressed TSPAN CD63, it remains to be determined whether CD63 expression promoted chemoresistance, as seen in the case of CD9-bearing exosomes that promote cancer cell migration.60
Malignant cell–derived exosomes can also modulate the organizational makeup of the BM. After exosome release from chronic lymphocytic leukemia cells, stromal cells underwent a transition into cancer-associated fibroblasts, whose increased cytokine secretions favored malignant cell growth.61 Induction of angiogenesis can also result from exosome release and favor the growth and survival of hematologic malignancies. In multiple myeloma, exosomes containing miR-135b were identified to be the mediators of factor-inhibiting hypoxia-inducible factor 1 expression and subsequent angiogenic formations.62
Immune evasion/suppression is another key aspect of malignant cell survival and progression. Exosomes derived from tumor cells are known to expand regulatory T-cell populations, thus preventing immune cell targeting of leukemic cells.63,64 It is intriguing to note that CD81 expression has been identified as a critical regulator of regulatory T-cell function.65 Because of this and the prominent expression of TSPANs on exosomes, it is possible that TSPANs could play a large role in cancer progression via immunosuppression. However, further investigation is still required.
Targeting TSPANs
TSPANs are potent mediators of BM interactions and cell signaling. Because of these effects, TSPANs make good therapeutic targets to improve treatment efficacy in hematologic malignancies (Figure 5).
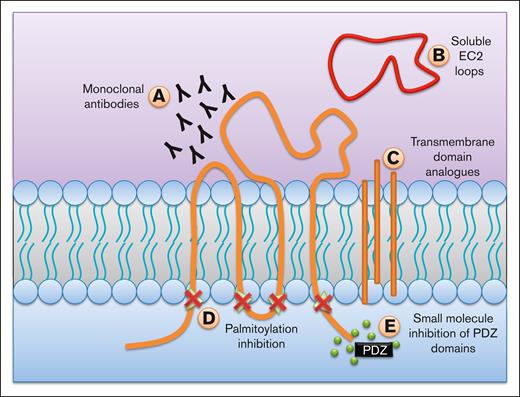
Possible methods to target TSPANs. There have been multiple proposed mechanisms on how to target TSPANs and inhibit their functions. (A) mAbs can be used to antagonize TSPANs either directly, or by inhibiting its lateral interactions. (B) Soluble EC2 loops can be used to disrupt the unique interactions of each TSPAN. (C) Analogues to transmembrane regions can prevent the formation of TEMs, thereby disrupting their effects. (D) Inhibiting palmitoylation can also be used as a mechanism to prevent TEM formation. (E) Many TSPANs have a motif in their C-terminus that can interact with PDZ domains on different signaling proteins. Small molecule inhibitors to these domains can prevent their activation, and therefore the cellular responses.
Possible methods to target TSPANs. There have been multiple proposed mechanisms on how to target TSPANs and inhibit their functions. (A) mAbs can be used to antagonize TSPANs either directly, or by inhibiting its lateral interactions. (B) Soluble EC2 loops can be used to disrupt the unique interactions of each TSPAN. (C) Analogues to transmembrane regions can prevent the formation of TEMs, thereby disrupting their effects. (D) Inhibiting palmitoylation can also be used as a mechanism to prevent TEM formation. (E) Many TSPANs have a motif in their C-terminus that can interact with PDZ domains on different signaling proteins. Small molecule inhibitors to these domains can prevent their activation, and therefore the cellular responses.
One way of targeting TSPANs is the use of mAbs, which have been widely used against CAMs in hematologic malignancies to improve outcomes.66 In most cases, these mAbs prevent a specific interaction between CAMs and their targets, either on other cells or in the ECM. mAbs against TSPANs can be antagonistic by diminishing associated cellular functions, either directly or by blocking lateral interactions (Table 2). For example, a unique CD81-targeting mAb that promotes CD81 clustering reportedly induces cytotoxicity in lymphoma cells via direct killing or via activation of the innate immune system.67,68 CD82-blocking mAbs potentiated the effect of cytarabine by disrupting AML cell adhesion to the BMM and improved survival in mice with AML cell engraftment.69 CD37 mAbs displayed proapoptotic effects in B-cell malignancies.70 A bifunctional mAb pair recognizing CD37 and CD20 mixed in a 1:1 ratio (PSB202)71 is currently being evaluated in the clinic. Moreover, CD37-targeting mAbs have been used in combination with PI3K inhibitors in preclinical studies to improve efficacy in chronic lymphocytic leukemia.72,73
Current TSPAN-targeting therapies in hematologic malignancies
| Therapeutic . | Target and effects . | Model . | Reference . |
|---|---|---|---|
| Anti-CD82 | CD82: mobilizing agent, potentiates effects of anticancer therapy | Preclinical | 69 |
| 5A6 | CD81: mAb selectively kills B-cell lymphoma cells via direct cell death and innate immune system activation | Preclinical | 67 |
| PSB202 | CD37-CD20: bifunctional mAb pair to enable direct killing of B cells in lymphomas | Clinical | NCT05003141 |
| CAR-37 | CD37: CAR T cells for CD37+ hematologic malignancies | Clinical | NCT04136275 |
| Therapeutic . | Target and effects . | Model . | Reference . |
|---|---|---|---|
| Anti-CD82 | CD82: mobilizing agent, potentiates effects of anticancer therapy | Preclinical | 69 |
| 5A6 | CD81: mAb selectively kills B-cell lymphoma cells via direct cell death and innate immune system activation | Preclinical | 67 |
| PSB202 | CD37-CD20: bifunctional mAb pair to enable direct killing of B cells in lymphomas | Clinical | NCT05003141 |
| CAR-37 | CD37: CAR T cells for CD37+ hematologic malignancies | Clinical | NCT04136275 |
CAR, chimeric antigen receptor.
Although mAbs offer a simple way to target TSPANs, it is not as simplistic as it may sound. Although many TSPANs are expressed at high levels in hematologic malignancies, they are also expressed on a variety of cell types throughout the body, both hematologic and nonhematologic, leading to off-target effects. However, studies using transgenic mice have identified that, despite knock out of these proteins, mice generally avoid overly serious side effects (Table 3). Encouragingly, targeting CD81 using 5A6 mAb selectively killed lymphoma cells while sparing normal hematopoietic cells, likely because of the higher CD81 expression in lymphoma.67 Another issue is that it can be difficult to know which cellular functions may be affected by using a mAb targeting a specific epitope. TSPANs can mediate a multitude of different processes, therefore, it can be hard to discern whether an antagonistic or agonistic response against a specific biologic process will be achieved.74,75
Physiologic effects of different TSPAN knockouts in mouse models
| Protein . | KO phenotype . | Physiologic consequence . | Reference . |
|---|---|---|---|
| CD9 | Enhanced lung inflammation | Reduced response to statins | 76 |
| Decreased lymphangiogenesis | Abnormal development of lymphatic vasculature | 77 | |
| CD37 | Deficiencies in T-cell–dependent B-cell responses | Reduced antibody responses to antigens | 78 |
| Dysregulation of TCR signaling | Hyperproliferative T cells | 79 | |
| Increased antigen presentation by dendritic cells | Hyperstimulation of T cells | 80 | |
| Impaired neutrophil recruitment | Decreased inflammatory response | 81 | |
| CD63 | Significant reduction in leukocyte rolling, recruitment, and extravasation | Decreased inflammatory response | 82 |
| CD81 | Lower expression of CD19 on B cells, leading to lower Ca2+ mobilization after CD19 stimulation | Abnormal B-cell antibody responses | 83 |
| Reduced production of IgG1 in Th2 immune responses | Reduced B1 lymphocyte response | 84 | |
| Decreased lymphangiogenesis | Abnormal development of lymphatic vasculature | 77 | |
| CD151 | Defects in hemostasis and in αIIbβ3 integrin signaling | Disrupted platelet function | 85 |
| Hyperproliferation of T cells in response to stimulation with mitogenic agents | Cellular hypersensitivity to activation | 86 |
| Protein . | KO phenotype . | Physiologic consequence . | Reference . |
|---|---|---|---|
| CD9 | Enhanced lung inflammation | Reduced response to statins | 76 |
| Decreased lymphangiogenesis | Abnormal development of lymphatic vasculature | 77 | |
| CD37 | Deficiencies in T-cell–dependent B-cell responses | Reduced antibody responses to antigens | 78 |
| Dysregulation of TCR signaling | Hyperproliferative T cells | 79 | |
| Increased antigen presentation by dendritic cells | Hyperstimulation of T cells | 80 | |
| Impaired neutrophil recruitment | Decreased inflammatory response | 81 | |
| CD63 | Significant reduction in leukocyte rolling, recruitment, and extravasation | Decreased inflammatory response | 82 |
| CD81 | Lower expression of CD19 on B cells, leading to lower Ca2+ mobilization after CD19 stimulation | Abnormal B-cell antibody responses | 83 |
| Reduced production of IgG1 in Th2 immune responses | Reduced B1 lymphocyte response | 84 | |
| Decreased lymphangiogenesis | Abnormal development of lymphatic vasculature | 77 | |
| CD151 | Defects in hemostasis and in αIIbβ3 integrin signaling | Disrupted platelet function | 85 |
| Hyperproliferation of T cells in response to stimulation with mitogenic agents | Cellular hypersensitivity to activation | 86 |
Rargeting TSPANs can be difficult because of their widespread expression on many cell types; despite these complications, many of the effects of TSPAN-knockout models can be mild, lending support to the development of anti-TSPAN therapies.
IgG, immunoglobulin G; TCR, T-cell receptor; Th2, T helper 2 cell.
Soluble EC2 loops are another way to target TSPANs by disrupting the specific interactions of each TSPAN protein.87 The lateral effects of TSPANs, like inducing VLA-4 binding to vascular cell adhesion molecule-1, can be diminished by this approach, thereby inhibiting responses to microenvironmental support. EC2 loops for CD9 and CD151 have previously been used to prevent extravasation by blocking endothelial cell interactions with leukocytes.88
The transmembrane regions of TSPANs are important for the development and stability of TEMs, which are a key part of the effects of TSPANs. Although no current studies have identified their effectiveness, structural analogues to these transmembrane regions have been proposed to be a possible way to disrupt TEM formation.89
Palmitoylation is critical for the formation of new TEMs; therefore, targeting TSPAN palmitoylation can also be used as a mechanism to prevent TEM formation. One way to achieve this is by inhibiting the activity of protein acyl transferases such as DHHC2, which reportedly affect the palmitoylation and stability of CD9 and CD151.90
Targeting the C-terminus can prevent the downstream effects of TSPANs. Specifically, many TSPANs have a motif in their C-terminus that can bind to PDZ domains present on cytoskeletal signaling proteins such as syntenin-1.91 Small-molecule inhibitors to these PDZ domains can prevent the transduction of extracellular stimuli into the cell, and prevent the corresponding cellular responses.92
Although most of the aforementioned targeting mechanisms focus on inhibiting TSPAN functions, some effects of TSPANs can be beneficial, and therefore, should be encouraged. This can be achieved by either adenoviral transductions or via targeted knock down of proteins known to mediate TSPAN degradation, such as gp78 ubiquitin ligase, which degrades CD82.93,94 Furthermore, TSPANs like CD81 and CD82 can have negative effects on cell survival and invasiveness in hematologic malignancies like multiple myeloma.95 Treatment with hypomethylating agents such 5-aza-2′-deoxycytidine can potentially elevate their expression by desilencing these genes and show a positive outcome.
TSPANs can be targeted in a multitude of ways, although many of the therapies are still in their infancy, especially in hematologic malignancies. If more targeted therapies can be successfully developed, they could go a long way in improving the overall efficacy of treatment.
Final thoughts
It is worth noting that, although TSPANs have not been identified to act as typical receptors and have no known cell-to-cell binding partners, it is possible that TSPANs could participate in homophilic or heterophilic binding via their EC2 loops. As mentioned earlier, the conserved region of the EC2 loop is responsible for mediating lateral homodimerization within the cell membrane9; however, it may be possible that these effects are seen in cell-to-cell contact as well. Moreover, the variable region of the EC2 loop dictates the specific interactome of each TSPAN. This region could possibly also mediate cell-to-cell contact by binding with TSPANs on other cells, or with other CAMs such as integrins through arginine-glycine-aspartic acid domains, which are known to interact with TSPANs.96 The BM plays a critical role in the progression and persistence of hematologic malignancies. By complexing with many different CAMs such as integrins and immunoglobulin superfamily members, and by using their ability to form dynamic membrane signaling entities (TEMs), TSPANs are potentially key mediators of interactions within the BM. Although different targeted therapies developed against individual CAMs have achieved moderate success, targeting TSPANs instead or in addition to individual CAMs could provide more comprehensive success. Further studies are needed not only to understand the role of TSPANs in hematologic malignancies but also in developing robust therapies that can target them and mitigate their effects.
Acknowledgments
The authors gratefully acknowledge the funding support from the Andrew McDonough B+ Foundation, the Lisa Dean Moseley Foundation, the Leukemia Research Foundation of Delaware, and the Nemours Foundation.
Authorship
Contribution: A.Q. researched and developed first draft and figures; A.G. conceptualized and edited draft manuscript; and S.P.B. conceptualized, researched, and edited the draft of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
The current affiliation for A.Q. is Dr. Kiran C. Patel College of Allopathic Medicine, Nova Southeastern University, Fort Lauderdale, FL.
Correspondence: Sonali P. Barwe, Nemours Children’s Hospital, Rm 5218, E400 Dupont Experimental Station, 200 Powder Mill Rd, Wilmington, DE 19803; e-mail: sbarwe@nemours.org.

![TEMs play a key role in TSPAN function. TEMs are dynamic membrane entities that play a key role in mediating interactions with the BM. TSPANs act as scaffolding proteins to bring together many proteins with similar functions such as CAMs (like integrins and immunoglobulin superfamily [IgSF] members) and signaling receptors (G protein–coupled receptors [GPCRs] and receptor tyrosine kinases [RTKs]). The crosslinking of TSPANs creates a large secondary signaling network, which can effectively transduce extracellular stimuli inside to intracellular signaling pathways.](https://ash.silverchair-cdn.com/ash/content_public/journal/bloodadvances/7/16/10.1182_bloodadvances.2023010476/2/m_blooda_adv-2023-010476-gr2.jpeg?Expires=1765906510&Signature=sitrPwX6Z75LYte7JAbo4fsToqjwPmx3OP~0nGndZSRYQPYl6NHo2fzWfDV2vfJ0ed6UelB75AbMu1W5Q1sMO7zPzKPe1aJlbXKPYC3ZgOdeOw3m0mdeUNWVlvdEXaoiGqy60wJpdb0CSriolE3W1popCsj4QTFXeMAdfzT6ciZmCrVRAJ~NfTZDyjYGpRYFEaTIxoz-B3AFQEZmrmk7nm1mBpBCF2JWMV5Ly0enxmUmbd6gJXEI6eK2jBj2tyPNv5eA~obzpFK5VJ0e6ZEtns0wGo9q2YHEn609ML0UrckHTymY2Cb9L4eV00boQJBONrUnScGVuNOG5jYhyGiPBw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)