Key Points
Adding sorafenib to CLAG-M is safe and leads to high rates of MRD– CR.
Compared with matched patients who received CLAG-M alone, CLAG-M/sorafenib led to longer multivariable-adjusted OS and EFS.
Abstract
The multikinase inhibitor sorafenib improves event-free survival (EFS) when used with 7 + 3 in adults with newly-diagnosed acute myeloid leukemia (AML), irrespective of the FLT3-mutation status. Here, we evaluated adding sorafenib to cladribine, high-dose cytarabine, granulocyte colony–stimulating factor, and mitoxantrone (CLAG-M) in a phase 1/2 trial of 81 adults aged ≤60 years with newly diagnosed AML. Forty-six patients were treated in phase 1 with escalating doses of sorafenib and mitoxantrone. No maximum tolerated dose was reached, and a regimen including mitoxantrone 18 mg/m2 per day and sorafenib 400 mg twice daily was declared the recommended phase 2 dose (RP2D). Among 41 patients treated at RP2D, a measurable residual disease–negative complete remission (MRD– CR) rate of 83% was obtained. Four-week mortality was 2%. One-year overall survival (OS) and EFS were 80% and 76%, without differences in MRD– CR rates, OS, or EFS between patients with or without FLT3-mutated disease. Comparing outcomes using CLAG-M/sorafenib with those of a matched cohort of 76 patients treated with CLAG-M alone, multivariable-adjusted survival estimates were improved for 41 patients receiving CLAG-M/sorafenib at RP2D (OS: hazard ratio,0.24 [95% confidence interval, 0.07-0.82]; P = .023; EFS: hazard ratio, 0.16 [95% confidence interval, 0.05-0.53]; P = .003). Benefit was limited to patients with intermediate-risk disease (univariate analysis: P = .01 for OS; P = .02 for EFS). These data suggest that CLAG-M/sorafenib is safe and improves OS and EFS relative to CLAG-M alone, with benefits primarily in patients with intermediate-risk disease. The trial was registered at www.clinicaltrials.gov as #NCT02728050.
Introduction
Despite the approval of several new drugs since 2017, the standard of care for medically fit adults with newly diagnosed acute myeloid leukemia (AML) has remained unchanged for 50 years and includes intensive chemotherapy, most commonly with standard-dose cytarabine-containing regimens, such as “7 + 3.”1,2 Although many patients will achieve complete remission (CR) with these therapies, the majority will ultimately relapse, and only ∼30% of patients will be alive 3 to 5 years after diagnosis.1,2 Therefore, the need for new first-line therapeutics to improve the outcomes with multiagent AML chemotherapy is unquestioned.
The orally administered multikinase inhibitor sorafenib may be such a therapeutic agent. Sorafenib inhibits kinases critical for tumor cell proliferation, including the serine/threonine kinase BRAF and the receptor tyrosine kinases RET, FLT3, and KIT, and targets the vascular endothelial growth factor receptor family (VEGFR-2/3).3 A randomized trial of 276 adults aged <60 years with newly diagnosed AML (SORAML) demonstrated that the addition of sorafenib 400 mg orally twice daily to 7 + 3 improved event-free survival (EFS) and relapse-free survival compared with 7 + 3 alone, a benefit that was observed irrespective of the FLT3 mutation status of the leukemia.4,5 This finding is particularly significant because ∼70% of patients with newly diagnosed AML do not harbor FLT3 abnormalities.
We previously showed in a phase 1/2 trial that cladribine, high-dose cytarabine, granulocyte colony–stimulating factor, and dose-escalated mitoxantrone (CLAG-M) led to a higher rate of CR without measurable residual disease (MRD– CR), an important predictor of long-term survival,6 relative to a historical group of medically matched patients treated with 7 + 3.7 Because the effects of sorafenib in the context of high-dose cytarabine-based induction chemotherapy are unknown, we conducted a single-arm phase 1/2 study evaluating the safety and preliminary efficacy of sorafenib when added to CLAG-M (CLAG-M/sorafenib) in adults aged <60 years with newly diagnosed AML or other high-grade myeloid neoplasms.
Patients and methods
Study population
We included adults aged 18 to 60 years with AML (based on the World Health Organization 2016 criteria, excluding acute promyelocytic leukemia) or other high-grade myeloid neoplasms (defined as ≥10% blasts in the peripheral blood or bone marrow). The patients were required to have a treatment-related mortality (TRM) score of ≤13.1. This score (online calculator: https://trmcalculator.fredhutch.org) corresponds to a predicted ≤13.1% risk of death by day 28 after intensive induction chemotherapy.8 Patients were also required to have adequate organ function, as defined by a left ventricular ejection fraction ≥ 45%, serum creatinine ≤ 2.0 mg/dL, and total bilirubin ≤ 2 times the upper limit of normal. Patients with prior exposure to azacitidine, decitabine, or other cytotoxic therapy for a hematologic malignancy, uncontrolled infection, concomitant illness with an expected survival of <1 year, and clinically significant cardiac disease (including myocardial infarction within 6 months of study or arrhythmias requiring antiarrhythmic therapy other than beta-blockers) were excluded. Hydroxyurea and up to 2 doses of cytarabine 500 mg/m2 for urgent cytoreduction before enrollment were allowed. All patients were treated at the Fred Hutchinson Cancer Center and the University of Washington Medical Center.
CLAG-M/sorafenib treatment plan
In phase 1, we assigned cohorts of 6 to 12 subjects to 1 of 6 dose levels that combined sorafenib doses between 200 and 400 mg twice daily on days 10 to 20, with escalating doses of mitoxantrone (10, 12, 15, or 18 mg/m2 per day on days 1-3, as previously studied7; Table 1). Sorafenib dosing was chosen based on the schedule of 7 + 3 in the SORAML trial.4 Other drug doses were fixed for all dose levels at: granulocyte colony–stimulating factor 5 μg/kg per day (rounded to the closest of 300 or 480 μg) on days 1 to 5, cladribine 5 mg/m2 per day on days 1 to 5, and cytarabine 2000 mg/m2 per day from on days 1 to 5. Consistent with our institutional standard practice, bone marrow specimens for response assessment after the first cycle were obtained at the time of blood count recovery (absolute neutrophil count [ANC] > 1000/μL and platelet count > 100 000/μL) or between days 28 and 35 after the start of CLAG-M, whichever occurred first. If MRD– CR was not achieved after the first course of therapy, a second identical cycle of induction therapy was administered. If CR or CR with incomplete hematologic recovery (CRi) was achieved with 1 or 2 courses of induction therapy, up to 4 cycles of postremission therapy with CLAG/sorafenib (mitoxantrone omitted) were allowed, with sorafenib administered from day 8 to 27 at the same dose as used during induction. Treatment in phase 2 included mitoxantrone and sorafenib administered at the recommended phase 2 dose (RP2D), as defined in phase 1; the number of induction and postremission courses was identical. The protocol allowed for maintenance therapy with sorafenib for 1 year after the completion of postremission therapy. Patients were excluded from the trial if they did not achieve MRD– CR after 3 courses of therapy, had persistent aplasia (ANC < 500/μL or platelets < 50 000/μL without evidence of leukemia after day 49), or underwent allogeneic hematopoietic cell transplantation (HCT). Toxicities were evaluated based on the National Cancer Institute Common Terminology Criteria for Adverse Events version 5.0 (http://ctep.cancer.gov).
Dose escalation schema, DLTs, and responses based on dose level in the CLAG-M/sorafenib phase 1 cohort (n = 46)
| Dose level . | Mitoxantrone dose (mg/m2)∗ . | Sorafenib dose (mg) . | Response . | DLT (n = 1) . |
|---|---|---|---|---|
| 1 (n = 6) | 10 | 200, daily | 3 MRD– CR 2 MRD+ CR 1 MRD– CRi | None |
| 2 (n = 6) | 12 | 200, bid | 6 MRD– CR | Intracranial hemorrhage |
| 3 (n = 11) | 15 | 200, bid | 8 MRD– CR 2 MLFS 1 resistant disease | Prolonged marrow aplasia |
| 4 (n = 8) | 18 | 200, bid | 4 MRD– CR 1 MRD– CRi 3 Resistant disease | Severe sepsis |
| 5 (n = 9) | 18 | 400, qAM, 200, qPM | 9 MRD– CR | None |
| 6 (n = 6) | 18 | 400 mg bid | 6 MRD– CR | Cardiomyopathy |
| Dose level . | Mitoxantrone dose (mg/m2)∗ . | Sorafenib dose (mg) . | Response . | DLT (n = 1) . |
|---|---|---|---|---|
| 1 (n = 6) | 10 | 200, daily | 3 MRD– CR 2 MRD+ CR 1 MRD– CRi | None |
| 2 (n = 6) | 12 | 200, bid | 6 MRD– CR | Intracranial hemorrhage |
| 3 (n = 11) | 15 | 200, bid | 8 MRD– CR 2 MLFS 1 resistant disease | Prolonged marrow aplasia |
| 4 (n = 8) | 18 | 200, bid | 4 MRD– CR 1 MRD– CRi 3 Resistant disease | Severe sepsis |
| 5 (n = 9) | 18 | 400, qAM, 200, qPM | 9 MRD– CR | None |
| 6 (n = 6) | 18 | 400 mg bid | 6 MRD– CR | Cardiomyopathy |
bid, twice daily; MLFS, morphologic leukemia–free state; qAM, every morning; qPM, every evening.
Dosing based on body surface area, using actual patient weight.
Comparison between CLAG-M/sorafenib and CLAG-M
For a nonrandomized comparison, we retrospectively identified adults aged ≥18 years with newly diagnosed nonacute promyelocytic leukemia AML or other high-grade myeloid neoplasms previously treated with CLAG-M with escalated-dose mitoxantrone (18 mg/m2 per dose) at our institution. Outcomes of some patients treated as part of our phase 1/2 CLAG-M study have been previously reported.7 For medical fitness matching, patients were required to be between 18 and 60 years old; have a TRM score of ≤13.1, left ventricular ejection fraction ≥ 45%, serum creatinine ≤ 2.0 mg/dL, total bilirubin ≤ 2 times the upper limit of normal; and to not have received prior therapy (including no prior exposure to hypomethylating agents). We collected baseline data, including age, sex, pretreatment cytogenetic/molecular risk, type of disease (de novo vs secondary), and TRM score and its components, along with clinical outcomes from electronic medical records.
Disease and response classification
The disease was risk-stratified using the refined Medical Research Council/National Cancer Research Institute9 and the 2017 European LeukemiaNet (ELN2017) criteria.10 Secondary disease was defined as either AML transformed from an antecedent hematologic disorder or AML/myelodysplastic syndrome after prior cytotoxic therapy or radiation. The best responses were measured after up to 2 cycles of induction therapy, and response and relapse were defined according to the ELN 2017 criteria.10 MRD was assessed using a multiparametric flow cytometry-based assay,11 which, in a large majority of cases, detects MRD at a level ≥0.1% and in progressively smaller subsets of patients, as the level of residual disease decreases below that level. Consistent with the performance characteristics of the assay and our approach in previous analyses, any level of MRD was considered MRD+.12-15
Statistical considerations
In line with the approach taken in our institutional phase 1/2 trials testing CLAG-M and CLAG-M in combination with gemtuzumab ozogamicin,7,16,17 dose-limiting toxicities (DLTs) in phase 1 were defined as (1) any grade 3 nonhematologic toxicity lasting >48 hours leading to a >7-day delay in the next cycle or (2) any grade ≥4 nonhematologic toxicity, if no recovery to grade ≤2 was observed in 14 days (both excluding febrile neutropenia/infection). In addition, we considered prolonged myelosuppression (ANC < 500/μL or platelet count < 50 000/μL for >49 days) after CLAG-M/sorafenib without any evidence of MRD or persistent disease a DLT. Doses were escalated up to dose level 6 if <2 of 6 patients in each cohort had a DLT (some cohorts were expanded up to 12 patients while awaiting completion of DLT monitoring periods). The level at which dose escalation was stopped was defined as RP2D. Patients treated in phase 1 at the RP2D were included in the RP2D analysis.
The primary end point in phase 2 was the rate of MRD– CR following CLAG-M/sorafenib administration, given the strong correlation of this end point with overall survival (OS).6,18 The secondary end points included the rate of CR (regardless of MRD status), overall response rate (defined as CR + CRi), OS, EFS (defined as either failure to achieve CR after 2 cycles of therapy, relapse, or death), and toxicity profile. The null hypothesis was an MRD– CR rate of 60% (based on data from patients treated with CLAG-M alone at the time of the study design), whereas the alternative hypothesis was an MRD– CR rate of 80%. The design had a 1-sided type 1 error rate of 10% and a power of 80%. Wilcoxon rank sum and Fisher exact tests were used to evaluate the associations between quantitative and categorical variables. EFS and OS were estimated using the Kaplan-Meier method. Multivariable logistic (for CR) and Cox regression models (for EFS and OS) were used to compare the outcomes between CLAG-M/sorafenib and CLAG-M.
The protocol (#NCT02728050) was approved by the Fred Hutchinson Cancer Center institutional review board, and the patients gave written informed consent in accordance with the Declaration of Helsinki. The data cutoff for the analysis was 25 January 2023.
Results
Study cohort and treatment
84 patients were enrolled in phases 1 and 2 of the CLAG-M/sorafenib trial between 8 December 2016 and 7 October 2021. Among these, 1 patient in phase 1 (who received CLAG-M at the RP2D dosing) and 2 patients in phase 2 did not receive any sorafenib: 2 because of the identification of an FLT3 TKD mutation (taken off protocol therapy to recieve midostaurin), and 1 because of personal preference; these 3 patients were omitted from the analysis. None of the patients received sorafenib maintenance therapy, largely because patients underwent allogeneic HCT or received nonprotocol postremission chemotherapy. The baseline characteristics of the remaining 81 patients are summarized in Table 2.
Baseline characteristics of the CLAG-M/sorafenib study cohort and historical cohort of patients treated with CLAG-M
| . | CLAG-M/sorafenib . | CLAG-M . | P value∗ . | ||
|---|---|---|---|---|---|
| All patients (N = 81) (%) . | Phase 1 cohort (n = 46) (%) . | RP2D cohort (n = 41) (%) . | All patients (N = 76) (%) . | ||
| Age, median (range), y | 50 (21-60) | 48 (22-60) | 50 (21-60) | 46 (18-60) | .16 |
| ECOG performance status | 1.00 | ||||
| 0-1 | 75 (93) | 43 (93) | 38 (93) | 71 (93) | |
| 2-3 | 6 (7) | 3 (7) | 3 (7) | 5 (7) | |
| Laboratory values at diagnosis | |||||
| White blood cell count | 10.2 (0.7-183.3) | 9.68 (0.94-183.3) | 11.1 (0.73-351.0) | 5.53 (0.58-240.4) | .17 |
| Platelet count | 47 (9-361) | 47 (9-257) | 58 (12-351) | 49 (6-794) | .39 |
| Creatinine | 0.84 (0.44-1.63) | 0.84 (0.44-1.63) | 0.84 (0.47-1.45) | 0.85 (0.34-1.37) | .89 |
| Bilirubin | 0.6 (0.2-1.6) | 0.6 (0.2-1.6) | 0.5 (0.3-1.6) | 0.6 (0-2.2) | .99 |
| TRM score, median (range) | 2.08 (0.19-12.26) | 2.06 (0.20-12.26) | 2.28 (0.20-8.52) | 1.99 (0.01-10.95) | .33 |
| Disease | .62 | ||||
| AML | 66 (81) | 39 (85) | 33 (80) | 64 (84) | |
| MDS-EB-2 | 12 (15) | 7 (15) | 8 (20) | 12 (16) | |
| Other | 3 (4) | 0 | 0 | 0 | |
| Secondary disease† | 12 (15) | 6 (13) | 6 (15) | 15 (20) | .62 |
| Cytogenetic risk (MRC) | .71 | ||||
| Favorable | 4 (5) | 1 (2) | 3 (7) | 9 (12) | |
| Intermediate | 59 (73) | 36 (78) | 29 (71) | 46 (62) | |
| Adverse | 18 (22) | 9 (20) | 9 (22) | 17 (23) | |
| Unknown | 0 | 0 | 0 | 8 (11) | |
| Cytogenetic/molecular risk (ELN 2017) | .02 | ||||
| Favorable | 24 (30) | 14 (30) | 11 (27) | 30 (39) | |
| NPM1 | 16 | 9 | 7 | 20 | |
| CEBPA | 6 | 4 | 2 | 5 | |
| Intermediate | 25 (31) | 13 (28) | 16 (39) | 14 (18) | |
| Adverse | 31 (38) | 18 (39) | 14 (34) | 24 (32) | |
| Unknown | 1 (1) | 1 (2) | 0 | 8 (11) | |
| FLT3-ITD, n (%) | 21 (26) | 11 (24) | 13 (32) | 9 (13) | .03 |
| FLT3-TKD, n (%) | 4 (5) | 3 (7) | 2 (5) | 5 (19) | .11 |
| . | CLAG-M/sorafenib . | CLAG-M . | P value∗ . | ||
|---|---|---|---|---|---|
| All patients (N = 81) (%) . | Phase 1 cohort (n = 46) (%) . | RP2D cohort (n = 41) (%) . | All patients (N = 76) (%) . | ||
| Age, median (range), y | 50 (21-60) | 48 (22-60) | 50 (21-60) | 46 (18-60) | .16 |
| ECOG performance status | 1.00 | ||||
| 0-1 | 75 (93) | 43 (93) | 38 (93) | 71 (93) | |
| 2-3 | 6 (7) | 3 (7) | 3 (7) | 5 (7) | |
| Laboratory values at diagnosis | |||||
| White blood cell count | 10.2 (0.7-183.3) | 9.68 (0.94-183.3) | 11.1 (0.73-351.0) | 5.53 (0.58-240.4) | .17 |
| Platelet count | 47 (9-361) | 47 (9-257) | 58 (12-351) | 49 (6-794) | .39 |
| Creatinine | 0.84 (0.44-1.63) | 0.84 (0.44-1.63) | 0.84 (0.47-1.45) | 0.85 (0.34-1.37) | .89 |
| Bilirubin | 0.6 (0.2-1.6) | 0.6 (0.2-1.6) | 0.5 (0.3-1.6) | 0.6 (0-2.2) | .99 |
| TRM score, median (range) | 2.08 (0.19-12.26) | 2.06 (0.20-12.26) | 2.28 (0.20-8.52) | 1.99 (0.01-10.95) | .33 |
| Disease | .62 | ||||
| AML | 66 (81) | 39 (85) | 33 (80) | 64 (84) | |
| MDS-EB-2 | 12 (15) | 7 (15) | 8 (20) | 12 (16) | |
| Other | 3 (4) | 0 | 0 | 0 | |
| Secondary disease† | 12 (15) | 6 (13) | 6 (15) | 15 (20) | .62 |
| Cytogenetic risk (MRC) | .71 | ||||
| Favorable | 4 (5) | 1 (2) | 3 (7) | 9 (12) | |
| Intermediate | 59 (73) | 36 (78) | 29 (71) | 46 (62) | |
| Adverse | 18 (22) | 9 (20) | 9 (22) | 17 (23) | |
| Unknown | 0 | 0 | 0 | 8 (11) | |
| Cytogenetic/molecular risk (ELN 2017) | .02 | ||||
| Favorable | 24 (30) | 14 (30) | 11 (27) | 30 (39) | |
| NPM1 | 16 | 9 | 7 | 20 | |
| CEBPA | 6 | 4 | 2 | 5 | |
| Intermediate | 25 (31) | 13 (28) | 16 (39) | 14 (18) | |
| Adverse | 31 (38) | 18 (39) | 14 (34) | 24 (32) | |
| Unknown | 1 (1) | 1 (2) | 0 | 8 (11) | |
| FLT3-ITD, n (%) | 21 (26) | 11 (24) | 13 (32) | 9 (13) | .03 |
| FLT3-TKD, n (%) | 4 (5) | 3 (7) | 2 (5) | 5 (19) | .11 |
CEBPA, CCAAT enhancer-binding protein alpha; EB, excess blasts; ECOG, Eastern Cooperative Oncology Group; MDS, myelodysplastic syndrome; MRC, Medical Research Council; TRM, treatment-related mortality score.
Comparison CLAG-M/sorafenib RP2D cohort vs CLAG-M cohort.
Defined as AML transformed from antecedent hematologic disorder or AML/myelodysplastic syndrome in a patient who had previously received cytotoxic therapy.
Phase 1
Of the 81 patients treated with CLAG-M/sorafenib, 46 patients with a median age of 48 years (range, 22-60 years) were enrolled in phase 1 of the study. ELN2017 cytogenetic/molecular risk was favorable in 14 patients, intermediate in 13, and adverse in 18; 31% had an FLT3-internal tandem duplication (ITD) (n = 11) or TKD mutation (n = 3) (Table 2). One DLT event occurred at dose level 2 (intracranial hemorrhage), dose level 3 (prolonged aplasia), dose level 4 (severe sepsis), and dose level 6 (cardiomyopathy; Table 1). Other treatment-emergent adverse events are described subsequently. None of the patients died within 4 weeks of starting therapy. This established CLAG-M with mitoxantrone at 18 mg/m2 per day and sorafenib at 400 mg twice daily as the RP2D. Six of the 46 phase 1 patients received CLAG-M/sorafenib at R2PD. Across all cohorts, 36 patients treated in phase 1 (78%; 95% confidence interval [95% CI], 64-89%) achieved MRD– CR. Thirty-eight patients (83%) achieved CR, 2 (4%) achieved MRD– CRi, 2 (4%) achieved a morphologic leukemia–free state, and 4 (9%) had resistant disease.
RP2D cohort
Forty-one patients with a median age of 50 years (range, 21-60 years) were treated at the RP2D, including 6 patients treated in phase 1 (Table 2). ELN2017 disease risk was favorable in 27%, intermediate in 39%, and adverse in 34% of the patients. FLT3-ITD and TKD mutations were present in 32% and 5% of the patients, respectively. Patients received a median of 2 cycles (range, 1-3) of on-protocol therapy. Among these 41 patients, 34 (83%, 95% CI, 68-93) achieved MRD– CR (Table 3), meeting our primary end point of an MRD– CR rate of 80%. Two of the 34 patients (6%) who achieved MRD– CR required 2 courses of therapy to obtain this response. Two additional patients achieved MRD+ CR and 2 achieved CRi, with an overall response rate of 93% (95% CI, 80-98). Two patients had resistant disease, and 1 died before the disease status was reassessed. The mortality at 28 days was 2% (95% CI, 0-13%). The MRD– CR rates in patients with and without FLT3 mutations (excluding the subject without baseline mutational data) were similar (93% vs 80%; P = .38). With a median follow-up of 34 months (range, 15-55 months), median OS and EFS were not reached. Twelve-month OS and EFS were 80% and 76%, respectively (Figure 1A-B). There was no difference in OS and EFS between patients with FLT3-mutated and FLT3-wild-type disease (Figure 1C-D). Sixty-three percent of patients underwent allogeneic HCT a median of 120 days (range, 73-532 days) after the start of the protocol therapy. Reasons patients who did not undergo HCT included favorable-risk disease (n = 6), patient choice/COVID-related choice (n = 4), death before transplantation (n = 3), refractory or relapsed disease before HCT (n = 1), and lack of a suitable caregiver (n = 1).
Best response after 1 or 2 cycles of study therapy
| Response, n (%) . | CLAG-M/sorafenib RP2D cohort (n = 41), (%) . | CLAG-M control cohort (n = 76), (%) . | P value . |
|---|---|---|---|
| MRD– CR | 34 (83) | 58 (76) | .48 |
| MRD+ CR | 2 (5) | 2 (3) | — |
| CRi | — | ||
| MRD– | 1 (2) | 2 (3) | |
| MRD+ | 1 (2) | 1 (1) | |
| ORR (CR + CRi) | 38 (93) | 63 (83) | .17 |
| MLFS | — | ||
| MRD– | 0 | 4 (5) | |
| Resistant disease | 2 (5) | 5 (7) | — |
| Death indeterminate cause | 1 (2) | 4 (5) | — |
| 4-wk mortality | 1 (2) | 3 (4) | 1.00 |
| 8-wk mortality | 1 (2) | 5 (7) | .42 |
| 1-y OS | 80 | 72 | .20 |
| 1-y EFS | 76 | 64 | .06 |
| D to ANC ≥ 500/μL | 29 (20-50) | 27 (17-44) | .02 |
| D to ANC ≥ 1000/μL | 32 (21-50) | 29 (18-45) | .02 |
| D to platelets ≥ 50 000/μL | 27 (17-50) | 23 (17-42) | .06 |
| D to platelets ≥ 100 000/μL | 31 (18-50) | 26 (19-51) | .03 |
| Response, n (%) . | CLAG-M/sorafenib RP2D cohort (n = 41), (%) . | CLAG-M control cohort (n = 76), (%) . | P value . |
|---|---|---|---|
| MRD– CR | 34 (83) | 58 (76) | .48 |
| MRD+ CR | 2 (5) | 2 (3) | — |
| CRi | — | ||
| MRD– | 1 (2) | 2 (3) | |
| MRD+ | 1 (2) | 1 (1) | |
| ORR (CR + CRi) | 38 (93) | 63 (83) | .17 |
| MLFS | — | ||
| MRD– | 0 | 4 (5) | |
| Resistant disease | 2 (5) | 5 (7) | — |
| Death indeterminate cause | 1 (2) | 4 (5) | — |
| 4-wk mortality | 1 (2) | 3 (4) | 1.00 |
| 8-wk mortality | 1 (2) | 5 (7) | .42 |
| 1-y OS | 80 | 72 | .20 |
| 1-y EFS | 76 | 64 | .06 |
| D to ANC ≥ 500/μL | 29 (20-50) | 27 (17-44) | .02 |
| D to ANC ≥ 1000/μL | 32 (21-50) | 29 (18-45) | .02 |
| D to platelets ≥ 50 000/μL | 27 (17-50) | 23 (17-42) | .06 |
| D to platelets ≥ 100 000/μL | 31 (18-50) | 26 (19-51) | .03 |
HMA, hypomethylating agents (ie, azanucleosides); MLFS, morphologic leukemia–free state; ORR, overall response rate.
Estimates of overall survival and event-free survival for patients treated at the RP2D of CLAG-M/sorafenib. Estimates of (A,C) OS and (B,D) EFS for patients treated with CLAG-M/sorafenib for (A-B) the entire RP2D cohort and (C-D) the RP2D cohort stratified based on FLT3 mutation status. Wt, wild-type.
Estimates of overall survival and event-free survival for patients treated at the RP2D of CLAG-M/sorafenib. Estimates of (A,C) OS and (B,D) EFS for patients treated with CLAG-M/sorafenib for (A-B) the entire RP2D cohort and (C-D) the RP2D cohort stratified based on FLT3 mutation status. Wt, wild-type.
Treatment-emergent adverse events
Table 4 summarizes the number of individual patients affected by grades 3 to 5 (National Cancer Institute Common Terminology Criteria for Adverse Events version5.0) treatment-emergent adverse events during the first cycle among the 46 patients treated in phase 1 and 41 patients treated at the RP2D. Two patients treated at the RP2D experienced grade 5 adverse events (ventricular fibrillation and cardiac arrest in 1 and fungal sinusitis in 1). The most common treatment-emergent adverse events were febrile neutropenia with 78% of subjects in phase 1 and 71% of subjects in the RP2D cohort having at least one instance of febrile neutropenia, and maculopapular rash in 37% of phase 1 patients and 32% of RP2D patients. At the RP2D, cardiac toxicities, including cardiac arrest, hypertension, and cardiomyopathy, occurred in 5 (12%) patients; however, no grade ≥3 bleeding events were identified.
Grades 3 to 5 treatment-emergent adverse events occurring with CLAG-M/sorafenib during the first treatment cycle in phase 1 and at the RP2D
| Adverse events based on the organ system class, n (% cycles) . | Phase 1 cohort (n = 46) . | RP2D cohort∗ (n = 41) . |
|---|---|---|
| Blood and lymphatic system disorders† | ||
| Febrile neutropenia | 52 | 40 |
| Infections and infestations | ||
| Bacteremia | 1 | 1 |
| Clostridium difficile | 1 | — |
| Enterocolitis/typhlitis | — | 2 |
| Lung infection | — | 2 |
| Oral infection | 1 | — |
| Sepsis | 2† | — |
| Sinusitis | 1 | 2‡ |
| Skin/soft-tissue infection | 3 | 2 |
| Upper respiratory tract infection | 1 | |
| Cardiac disorders | ||
| Cardiac arrest | — | 1‡ |
| Cardiac troponin increase | 1 | |
| Cardiomyopathy | — | 2 |
| Hypertension | 2 | 2 |
| Hypotension | 1 | — |
| Pericarditis | 1 | — |
| Myocardial infarction | 1 | — |
| Ventricular fibrillation | — | 1‡ |
| Gastrointestinal disorders | ||
| Abdominal pain | — | 1 |
| Colon adenocarcinoma | 1 | — |
| Dental caries/tooth pain | 1 | 1 |
| Diarrhea | 1 | 1 |
| Fistula/fissure, anorectal | 1 | 2 |
| Rectal pain | — | 1 |
| Investigations | ||
| aPTT prolonged | 1 | — |
| Alanine aminotransferase increased | — | 1 |
| Aspartate aminotransferase increased | — | 1 |
| Blood bilirubin increased | 1 | 1 |
| Metabolism and nutrition disorders | ||
| Hyperglycemia | — | 3 |
| Hypokalemia | 1 | 2 |
| Hyponatremia | 2 | — |
| Tumor lysis | 3 | 1 |
| Psychiatric disorders | ||
| Delirium | 1 | — |
| Nervous system disorders | ||
| Depressed level of consciousness | 1† | — |
| Headache | 1 | — |
| Intracranial hemorrhage | 1† | — |
| Paresthesia | — | 1 |
| Seizure | — | 1 |
| Syncope | 1 | 2 |
| Renal and urinary disorders | ||
| Acute kidney injury | — | 1 |
| Hematuria | 1 | — |
| Respiratory, thoracic, and mediastinal disorders | ||
| Hypoxia | — | 1† |
| Pulmonary edema/effusion | — | 2† |
| Pulmonary embolism | 2 | |
| Skin and subcutaneous tissue disorders | ||
| Rash maculo-papular | 17 | 13 |
| Pruritis | — | 1 |
| Other | ||
| Eye disorders: blurred vision | 1 | — |
| Ear and labyrinth disorders: Bell palsy | 1 | — |
| Infusion reaction, blood product | — | 2 |
| Musculoskeletal: joint or extremity pain | 2 | — |
| Vascular: catheter-associated thrombosis | 4 | 3 |
| Adverse events based on the organ system class, n (% cycles) . | Phase 1 cohort (n = 46) . | RP2D cohort∗ (n = 41) . |
|---|---|---|
| Blood and lymphatic system disorders† | ||
| Febrile neutropenia | 52 | 40 |
| Infections and infestations | ||
| Bacteremia | 1 | 1 |
| Clostridium difficile | 1 | — |
| Enterocolitis/typhlitis | — | 2 |
| Lung infection | — | 2 |
| Oral infection | 1 | — |
| Sepsis | 2† | — |
| Sinusitis | 1 | 2‡ |
| Skin/soft-tissue infection | 3 | 2 |
| Upper respiratory tract infection | 1 | |
| Cardiac disorders | ||
| Cardiac arrest | — | 1‡ |
| Cardiac troponin increase | 1 | |
| Cardiomyopathy | — | 2 |
| Hypertension | 2 | 2 |
| Hypotension | 1 | — |
| Pericarditis | 1 | — |
| Myocardial infarction | 1 | — |
| Ventricular fibrillation | — | 1‡ |
| Gastrointestinal disorders | ||
| Abdominal pain | — | 1 |
| Colon adenocarcinoma | 1 | — |
| Dental caries/tooth pain | 1 | 1 |
| Diarrhea | 1 | 1 |
| Fistula/fissure, anorectal | 1 | 2 |
| Rectal pain | — | 1 |
| Investigations | ||
| aPTT prolonged | 1 | — |
| Alanine aminotransferase increased | — | 1 |
| Aspartate aminotransferase increased | — | 1 |
| Blood bilirubin increased | 1 | 1 |
| Metabolism and nutrition disorders | ||
| Hyperglycemia | — | 3 |
| Hypokalemia | 1 | 2 |
| Hyponatremia | 2 | — |
| Tumor lysis | 3 | 1 |
| Psychiatric disorders | ||
| Delirium | 1 | — |
| Nervous system disorders | ||
| Depressed level of consciousness | 1† | — |
| Headache | 1 | — |
| Intracranial hemorrhage | 1† | — |
| Paresthesia | — | 1 |
| Seizure | — | 1 |
| Syncope | 1 | 2 |
| Renal and urinary disorders | ||
| Acute kidney injury | — | 1 |
| Hematuria | 1 | — |
| Respiratory, thoracic, and mediastinal disorders | ||
| Hypoxia | — | 1† |
| Pulmonary edema/effusion | — | 2† |
| Pulmonary embolism | 2 | |
| Skin and subcutaneous tissue disorders | ||
| Rash maculo-papular | 17 | 13 |
| Pruritis | — | 1 |
| Other | ||
| Eye disorders: blurred vision | 1 | — |
| Ear and labyrinth disorders: Bell palsy | 1 | — |
| Infusion reaction, blood product | — | 2 |
| Musculoskeletal: joint or extremity pain | 2 | — |
| Vascular: catheter-associated thrombosis | 4 | 3 |
Hematologic treatment-emergent adverse events were not collected, except for febrile neutropenia and bleeding events ≥grade 3.
Six patients are represented in both the phase 1 and RP2D cohorts.
One of the events is a grade 4 event.
One of the events is a grade 5 event: 1 each for the adverse events marked.
Duration of cytopenias
To minimize confounding by residual leukemia, we assessed the time to peripheral blood count recovery after CLAG-M/sorafenib in a subset of patients treated with RP2D who achieved morphologic CR after the first course of induction therapy. Among these 36 subjects, the median time periods to ANC recovery of 500/μL and platelet recovery of 50 000/μL were 29 days (range, 20-50 days) and 27 days (range, 17-50 days), respectively. The median time to recovery of ANC ≥ 1000/μL and platelets ≥ 100 000/μL was 32 days (range, 21-50 days) and 31 days (range, 18-50 days), respectively.
Comparison between CLAG-M/sorafenib and CLAG-M alone
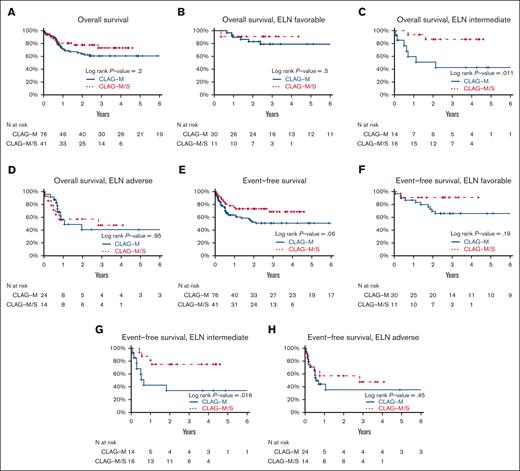
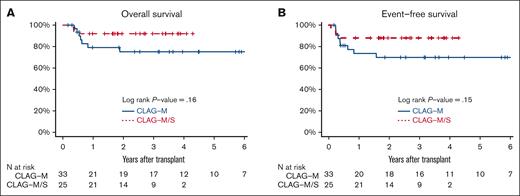
Finally, we compared the characteristics and outcomes of 41 patients treated with CLAG-M/sorafenib at the RP2D with those of a historical cohort of 76 newly diagnosed patients treated with CLAG-M alone, matching control patients for mitoxantrone dose, age, TRM score, organ function, and treatment history (Table 2). These 2 patient cohorts were similar across most baseline variables, except that the proportion of patients with favorable-risk disease and FLT3-tyrosine kinase domain (TKD) mutations was higher, and the proportion of patients with FLT3-ITD mutations was lower in the CLAG-M group. A comparison of response rates and survival is shown in Table 3. In univariate analysis, the rates of MRD– CR, OS, and EFS were not significantly different (P = .48; P = .20; and P = .06, respectively; Figure 2). Similarly, no statistically significant difference was identified in OS (P = .08) or EFS (P = .21) between patients with mutated FLT3 who received CLAG-M/sorafenib and those who received CLAG-M. In the univariate subgroup analysis, we observed a benefit of CLAG-M/sorafenib for both OS (P = .01) and EFS (P = .02) among patients with ELN2017 intermediate-risk disease (Figure 2). No benefit of CLAG-M/sorafenib vs CLAG-M alone was observed among patients with adverse risk disease for either OS or EFS. The analysis of patients with favorable-risk AML was limited by the small sample size. Multivariable models comparing CLAG-M/sorafenib to CLAG-M alone (Table 5) and controlling for age, secondary disease, FLT3 mutation status, ELN 2017 cytogenetic/molecular risk, and number of induction cycles required to achieve the best response showed improved OS (HR, 0.24; 95% CI, 0.07-0.82; P = .023) and EFS (HR, 0.16; 95% CI, 0.05-0.53; P = .003) following CLAG-M/sorafenib; however, the odds ratio (OR) for MRD– CR with CLAG-M/sorafenib was not statistically significant (OR, 1.95; 95% CI, 0.57-6.63; P = .28). The sample size precluded interaction analysis between the treatment group and disease risk. The median time to ANC of 500/μL and 1000/μL was 2 and 3 days longer, respectively, in the CLAG-M/sorafenib group (P = .02 for both), and the median time to platelets 50 000/μL and 100 000/μL was 4 and 5 days longer, respectively (P = .06 and P = .03, respectively; Table 3). Transplantation rates were 63% in our RP2D CLAG-M/sorafenib cohort and 58% in the CLAG-M alone cohort, including 61% and 43% of patients, respectively undergoing HCT in first remission. Thus, we built additional multivariable models for EFS and OS, in which we included transplantation as a time-dependent covariate. In these models, EFS remained significantly longer for the CLAG-M/sorafenib group (HR, 0.15; 95% CI, 0.04-0.52; P = .0027). For OS, the HR remained low at 0.46 (95% CI, 0.20-1.09; P = .08), with the P value at least partially driven by the modest number of events. The post-HCT outcomes of the 33 patients who received CLAG-M/sorafenib and 25 patients who received CLAG-M alone are depicted in Figure 3.
Comparison of overall and event-free survival of patients who recieved CLAG-M/sorafenib vs medically matched patients who received CLAG-M alone. Estimates of (A-D) OS and (E-H) EFS for 41 patients who received CLAG-M/sorafenib at the RP2D and 76 medically matched patients who received CLAG-M alone are shown (A,E) for all patients stratified based on (B,F) favorable, (C,G) intermediate, and (D,H) adverse cytogenetic/molecular disease risk. CLAG-M/S, CLAG-M/sorafenib.
Comparison of overall and event-free survival of patients who recieved CLAG-M/sorafenib vs medically matched patients who received CLAG-M alone. Estimates of (A-D) OS and (E-H) EFS for 41 patients who received CLAG-M/sorafenib at the RP2D and 76 medically matched patients who received CLAG-M alone are shown (A,E) for all patients stratified based on (B,F) favorable, (C,G) intermediate, and (D,H) adverse cytogenetic/molecular disease risk. CLAG-M/S, CLAG-M/sorafenib.
Multivariable models evaluating MRD– CR, OS, and EFS for CLAG-M/sorafenib RP2D vs historical CLAG-M cohort
| Covariate . | OR for MRD– CR . | 95% CI . | P value . | HR for OS . | 95% CI . | P value . | HR for EFS . | 95% CI . | P value . |
|---|---|---|---|---|---|---|---|---|---|
| CLAG-M/Sorafenib (ref = CLAG-M) | 1.95 | 0.57-6.63 | .28 | 0.24 | 0.07-0.82 | .023 | 0.16 | 0.05-0.53 | .003 |
| Age | 0.94 | 0.89-1.00 | .05 | 1.04 | 1.00-1.09 | .05 | 1.05 | 1.01-1.09 | .02 |
| Secondary disease∗ | 0.68 | 0.18-2.63 | .58 | 2.52 | 01.02-6.23 | .04 | 1.34 | 0.58-3.12 | .49 |
| FLT3-mutated | 2.21 | 0.40-12.15 | .36 | 0.85 | 0.27-2.66 | .77 | 0.69 | 0.23-2.04 | .50 |
| Intermediate risk† | 0.18 | 0.03-1.01 | .05 | 3.46 | 1.12-10.65 | .03 | 3.15 | 1.18-8.37 | .02 |
| Adverse risk† | 0.14 | 0.03-0.74 | .02 | 3.02 | 1.01-9.02 | .05 | 2.50 | 1.00-6.22 | .05 |
| Two induction cycles (ref = 1 induction cycle) | —‡ | 0.77 | 0.22-2.68 | .69 | 1.36 | 0.51-3.63 | .54 |
| Covariate . | OR for MRD– CR . | 95% CI . | P value . | HR for OS . | 95% CI . | P value . | HR for EFS . | 95% CI . | P value . |
|---|---|---|---|---|---|---|---|---|---|
| CLAG-M/Sorafenib (ref = CLAG-M) | 1.95 | 0.57-6.63 | .28 | 0.24 | 0.07-0.82 | .023 | 0.16 | 0.05-0.53 | .003 |
| Age | 0.94 | 0.89-1.00 | .05 | 1.04 | 1.00-1.09 | .05 | 1.05 | 1.01-1.09 | .02 |
| Secondary disease∗ | 0.68 | 0.18-2.63 | .58 | 2.52 | 01.02-6.23 | .04 | 1.34 | 0.58-3.12 | .49 |
| FLT3-mutated | 2.21 | 0.40-12.15 | .36 | 0.85 | 0.27-2.66 | .77 | 0.69 | 0.23-2.04 | .50 |
| Intermediate risk† | 0.18 | 0.03-1.01 | .05 | 3.46 | 1.12-10.65 | .03 | 3.15 | 1.18-8.37 | .02 |
| Adverse risk† | 0.14 | 0.03-0.74 | .02 | 3.02 | 1.01-9.02 | .05 | 2.50 | 1.00-6.22 | .05 |
| Two induction cycles (ref = 1 induction cycle) | —‡ | 0.77 | 0.22-2.68 | .69 | 1.36 | 0.51-3.63 | .54 |
Defined as AML transformed from antecedent hematologic disorder or AML/myelodysplastic syndrome in a patient who had previously received cytotoxic therapy.
Based on ELN 2017 cytogenetic/molecular criteria.
The number of induction cycles is not included in multivariable model because of model instability resulting from small sample sizes in subgroups.
Estimate of posttransplant OS and EFS for the patients who recieved CLAG-M/sorafenib vs CLAG-M alone. Estimates of posttransplantation (A) OS and (B) EFS OS for the 33 patients who received CLAG-M/sorafenib at the RP2D and the 25 medically matched patients who received CLAG-M alone and underwent allogeneic HCT in the first morphologic remission.
Estimate of posttransplant OS and EFS for the patients who recieved CLAG-M/sorafenib vs CLAG-M alone. Estimates of posttransplantation (A) OS and (B) EFS OS for the 33 patients who received CLAG-M/sorafenib at the RP2D and the 25 medically matched patients who received CLAG-M alone and underwent allogeneic HCT in the first morphologic remission.
Discussion
Sorafenib, an oral multikinase inhibitor that is relatively well tolerated, is an appealing drug to complement high-dose AML chemotherapy backbones. It is currently approved for hepatocellular, renal, and thyroid carcinomas and has been studied in patients with FLT3-mutated AML in both pre and postHCT settings.19,20 Possible mechanisms underlying the antileukemic activity of sorafenib include the inhibition of multiple kinases active in leukemic cells, antiangiogenic activity through the inhibition of vascular endothelial growth factor and platelet-derived growth factor, and stimulation of antileukemic immune responses through increased production of interleukin-15, a cytokine known to enhance T-cell persistence and cytolytic activity.21
Here, we tested the addition of sorafenib to CLAG-M chemotherapy. The findings from our phase 1/2 trial that combining sorafenib with CLAG-M support 3 main conclusions. Firstly, the addition of sorafenib to CLAG-M was safe, with a treatment-emergent adverse event/toxicity profile similar to that expected using CLAG-M alone. Secondly, treatment with CLAG-M/sorafenib yielded high rates of MRD– CR (83%) in patients aged between 18 and 60 years with newly diagnosed AML or other high-grade myeloid neoplasms. Thirdly, after multivariable adjustment, the survival outcomes observed after CLAG-M/sorafenib were better than those observed with CLAG-M in the historical, matched control cohort. Although interaction analyses between treatment and disease risk were not possible because of the relatively small cohort size, univariate analyses indicated that this benefit of CLAG-M/sorafenib was most pronounced among patients with ELN2017 intermediate-risk disease. Given the potential difference in the transplantation rate between cohorts, we also built additional multivariable models for EFS and OS in which we included transplantation as a time-dependent covariate. These models support the conclusion that the improved outcomes observed with CLAG-M/sorafenib vs CLAG-M are at least partly due to the benefit provided by sorafenib and are not entirely explained by the increased frequency of allografting in the CLAG-M/sorafenib cohort.
With the addition of drugs to intensive multidrug regimens, the risk of excess toxicity must be weighed against the expectation of benefits. In the SORAML trial comparing 7 + 3 alone to 7 + 3 with sorafenib, higher rates of fever, bleeding, and hand-foot syndrome were observed in the sorafenib arm.4 In contrast, in our study, we did not observe grade 3 or higher hand-foot syndrome (although generalized papular rash was common, documented in about one-third of the patients). Furthermore, we did not observe any grade 3 or higher bleeding events. Moreover, although the rates of neutropenic fever with CLAG-M/sorafenib were high, they were comparable with the published rates of neutropenic fever with CLAG-M alone (78% vs 71% respectively), and we did not observe an increased rate of early death with the addition of sorafenib.7 The major excess toxicities we observed that were attributed to sorafenib (specifically maculopapular rash and hypertension) were manageable with supportive care and not life-threatening. However, as a reflection of the added myelosuppression, the times to ANC and platelet count recovery were slightly and statistically significantly longer with CLAG-M/sorafenib than with CLAG-M alone.
Our data indicate that the combination of CLAG-M and sorafenib has very high antileukemic efficacy, with an MRD– CR rate of 83%. This is clinically relevant because deep remissions, which are remissions without MRD associated with a negative MRD result, have repeatedly been associated with a decreased risk of relapse and prolonged survival in both the nontransplantation and transplantation settings.22,23 To better understand whether sorafenib provided additional antileukemic efficacy beyond that of CLAG-M alone, we compared patients treated with CLAG-M/sorafenib with a historical control cohort of matched patients who received only CLAG-M. Although the proportion of patients achieving MRD– CR was not statistically significantly different in multivariable analysis, patients treated with CLAG-M/sorafenib experienced longer EFS and OS than those treated with CLAG-M, with no apparent difference in this benefit between the patient subsets with FLT3-mutated and FLT3-wild-type disease. Importantly, our data indicated that the benefit provided by sorafenib was not uniform across the cytogenetic/molecular disease spectrum. Improved outcomes with CLAG-M/sorafenib compared with CLAG-M alone were most marked in patients with intermediate-risk disease. Notably, this benefit was not explained by patients with FLT3-mutated disease, as such patients were not enriched in the current study. Thus, our data suggest that the benefit of sorafenib may, at least partly, be related to its effects beyond its inhibition of mutant FLT3 signaling. This may be of clinical relevance because half of the patients with AML do not harbor mutations for which there is a Food and Drug Administration–approved, molecularly targeted drug available at this time, constituting a subset of patients for which a drug such as sorafenib may be of particular interest.24
Three previous trials have evaluated the addition of sorafenib to standard intensive AML therapies, irrespective of the FLT3 mutation status. One group combined sorafenib with high-dose cytarabine and idarubicin in patients aged <65 years with newly diagnosed AML and observed a CR rate of 79% and an early death rate of 2%.25 In the SORAML trial,4 276 adults (17% FLT3 mutated) age <60 years were randomized to 7 + 3 with placebo or sorafenib at 400 mg twice daily. The CR rates were similar: 60% in the sorafenib group and 59% in the placebo group. The median EFS at 3 years was longer in the sorafenib group (40% vs 22%; P = .01), although no difference was observed in the OS. Similar to our study, this improved outcome was not driven by patients with FLT3 mutation. Comparably, acknowledging nonmatched patient populations, our CR rate (irrespective of MRD) was higher, at 88%, than that of 7 + 3 + sorafenib, and we observed both an EFS and OS benefit in our study cohort compared with a matched historical control cohort. In addition, we observed similarly low early death and acceptable toxicity rates. In contrast, Serve et al randomly assigned patients >60 years of age to 7 + 3 with placebo vs sorafenib and found nonsignificantly lower CR rates and higher early death rates in the sorafenib arm vs placebo (17% vs 7%; P = .05).26 Given the high toxicity rates noted in older patients, we excluded patients aged >60 years from our study and thus have less data regarding the use of nonselected tyrosine kinase inhibitors in this population.
We acknowledge several limitations. Firstly, our trial was limited by its single-arm design without the inclusion of a randomized control group. To address this, we compared the observed outcomes to a matched historical control treated with CLAG-M alone and used multivariable modeling to control for confounding variables. Secondly, this phase 1/2 study was conducted as a single-institution trial. In this setting, we did not observe any increase in early mortality with CLAG-M/sorafenib relative to that observed historically with CLAG-M alone, despite the longer duration of severe neutropenia and thrombocytopenia with CLAG-M/sorafenib. Whether our trial findings can be immediately extrapolated to a multicenter setting is unknown. Thirdly, our subgroup analysis of the benefit of CLAG-M/sorafenib in the favorable-risk subgroup was limited by the small sample size of this group, partially related to a concurrent trial at our institution evaluating CLAG-M with gemutuzumab ozogamicin, which treating physicians preferred for patients with favorable risk disease. Finally, although CLAG-M is an established regimen for the treatment of relapsed/refractory AML, only a limited number of institutions use CLAG-M in the upfront setting, limiting the overall impact that our findings may have in the field of AML.
In summary, our findings build on previous data from the SORAML study, supporting the conclusion that the addition of sorafenib to intensive AML chemotherapy, including high-dose cytarabine-based regimens, improves outcomes compared with conventional multiagent induction therapy alone, at least in selected patient subsets. In particular, for patients with intermediate-risk disease for whom no other targeted agents are available, the inclusion of sorafenib during induction and postremission therapy may be an appealing treatment option. However, sorafenib is currently not approved for treating AML, and further development of this drug for clinical application may focus on malignancies other than AML. Furthermore, recognizing the availability of many other approved and investigational small-molecule inhibitors, including other tyrosine kinase inhibitors, a randomized trial testing sorafenib for intermediate-risk AML may not be feasible or desirable. Acknowledging this uncertainty, the data from this phase 1/2 trial provide justification for a randomized, possibly multicenter trial testing the addition of sorafenib to a high-dose cytarabine-containing chemotherapy regimen in adults with intermediate-risk AML.
Acknowledgments
The CLAG-M/sorafenib trial was supported by Bayer Pharmaceuticals Inc. A.B.H. was supported by an ASCO Young Investigator award.
Authorship
Contribution: A.B.H. and R.B.W. designed and performed the research, analyzed the data, and wrote the manuscript; E.R.-A. performed research and contributed to the manuscript; M.O. designed the research, analyzed the data, and contributed to the manuscript; K.-L.A.G. performed research and contributed to the data; M.-E.M.P., R.D.C., V.G.O., P.S.B., J.S.A., J.L.A., J.J.O., S.B.K., P.C.H., B.L.S., and M.C.G. performed research and contributed to the manuscript; and E.H.E. designed and performed the research.
Conflict-of-interest disclosure: A.B.H. receives research funding from Imago BioSciences, Bayer Pharmaceuticals, Gilead Sciences, Jazz Pharmaceuticals, Incyte Pharmaceuticals, Karyopharm Therapeutics, and Disc Medicine, and has consulted for AbbVie Pharmaceuticals and Notable Labs. E.R.-A. reports travel and conference funding from Jazz Pharmaceuticals, AbbVie, and Gilead. K.-L.A.G. is an employee at WIRB-Copernicus Group. M.-E.M.P. reports research funding from AbbVie, Ascentage, Astex, Biosight, Bristol Myers Squibb (BMS)/Celgene, Cardiff Ongolocy, Glycomimetics, Pfizer, Telios, and Trillium. R.D.C. reports research funding from Amgen, Kite/Gilead, Merck, Pfizer, Servier, and Vanda Pharmaceuticals; receives honoraria/consulting from Amgen, Jazz, Kite/Gilead, and Pfizer; is a member of the data and safety monitoring board for Pepromene Bio; serves on the independent response review committee for Autolus; and his spouse has been employed by and owns stock in Seagen. V.G.O. reports research funding from Pfizer Inc., and consults for Pfizer and Novartis. P.S.B. reports research support GlycoMimetics, Pfizer, Notable Labs, and GPCR, and is a medical adviser for Accordant Health Services/CVS Caremark. J.J.O. receives research funding from Actinium Pharmaceuticals Inc. S.B.K. provides consultancy for Disc Medicine. R.B.W. received laboratory research grants and/or clinical trial support from Amgen, Aptevo, Celgene, ImmunoGen, Janssen, Jazz, Kura, MacroGenics, and Pfizer; has ownership interests in Amphivena; and is (or has been) a consultant to AbbVie, Adicet, Amphivena, BerGenBio, BMS, GlaxoSmithKline, ImmunoGen, Kura, and Orum. None of these disclosures are related to the drugs described in the manuscript (except for the Bayer funding of this research study). The remaining authors declare no competing financial interests.
The current affiliation for P.S.B. is Division of Leukemia, Department of Hematology & Hematopoietic Cell Transplantation, City of Hope, Duarte, CA.
Elihu H. Estey died on 8 October 2021.
Correspondence: Anna B. Halpern, Division of Hematology, Department of Medicine, University of Washington, 825 Eastlake Ave E, Box LG-700 Seattle, WA 98109; e-mail: halpern2@uw.edu.
References
Author notes
The protocol is available on request from corresponding author, Anna B. Halpern (halpern2@uw.edu).