TO THE EDITOR:
In multiple myeloma (MM), plasmacytoid dendritic cells (pDCs) interact with tumor cells to promote their growth and contribute to the immunosuppressive microenvironment.1-3 However, the mechanisms of this interaction are not fully understood. Here, we performed single-cell RNA sequencing on bone marrow (BM) cells from relapsed/refractory patients with MM and healthy donors (n = 3 each; supplemental Figure 1A) and found a striking perturbation in the sialylation-recognition pathway in pDCs (Figure 1A).
MM tumor cells and MM-pDCs in the bone marrow microenvironment exhibit hypersialylation. (A) Hypersialylation in MM tumor cells results in strong interactions with Siglecs on immune cells in the microenvironment. (B) NESs that were significantly different between the MM-pDCs and normal pDCs based on GSEA MSigDB and WebGestalt analysis (false discovery rate <0.05; NES, ±1.5 as the cutoff). GSEA identified several downregulated (red) and upregulated (green) pathways. (C) Upregulation of SIGLEC6/CD327 in MM-pDCs (FC, 2.439; MM-pDC vs normal pDC; n = 3 each; adjusted P = .009283). (D) Total BM-MNCs were isolated from the BM of patients with relapsed/refractory MM and healthy donors (n = 3 each). pDCs were separated using CD304 magnetic beads on a Miltenyi autoMacs Neo machine and collected. MM.1S cells and BPDCN Cal-1 cell lines were used as controls. RNA was isolated using Qiagen RNeasy Mini kit. Poly RNA was subjected to Reverse Transcription Quantitative PCR (RT-qPCR) using Luna Universal 1-step RT-qPCR kit (New England Biolabs, Ipswich, MA) on an Applied Biosystems 7500 Fast Real-Time PCR System (Thermo Fisher Scientific Inc). SIGLEC6/CD327 gene expression was quantified from raw data using ΔΔCT method, using Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) as the reference. The bar graph denotes the relative expression of SIGLEC6 in different samples (normal, pDCs from normal healthy donor; MM-pDCs, pDCs from patients with relapsed/refractory with MM; Cal-1, BPDCN pDC cell line; MM.1S, dexamethasone-sensitive MM cell line; mean ± standard deviation [SD]; P < .05; all points done in triplicate). (E) CD303+/CD123+ MM-pDCs from MM BM-MNCs were examined by flow cytometry, and MFI of SIGLEC6/CD327 expression was determined using anti-Siglec-6 antibody (Ab) conjugated to Alexa Fluor 647. Panel shows representative histograms for SIGLEC6+ MM-pDCs (red), normal pDCs (black), and isotype control Ab conjugated to Alexa Fluor 647 (blue). (F-G) FC in MFI of biotinylated lectins from Sambucus nigra (SNA) and Maackia amurensis (MAL-II) bound to pDC (F) and MM.1R (G) cell surface. Fluorescence signal was detected by flow using secondary streptavidin conjugated to PE. The cells were first treated with biotinylated lectins (5 μg/mL; Vector Laboratories) for 45 to 60 minutes and washed, followed by staining with streptavidin-PE secondary (0.8-1.0 μg/mL) for 30 minutes. All samples were preblocked with Tru Fc blocker (BioLegend). 7-Aminoactinomycin D (7-AAD) dye was used to exclude dead cells, and signal was measured only on live cells. The data are presented as FC of biotinylated lectin–streptavidin-PE signal with respect to the fluorescence signal from cells treated with only secondary streptavidin-PE (mean ± SD; P < .05 for conjugated vs second signal). ER, Endoplasmic Reticulum; MFI, median fluorescence intensity; mRNA, messenger RNA; NES, normalized enrichment score; TLR10, Toll-like receptor 10.
MM tumor cells and MM-pDCs in the bone marrow microenvironment exhibit hypersialylation. (A) Hypersialylation in MM tumor cells results in strong interactions with Siglecs on immune cells in the microenvironment. (B) NESs that were significantly different between the MM-pDCs and normal pDCs based on GSEA MSigDB and WebGestalt analysis (false discovery rate <0.05; NES, ±1.5 as the cutoff). GSEA identified several downregulated (red) and upregulated (green) pathways. (C) Upregulation of SIGLEC6/CD327 in MM-pDCs (FC, 2.439; MM-pDC vs normal pDC; n = 3 each; adjusted P = .009283). (D) Total BM-MNCs were isolated from the BM of patients with relapsed/refractory MM and healthy donors (n = 3 each). pDCs were separated using CD304 magnetic beads on a Miltenyi autoMacs Neo machine and collected. MM.1S cells and BPDCN Cal-1 cell lines were used as controls. RNA was isolated using Qiagen RNeasy Mini kit. Poly RNA was subjected to Reverse Transcription Quantitative PCR (RT-qPCR) using Luna Universal 1-step RT-qPCR kit (New England Biolabs, Ipswich, MA) on an Applied Biosystems 7500 Fast Real-Time PCR System (Thermo Fisher Scientific Inc). SIGLEC6/CD327 gene expression was quantified from raw data using ΔΔCT method, using Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) as the reference. The bar graph denotes the relative expression of SIGLEC6 in different samples (normal, pDCs from normal healthy donor; MM-pDCs, pDCs from patients with relapsed/refractory with MM; Cal-1, BPDCN pDC cell line; MM.1S, dexamethasone-sensitive MM cell line; mean ± standard deviation [SD]; P < .05; all points done in triplicate). (E) CD303+/CD123+ MM-pDCs from MM BM-MNCs were examined by flow cytometry, and MFI of SIGLEC6/CD327 expression was determined using anti-Siglec-6 antibody (Ab) conjugated to Alexa Fluor 647. Panel shows representative histograms for SIGLEC6+ MM-pDCs (red), normal pDCs (black), and isotype control Ab conjugated to Alexa Fluor 647 (blue). (F-G) FC in MFI of biotinylated lectins from Sambucus nigra (SNA) and Maackia amurensis (MAL-II) bound to pDC (F) and MM.1R (G) cell surface. Fluorescence signal was detected by flow using secondary streptavidin conjugated to PE. The cells were first treated with biotinylated lectins (5 μg/mL; Vector Laboratories) for 45 to 60 minutes and washed, followed by staining with streptavidin-PE secondary (0.8-1.0 μg/mL) for 30 minutes. All samples were preblocked with Tru Fc blocker (BioLegend). 7-Aminoactinomycin D (7-AAD) dye was used to exclude dead cells, and signal was measured only on live cells. The data are presented as FC of biotinylated lectin–streptavidin-PE signal with respect to the fluorescence signal from cells treated with only secondary streptavidin-PE (mean ± SD; P < .05 for conjugated vs second signal). ER, Endoplasmic Reticulum; MFI, median fluorescence intensity; mRNA, messenger RNA; NES, normalized enrichment score; TLR10, Toll-like receptor 10.
We compared transcriptional changes in MM-pDCs vs normal pDCs and found 10 219 upregulated and 781 downregulated genes (adjusted P < .05). In contrast, only 290 and 384 genes were upregulated and downregulated (adjusted P < .05), respectively, in myeloid DCs, further highlighting the critical role of pDCs in MM pathogenesis (supplemental Figure 1B). In agreement with our earlier studies,1,4 these genes clustered into sets, with MM-pDCs downregulating “Interferon Type I Signaling,” “Toll-like Receptor Cascades,” and “GO_MP Basal_transcription_machinery_binding” gene sets, according to Gene Set Enrichment Analysis (GSEA) MSigDB and WebGestalt (Figure 1B).5-7 The latter set contains TCF4/E2-2, a transcription factor necessary for the development of normal pDCs,8-10which is downregulated in MM-pDCs (supplemental Figure 1C).
Regarding upregulated gene sets, we replicated an association to “Reactome Class A 1 Rhodopsin-like Receptors,” in which ADORA2A/2B was previously reported to be upregulated in MM-pDCs and tumor cells.11 Importantly, “SIGLEC2/CD22-mediated BCR regulation” (normalized enrichment score, 1.81; false discovery rate, <0.05) and “Immunoregulatory Interactions between a Lymphoid and a Non-Lymphoid cells” (normalized enrichment score, 1.6404; false discovery rate (FDR), <0.05) were upregulated, indicating a perturbed sialylation-recognition pathway.
Sialylation is the addition of sialic acid to glycoproteins and glycolipids embedded in the cell membrane.12,13 These modifications are recognized by their cognate Siglec (sialic acid–binding immunoglobulin-like lectins) receptors on immune cells, and this interaction suppresses immune cell activation, similar to Programmed death-ligand 1/Programmed cell death protein 1 (PD-L1/PD-1)12-21 (Figure 1A). Hypersialylation occurs in all cancer cells, including MM cells, and promotes drug resistance and immunosuppression.12-13 Specifically, MM tumor cells exhibit high sialyltransferase activity,13,15 which affects the homing of tumor cells into the BM.15-21 The immune cells in patients with MM express various Siglec receptors.14,12,22 Concordantly, we found several SIGLEC genes and several α-2,3- and α-2,6-sialyltransferases were upregulated in the MM BM (supplemental Figure 1D), of which ST3GAL6 was significantly overexpressed in MM-pDCs (log2 fold change [log2FC], 3.124 vs normal pDCs; adjusted P = .0009; supplemental Figure 2). This was replicated in a cohort of 360 patients with MM and 18 normal plasma cells (adjusted P < 2e-16; supplemental Figure 3) in data analyzed using the oncogenomics web portal MMreporter.23
Of note, SIGLEC6/CD327 was highly expressed in MM-pDCs (log2FC, 1.291 vs normal pDCs; adjusted P =.0001; Figure 1C), mirroring its expression in acute myeloid leukemia.24 This was validated by quantitative polymerase chain reaction using the blastic pDC neoplasm cell line Cal-1 as a positive control (n = 3 each; Figure 1D) and by flow cytometry, which found that MM-pDCs exhibited a significant increase in SIGLEC6/CD327 on their cell surface (median fluorescence intensity >1.5-fold vs normal; P < .05; Figure 1E) compared to normal pDCs. Conversely, SIGLEC6/CD327 expression on myeloma cell lines was insignificant (supplemental Figure 4A), yet the high-affinity ligand for SIGLEC6 (sialyl-Tn)25 was highly expressed on dexamethasone-resistant MM.1R cells and MM-pDCs (supplemental Figure 4B). Of note, based on our single-cell RNA sequencing results, SIGLEC6 was not significantly expressed in any other immune cells besides DCs.
Hypersialylation results in excessive accumulation of α-2,3– and α-2,6–linked sialic acids on cell surfaces, which function as ligands for preferential lectin binding. To assess sialic acid accumulation, we exposed pDCs and MM cells (MM.1R) to a biotinylated lectin that binds α-2,6–linked sialic acid (Sambucus nigra [SNAI]) and one that binds α-2,3–linked sialic acid (Maackia amurensis [MAL-II]), using other non–sialic acid–binding lectins as controls.26 The cells were treated with the lectins for 1 hour, stained with streptavidin-conjugated Phycoerythrin (PE), and assessed by flow cytometry. Both pDCs and MM.1R cells had very high expression of both sialic acids, with pDCs having similar levels of both sialic acids and MM cells having less α-2,3–linked sialic acid (Figure 1F-G).
Recent studies have demonstrated the efficacy of Siglec-6 as a therapeutic target in acute myeloid leukemia and chronic lymphocytic leukemia.24,27 Therefore, we hypothesized that Siglec receptors/sialic acid checkpoints could be targeted to reverse MM-pDC–mediated immunosuppression.11,28 We used our autologous coculture models2-4,29,30 of MM and BM cells to examine the following: (1) whether targeting the hypersialylation pathway with sialyltransferase inhibitors can improve anti-MM immunity; and (2) whether similar anti-MM immunity can be achieved by specifically targeting Siglec-6.
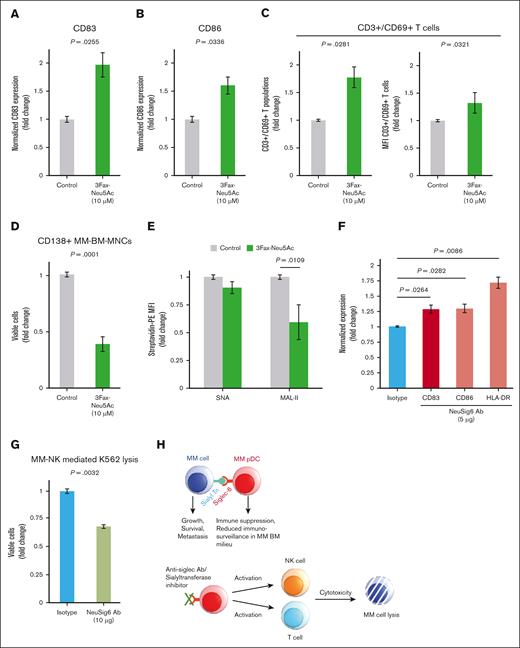
First, we used the sialyltransferase inhibitor 3Fax-Peracetyl Neu5Ac (3Fax-Neu5Ac). Treating total MM bone marrow-mononuclear cells (BM-MNCs) with 3Fax-Neu5Ac at different concentrations for 3 days triggered the activation of MM-pDCs, as indicated by the upregulation of CD83/CD86 on MM-pDCs (1.5-fold ± standard deviation; treated vs untreated, P < .05; Figure 2A-B). Subsequently, autologous CD3+ T cells were activated, as indicated by the upregulation of CD69 on CD3+ T cells and an increased population of CD3+/CD69+ T cells (percent T-cell proliferation, 1.77-fold; percent median fluorescence intensity, 1.328-fold; treated vs untreated, P < .05; Figure 2C). Treatment also decreased the population of viable autologous tumor cells (CD138+; viability, 38.76% [treated vs untreated]; n = 3; P < .05; Figure 2D), suggesting that sialyltransferase inhibition triggers an anti-MM immune response. Of note, 3Fax-Neu5Ac has no direct cytotoxic effect on MM cells and BM stromal cells at this concentration.
Blocking Siglec-6–sialyltransferase axis activates pDCs and CD3+ T cells. MM BM-MNCs were treated with the sialyltransferase inhibitor 3Fax-Neu5Ac for 24 hours, followed by the flow analysis of activation markers CD83 (A) and CD86 (B) on MM-pDCs (mean ± SD; FC, treated vs untreated; P < .05). (C) MM BM-MNCs were treated with the sialyltransferase inhibitor 3Fax-Neu5Ac for 48 hours, followed by the flow analysis of the activation marker CD69 on CD3+ T cells. Bar plots show the upregulation of CD3+/CD69+ T-cell populations (left) and change in MFI of CD69 on CD3+ T cells (right; mean ± SD; FC, treated vs untreated; P < .05). (D) MM BM-MNCs were treated with the sialyltransferase inhibitor 3Fax-Neu5Ac for 48 hours, followed by the flow analysis of autologous CD138+ tumor cells. After treatment, the cells were washed and stained with anti-CD138 conjugated to Fluorescein isothiocyanate (FITC), followed by staining with 7-AAD. Viable cells were gated out as 7-AAD–, and the viability of autologous CD138+ tumor cells was analyzed in the presence and absence of the inhibitor (mean ± SD; FC, treated vs untreated; P < .05). (E) FC in the MFI of biotinylated lectins SNA and MAL-II bound to pDC cell surface in the presence and absence of 3Fax-Neu5Ac. Fluorescence signal was detected by flow using secondary streptavidin conjugated to PE. The cells were cultured in the presence of increasing inhibitor concentrations (1-125 μM) for 48 hours. After this, the cells were washed and first treated with biotinylated lectins (5 μg/mL; Vector Laboratories) for 45 to 60 minutes. The cells were then washed, followed by staining with streptavidin-PE secondary (0.8-1.0 μg/mL) for 30 minutes. All samples were preblocked with Tru Fc blocker (BioLegend). 7-AAD dye was used to exclude dead cells, and signal was measured only on live cells. The data are presented as FC in biotinylated lectin–streptavidin-PE signal in the presence vs absence of the inhibitor (mean ± SD; P = .0109 for MAL-II). (F) MM BM-MNCs were treated with Siglec-6–neutralizing Ab (5 μg/mL) or appropriate isotype Ab control for 48 hours, followed by the flow analysis of activation markers CD83, CD86, and HLA-DR on MM-pDCs in the presence or absence of Siglec-6–neutralizing Ab (mean ± SD; FC, treated vs untreated; P <.05). (G) Total BM-MNCs from patients with MM were treated with Siglec-6–neutralizing Ab or isotype control (10 μg/mL) for 48 hours, then washed and resuspended, followed by the addition of CellTrace Violet–stained K562 cells. Cells were incubated for 1 day before multicolor flow analysis to assess K562 cell lysis. The bar plot shows the quantification of flow cytometry data (mean ± SD; treated vs untreated). (H) Siglec–sialic acid immune checkpoint axis-mediated immune dysfunction in myeloma (upper); and the therapeutic potential of Siglec–sialic acid immune checkpoint axis as an immunometabolic target (lower).
Blocking Siglec-6–sialyltransferase axis activates pDCs and CD3+ T cells. MM BM-MNCs were treated with the sialyltransferase inhibitor 3Fax-Neu5Ac for 24 hours, followed by the flow analysis of activation markers CD83 (A) and CD86 (B) on MM-pDCs (mean ± SD; FC, treated vs untreated; P < .05). (C) MM BM-MNCs were treated with the sialyltransferase inhibitor 3Fax-Neu5Ac for 48 hours, followed by the flow analysis of the activation marker CD69 on CD3+ T cells. Bar plots show the upregulation of CD3+/CD69+ T-cell populations (left) and change in MFI of CD69 on CD3+ T cells (right; mean ± SD; FC, treated vs untreated; P < .05). (D) MM BM-MNCs were treated with the sialyltransferase inhibitor 3Fax-Neu5Ac for 48 hours, followed by the flow analysis of autologous CD138+ tumor cells. After treatment, the cells were washed and stained with anti-CD138 conjugated to Fluorescein isothiocyanate (FITC), followed by staining with 7-AAD. Viable cells were gated out as 7-AAD–, and the viability of autologous CD138+ tumor cells was analyzed in the presence and absence of the inhibitor (mean ± SD; FC, treated vs untreated; P < .05). (E) FC in the MFI of biotinylated lectins SNA and MAL-II bound to pDC cell surface in the presence and absence of 3Fax-Neu5Ac. Fluorescence signal was detected by flow using secondary streptavidin conjugated to PE. The cells were cultured in the presence of increasing inhibitor concentrations (1-125 μM) for 48 hours. After this, the cells were washed and first treated with biotinylated lectins (5 μg/mL; Vector Laboratories) for 45 to 60 minutes. The cells were then washed, followed by staining with streptavidin-PE secondary (0.8-1.0 μg/mL) for 30 minutes. All samples were preblocked with Tru Fc blocker (BioLegend). 7-AAD dye was used to exclude dead cells, and signal was measured only on live cells. The data are presented as FC in biotinylated lectin–streptavidin-PE signal in the presence vs absence of the inhibitor (mean ± SD; P = .0109 for MAL-II). (F) MM BM-MNCs were treated with Siglec-6–neutralizing Ab (5 μg/mL) or appropriate isotype Ab control for 48 hours, followed by the flow analysis of activation markers CD83, CD86, and HLA-DR on MM-pDCs in the presence or absence of Siglec-6–neutralizing Ab (mean ± SD; FC, treated vs untreated; P <.05). (G) Total BM-MNCs from patients with MM were treated with Siglec-6–neutralizing Ab or isotype control (10 μg/mL) for 48 hours, then washed and resuspended, followed by the addition of CellTrace Violet–stained K562 cells. Cells were incubated for 1 day before multicolor flow analysis to assess K562 cell lysis. The bar plot shows the quantification of flow cytometry data (mean ± SD; treated vs untreated). (H) Siglec–sialic acid immune checkpoint axis-mediated immune dysfunction in myeloma (upper); and the therapeutic potential of Siglec–sialic acid immune checkpoint axis as an immunometabolic target (lower).
We next investigated whether 3Fax-Neu5Ac reduces cell surface accumulation of α-2,6– and α-2,3–linked sialic acids. MM BM-MNCs were treated with 3Fax-Neu5Ac for 48 hours, followed by staining with biotinylated SNA and MAL-II. We found that MAL-II binding (α-2,3–linked) was more inhibited in pDCs and total MM BM-MNCs (Figure 2E; supplemental Figure 5A) than SNA binding (α-2,6–linked), and these cells were more affected than MM cells. However, 3Fax-Neu5Ac can exhibit on-target off-tumor activities,26,31,32 which can potentially be overcome by directly targeting Siglec receptors.
We investigated whether directly targeting SIGLEC6 inhibits sialyltransferase activity in BM-MNCs. Treatment of MMBM-MNCs with anti–Siglec-6 antibody (5.0 μg/mL) upregulated CD83, CD86, and HLA-DR on MM-pDCs, indicating activation (1.29-fold to 1.71-fold; P < .05; Figure 2F). CD83 is an important activation marker of pDCs,1,4 and it is generally downregulated in MM-pDCs (log2FC, –2.42 vs normal pDCs; adjusted P = .0024; supplemental Figure 5B). We confirmed its upregulation after Small interfering RNA (siRNA)-mediated blocking of SIGLEC6 (∼1.75-fold vs control scrambled (scr) siRNA (scr-siRNA); P < .05; supplemental Figure 5C).
Hypersialylation and Siglec hyperexpression downregulate natural killer (NK) cell effector functions by suppressing their antitumor responses.22 Therefore, we investigated whether Siglec-6 blockade can restore NK cell–mediated anti-MM immunity. MM BM-MNCs were treated with anti–Siglec-6 antibody for 3 to 4 days before prestained K562 cells (chronic myelogenous leukemia) were added. Anti–Siglec-6 triggered NK cell–mediated lysis of the target K562 cells (viability, 60% [treated vs untreated]; n = 3; P < .05; Figure 2G-H). K562 cells have weak expression of major histocompatibility complex (MHC) class I but high expression of ligands for NK group 2 and natural cytotoxicity receptors. Thus, K562 is extensively used as a target for NK cell–mediated lysis.3,22,33 We conclude that anti-MM activity induced by Siglec/sialic acid blockade could partly be mediated by the disruption of pDC-NK interactions.3,22,33 Of note, at this concentration, the antibody did not affect the viability of K562 or NK cells.
In summary, we found that both MM-pDCs and tumor cells are hypersialylated, and reversing this with sialyltransferase inhibitor activates MM-pDCs, triggers autologous T-cell proliferation, and induces autologous tumor cell killing. In addition, SIGLEC6 activates MM-pDCs in a manner similar to that of the sialyltransferase inhibitor (Figure 2H). Our preclinical study suggests that therapeutic intervention in the Siglec/sialic acid axis in MM can help restore antimyeloma immunity.
Samples from patients with MM were collected at the Dana-Farber Cancer Institute (approved by institutional review board according to the Helsinki protocol).
Acknowledgments: This work was supported by the National Institutes of Health Specialized Programs of Research Excellence grant P50-100707 and P01 CA155258 grant was from National Institutes of Health/National Cancer Institute (NIH/NCI); as well as by the Adelson Medical Research Foundation and the Paula and Rodger Riney Foundation.
Contribution: A.R. designed the research strategy, performed experiments, analyzed data, and wrote the manuscript; L.B. helped with the bioinformatics analysis; X.W. helped with the quantitative polymerase chain reaction experiments; T.D., Y.S., S.C.P., M.A.M., T.F., and M.W. helped with the flow cytometry experiments; and K.C.A. provided clinical samples, reviewed data, and wrote the manuscript.
Conflict-of-interest disclosure: K.C.A. is an adviser for Janssen, Pfizer, and AstraZeneca; and has ownership interests in C4 Therapeutics, Dynamic Cell Therapies, Window Therapeutics, Starton Therapeutics, NextRNA, and OncoPep. The remaining authors declare no competing financial interests.
Correspondence: Kenneth C. Anderson, Department of Medical Oncology Dana-Farber Cancer Institute, Smith Building, Floor 6, 450 Brookline Ave, Boston, MA 02215; email: kenneth_anderson@dfci.harvard.edu; and Arghya Ray, Department of Medical Oncology Dana-Farber Cancer Institute, Smith Building, Floor 6, Room# SM608 450 Brookline Ave, Boston, MA 02215; email: arghya_ray@dfci.harvard.edu.
References
Author notes
Data are not available without request and institutional review board review due to patient confidentiality.
The full-text version of this article contains a data supplement.

![MM tumor cells and MM-pDCs in the bone marrow microenvironment exhibit hypersialylation. (A) Hypersialylation in MM tumor cells results in strong interactions with Siglecs on immune cells in the microenvironment. (B) NESs that were significantly different between the MM-pDCs and normal pDCs based on GSEA MSigDB and WebGestalt analysis (false discovery rate <0.05; NES, ±1.5 as the cutoff). GSEA identified several downregulated (red) and upregulated (green) pathways. (C) Upregulation of SIGLEC6/CD327 in MM-pDCs (FC, 2.439; MM-pDC vs normal pDC; n = 3 each; adjusted P = .009283). (D) Total BM-MNCs were isolated from the BM of patients with relapsed/refractory MM and healthy donors (n = 3 each). pDCs were separated using CD304 magnetic beads on a Miltenyi autoMacs Neo machine and collected. MM.1S cells and BPDCN Cal-1 cell lines were used as controls. RNA was isolated using Qiagen RNeasy Mini kit. Poly RNA was subjected to Reverse Transcription Quantitative PCR (RT-qPCR) using Luna Universal 1-step RT-qPCR kit (New England Biolabs, Ipswich, MA) on an Applied Biosystems 7500 Fast Real-Time PCR System (Thermo Fisher Scientific Inc). SIGLEC6/CD327 gene expression was quantified from raw data using ΔΔCT method, using Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) as the reference. The bar graph denotes the relative expression of SIGLEC6 in different samples (normal, pDCs from normal healthy donor; MM-pDCs, pDCs from patients with relapsed/refractory with MM; Cal-1, BPDCN pDC cell line; MM.1S, dexamethasone-sensitive MM cell line; mean ± standard deviation [SD]; P < .05; all points done in triplicate). (E) CD303+/CD123+ MM-pDCs from MM BM-MNCs were examined by flow cytometry, and MFI of SIGLEC6/CD327 expression was determined using anti-Siglec-6 antibody (Ab) conjugated to Alexa Fluor 647. Panel shows representative histograms for SIGLEC6+ MM-pDCs (red), normal pDCs (black), and isotype control Ab conjugated to Alexa Fluor 647 (blue). (F-G) FC in MFI of biotinylated lectins from Sambucus nigra (SNA) and Maackia amurensis (MAL-II) bound to pDC (F) and MM.1R (G) cell surface. Fluorescence signal was detected by flow using secondary streptavidin conjugated to PE. The cells were first treated with biotinylated lectins (5 μg/mL; Vector Laboratories) for 45 to 60 minutes and washed, followed by staining with streptavidin-PE secondary (0.8-1.0 μg/mL) for 30 minutes. All samples were preblocked with Tru Fc blocker (BioLegend). 7-Aminoactinomycin D (7-AAD) dye was used to exclude dead cells, and signal was measured only on live cells. The data are presented as FC of biotinylated lectin–streptavidin-PE signal with respect to the fluorescence signal from cells treated with only secondary streptavidin-PE (mean ± SD; P < .05 for conjugated vs second signal). ER, Endoplasmic Reticulum; MFI, median fluorescence intensity; mRNA, messenger RNA; NES, normalized enrichment score; TLR10, Toll-like receptor 10.](https://ash.silverchair-cdn.com/ash/content_public/journal/bloodadvances/9/19/10.1182_bloodadvances.2025015980/2/m_blooda_adv-2025-015980-gr1.jpeg?Expires=1765888322&Signature=4JeAbuBYD0SIdBU9UjFOfJNmjTgfuuom4N0LRYxSdNpFl54Z0Z4BZlDqXcy6RdF1~FBgZgBSYhZ1wI2SP9fDUybgUsVfxmdWZ~WfCTzRHcH6LrsfRad6UOdkflszrSKrOOaKEMRZp0bR-rrz8~axwr4bGbFzCGcb23ty0Oqctv18hKBjq-5J1Z-VOC1YXN-TW34EU6sY13SvlvboQkuJ9SCB2HeqS7-dlpAjr-uNHmYaudO5qCTJPU2P2ctbTpEzXUp3kKECprmjOJ3hG0WrHRmQHUOWUCW1MbiDYCgnxrl~OoIewmdxaVB4cjXHO4XHvSDhuzMhmj3L-umvMRN2Qw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)