TO THE EDITOR:
Non-immunoglobulin M (IgM) monoclonal gammopathy of undetermined significance (MGUS) is an asymptomatic precursor to multiple myeloma (MM) and related disorders.1 It is defined by <30 g/L monoclonal (M) protein, <10% bone marrow (BM) plasma cells, and no end-organ damage related to clonal plasma cells.2 MGUS affects ∼3% to 5% of adults over the age of 50,3,4 and progresses to malignancy at ∼1% annually.1 Smoldering MM (SMM) is an intermediate stage, defined by ≥10% BM plasma cells or ≥30 g/L M-protein, with a ∼10% yearly progression risk in the first 5 years.2 The risk of progression in MGUS is influenced by M-protein isotype and concentration, and free light-chain (FLC) ratio.5 IgA MGUS carries a higher risk of progression than IgG MGUS,1,5 yet the biological basis for this isotype-related difference remains unclear.
Multiparameter flow cytometry (MFC) enables a cellular-level assessment of plasma cell clonality by detecting aberrant immunophenotypes characteristic of both premalignant and malignant plasma cell disorders, providing diagnostic and prognostic value.6,7 Next-generation flow cytometry (NGF) builds upon MFC, offering standardized protocols and improved sensitivity.8 Although NGF is primarily used for measurable residual disease assessment in MM, its ability to provide sensitive, cell-based evaluation of plasma cell clonality may enhance risk stratification in early-stage or lower-risk MGUS.
We hypothesized that differences between IgA and IgG MGUS may be reflected in the presence of clonal plasma cells. We therefore, evaluated NGF-based detection of clonal plasma cells in BM in relation to isotype and clinical outcomes, to explore its utility in the diagnostic work-up of MGUS.
The study was conducted within the Iceland Screens, Treats, or Prevents Multiple Myeloma (iStopMM) trial, a population-based screening study for MGUS (ClinicalTrials.gov identifier: NCT03327597).9 Between 2016 to 2020, 75 422 adults in Iceland were screened by serum protein electrophoresis (SPEP) and FLC assay. Individuals with positive screening were randomized to observation, guideline-based follow-up, or intensive follow-up (arm 3), which included baseline BM sampling and annual monitoring.9 We included 122 participants in arm 3 with IgA and/or IgG M-protein who underwent NGF analysis at baseline (median 7 months after screening). Participants were selected by convenience sampling, enriched for IgA to offset IgG predominance. Individuals with multiple M-proteins of the same heavy-chain isotype (n = 7) were classified by that isotype; those with both IgA and IgG M-proteins (n = 5) as biclonal. M-protein concentration at screening was measured by the perpendicular drop method; the tangent skimming method was used for follow-up.10 The iStopMM protocol was approved by the Icelandic National Bioethics Committee (approval no. 16-022, 26 April 2016) and the Icelandic Data Protection Authority. All participants provided signed informed consent.
BM samples were stained with the standardized EuroFlow MM-MRD panel (2-tube, 8-color), and processed per EuroFlow protocols (www.euroflow.org).8 Samples were measured on a FACSCanto II and data analyzed using Infinicyt. Clonal plasma cells were identified by aberrant phenotype and light-chain restriction.11 The median Bone Marrow Quality Index12 was 2.7 (range, 0.11-7.5) and the median limit of detection was 2.2 × 10–6 (range, 1.4 × 10–6 to 5.8 × 10–6) for samples without clonal plasma cells.
Wilcoxon rank-sum and χ2 tests assessed group differences. Kaplan-Meier estimates with log-rank tests were used for time-to-event analyses (transient M-protein and progression). Transient M-protein was defined as the disappearance on SPEP and immunofixation. Progression was defined as development of a more advanced plasma cell disorder. A linear mixed-effects model assessed M-protein concentration changes over time among clonal-negative individuals with persistent M-proteins. Screening measurements were excluded due to method differences. Analyses were performed in R (version 4.3.2).
Of 122 individuals included, 39 (32.0%) had IgA, 78 (63.9%) had IgG, and 5 (4.1%) had biclonal IgA/IgG M-proteins. Median M-protein was 3.2 g/L (range, not quantifiable to 21.9 g/L), median age was 69 years (range, 45-86), and 59.8% were male. Diagnoses included 91 MGUS, 29 SMM, 1 MM, and 1 chronic lymphocytic leukemia (CLL). Abnormal FLC levels13,14 were present in 26 (21.3%) individuals (16 MGUS, 9 SMM, and 1 MM) (Table 1).
Baseline characteristics of study participants, overall and stratified by M-protein isotype
| Characteristic . | All (n = 122) . | IgA (n = 39) . | IgG (n = 78) . | IgA/IgG (n = 5) . |
|---|---|---|---|---|
| Age, y, median (range) | 69 (45-86) | 69 (45-85) | 66 (48-86) | 78 (71-83) |
| Sex, male, n (%) | 73 (59.8) | 21 (53.8) | 48 (61.5) | 4 (80.0) |
| M-protein, g/L, median (range) | 3.2 (n.q. to 21.9) | 1.68 (n.q. to 5.8) | 4.32 (n.q. to 21.9) | 5.0 (n.q. to 12.0) |
| I/U FLC ratio, median (range) | 1.55 (1.00-41.8) | 1.74 (1.00-35.9) | 1.47 (1.01-41.76) | 1.30 (1.05-14.2) |
| Abnormal FLC levels (revised), n (%) | 26 (21.3) | 10 (25.6) | 15 (19.2) | 1 (20.0) |
| BMPC, %, median (range)∗ | 8.0 (2.5-65.5) | 8.0 (2.5-35.5) | 8.0 (2.5-65.5) | 13.0 (4.0-25.5) |
| Baseline diagnosis, n (%) | ||||
| MGUS | 91 (74.6) | 30 (76.9) | 59 (75.6) | 2 (40.0) |
| SMM | 29 (23.8) | 9 (23.1) | 17 (21.8) | 3 (60.0) |
| MM | 1 (0.8) | 0 (0.0) | 1 (1.3) | 0 (0.0) |
| CLL | 1 (0.8) | 0 (0.0) | 1 (1.3) | 0 (0.0) |
| NGF analysis of BM | ||||
| Clonal plasma cells detected, n (%) | 103 (84.4) | 37 (94.9) | 61 (78.2) | 5 (100.0) |
| Clonal plasma cells, % of† | ||||
| Nucleated cells, median (range) | 0.33 (0.00047-7.9) | 0.34 (0.0070-3.0) | 0.29 (0.00047-7.9) | 0.48 (0.23-1.13) |
| Total plasma cells, median (range) | 69.2 (1.5-99.9) | 63.7 (1.5-99.2) | 65.7 (2.8-99.9) | 89.3 (73.2-95.1) |
| Characteristic . | All (n = 122) . | IgA (n = 39) . | IgG (n = 78) . | IgA/IgG (n = 5) . |
|---|---|---|---|---|
| Age, y, median (range) | 69 (45-86) | 69 (45-85) | 66 (48-86) | 78 (71-83) |
| Sex, male, n (%) | 73 (59.8) | 21 (53.8) | 48 (61.5) | 4 (80.0) |
| M-protein, g/L, median (range) | 3.2 (n.q. to 21.9) | 1.68 (n.q. to 5.8) | 4.32 (n.q. to 21.9) | 5.0 (n.q. to 12.0) |
| I/U FLC ratio, median (range) | 1.55 (1.00-41.8) | 1.74 (1.00-35.9) | 1.47 (1.01-41.76) | 1.30 (1.05-14.2) |
| Abnormal FLC levels (revised), n (%) | 26 (21.3) | 10 (25.6) | 15 (19.2) | 1 (20.0) |
| BMPC, %, median (range)∗ | 8.0 (2.5-65.5) | 8.0 (2.5-35.5) | 8.0 (2.5-65.5) | 13.0 (4.0-25.5) |
| Baseline diagnosis, n (%) | ||||
| MGUS | 91 (74.6) | 30 (76.9) | 59 (75.6) | 2 (40.0) |
| SMM | 29 (23.8) | 9 (23.1) | 17 (21.8) | 3 (60.0) |
| MM | 1 (0.8) | 0 (0.0) | 1 (1.3) | 0 (0.0) |
| CLL | 1 (0.8) | 0 (0.0) | 1 (1.3) | 0 (0.0) |
| NGF analysis of BM | ||||
| Clonal plasma cells detected, n (%) | 103 (84.4) | 37 (94.9) | 61 (78.2) | 5 (100.0) |
| Clonal plasma cells, % of† | ||||
| Nucleated cells, median (range) | 0.33 (0.00047-7.9) | 0.34 (0.0070-3.0) | 0.29 (0.00047-7.9) | 0.48 (0.23-1.13) |
| Total plasma cells, median (range) | 69.2 (1.5-99.9) | 63.7 (1.5-99.2) | 65.7 (2.8-99.9) | 89.3 (73.2-95.1) |
BMPC, bone marrow plasma cell; I/U, involved/uninvolved; n.q., not quantifiable.
Based on the midpoint of the higher reported percentage category from aspirate or trephine biopsy.
Calculated only among individuals with detectable clonal plasma cells.
NGF detected clonal BM plasma cells in 103/122 (84.4%) individuals, including 72/91 (79.1%) MGUS and all SMM, MM, and CLL. Detection was significantly more frequent in IgA MGUS (28/30, 93.3%) than IgG MGUS (42/59, 71.2%; P < .05). Among 40 MGUS individuals with repeat BM sampling (median 66 months; range, 13-71 months), clonal plasma cell status remained consistent (34 positive, 6 negative). Detection correlated with MGUS risk score,5 increasing from 19/42 (43.2%) in low, to 26/30 (86.7%) in low-intermediate, and 17/17 (100%) in high-intermediate risk (P < .01). Among MGUS individuals with abnormal FLC levels, 15/16 (93.8%) had detectable clonal plasma cells. In the individual with CLL, clonal plasma cells were detected alongside a clonal B-cell population, identified through additional testing.
IgG is the most predominant immunoglobulin type in serum, and small IgG M-proteins are more readily detected on SPEP/immunofixation than IgA due to higher background levels and favorable migration patterns.15 Furthermore, IgA MGUS is more likely to persist and progress than IgG MGUS,1,16 suggesting that IgG M-proteins may more often reflect reactive or transient responses.
Our findings indicate that clonal plasma cells are usually present in IgA MGUS but frequently absent in IgG MGUS, when assessed by NGF. A prior study on iStopMM participants, using a smaller, nonoverlapping cohort, similarly reported clonal plasma cell detection in all IgA MGUS but not all IgG MGUS.17 These isotype-related differences likely reflect a higher prevalence of true plasma cell neoplasia in IgA MGUS, offering a potential explanation for its consistently higher progression risk.1
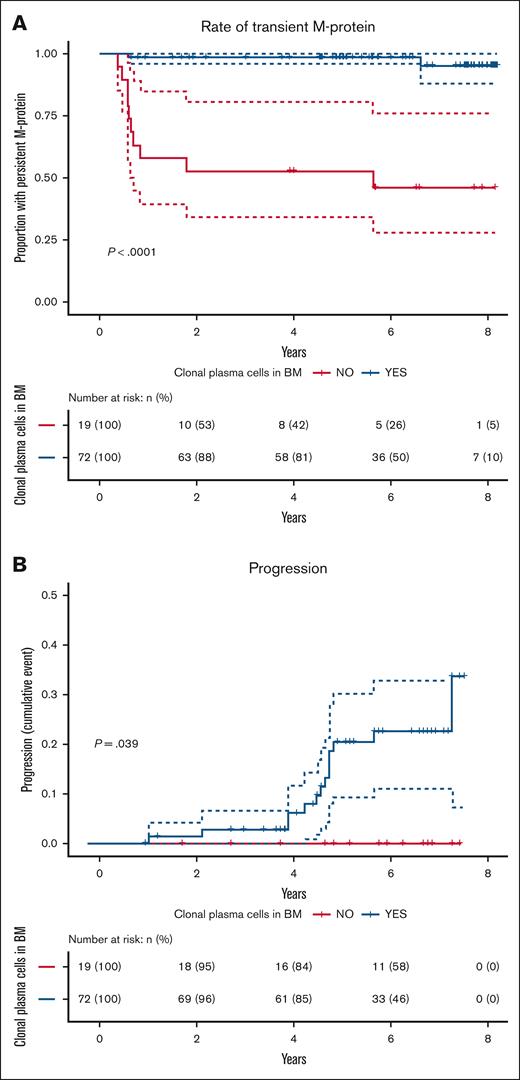
Next, we evaluated outcomes among MGUS individuals by clonal plasma cell status. Over a median follow-up of 6.2 years (median 7 repeated SPEP), M-proteins were transient in 10 of 19 (52.6%) individuals without clonal plasma cells, compared to 2 fo 72 (2.6%) with clonal plasma cells (P < .001; Figure 1A). Of the 12 transient M-proteins, 9 (75.0%) were detected only at screening, suggesting short-lived clonal expansions, rather than stable premalignant conditions. The remaining 9 clonal-negative individuals had persistent, stable, low-level IgG M-proteins (median 0.1 g/L at the diagnostic work-up; range, 0.1-1.1 g/L), with no significant change over time (estimate = −0.0017 g/L per year; P = .90).
Detection of clonal plasma cells by NGF cytometry stratifies MGUS outcomes. Kaplan-Meier analysis of (A) the probability of persistent M-protein (as detected by either SPEP or immunofixation) and (B) cumulative incidence of progression to a more advanced plasma cell disorder, comparing MGUS individuals with detectable (n = 72; blue) vs undetectable (n = 19; red) clonal plasma cells by NGF cytometry.
Detection of clonal plasma cells by NGF cytometry stratifies MGUS outcomes. Kaplan-Meier analysis of (A) the probability of persistent M-protein (as detected by either SPEP or immunofixation) and (B) cumulative incidence of progression to a more advanced plasma cell disorder, comparing MGUS individuals with detectable (n = 72; blue) vs undetectable (n = 19; red) clonal plasma cells by NGF cytometry.
Over 5.9 years median follow-up, none of the 19 clonal-negative individuals progressed. In contrast, 14/72 (19.4%) clonal-positive individuals progressed to SMM (n = 12), MM (n = 1), or follicular lymphoma (n = 1) (P < .05; Figure 1B). One individual initially had transient IgG M-protein and developed IgM MGUS at 4 years, progressing to Waldenström macroglobulinemia, representing a separate clonal process.
These findings suggest that the absence of detectable clonal plasma cells defines an indolent MGUS subset, characterized by transient or stable low-level IgG M-proteins and no progression. This aligns with prior studies indicating that a subset of MGUS, particularly those with low-level IgG M-protein, follow a stable or regressive course.1,16 Although current guidelines recommend lifelong follow-up of MGUS,18 NGF-based clonality assessment could help identify individuals unlikely to benefit from continued monitoring, though this requires BM sampling.
Limitations include the potential for NGF to miss rare clonal plasma cell populations lacking typical aberrancies, as well as B-cell clonality not captured by the assay. Longer follow-up is needed to confirm long-term outcomes.
In conclusion, NGF-based detection of clonal plasma cells identifies a clinically indolent subset of MGUS, typically IgG, with transient or stable, low-level M-proteins. These findings provide biological insight into isotype-related differences and highlight the diagnostic utility of NGF in identifying MGUS individuals, who may not require ongoing monitoring.
Acknowledgments: Screening tests were performed by The Binding Site Ltd, Birmingham, United Kingdom.
The Iceland Screens, Treats, or Prevents Multiple Myeloma study is funded by the Black Swan Research Initiative of the International Myeloma Foundation and the Icelandic Center for Research (grant agreements 173857, 217897-051, and 228521-051). This project has also received funding from the European Research Council under the European Union’s Horizon 2020 research and innovation programme (grant agreements 716677 and 101045549), the Leukemia & Lymphoma Society (grant number 2340-23), the International Myeloma Society, and the Rising Tide Foundation (grant agreement CCR-24-160). Additional funding was provided by the University of Iceland, Landspitali University Hospital, and the Icelandic Cancer Society.
Contribution: J.Þ.Ó. and S.Y.K. developed the study concept; J.Þ.Ó. was responsible for data analysis and wrote the original manuscript; S.T. and G.V. contributed to data interpretation; A.Ó. was responsible for data management; G.V., E.R.G., E.E., Á.J., B.V., P.T.Ö., B.A.A., M.S., I.Þ., and Í.Ó. contributed to data collection within the Iceland Screens, Treats, or Prevents Multiple Myeloma (iStopMM) study; S.Y.K. and S.J.H. developed the iStopMM study and S.R., S.T., and T.J.L. contributed to its practical design; S.Y.K. supervised the work and was responsible for data integrity; and all authors offered input to the manuscript and approved the final version.
Conflict-of-interest disclosure: S.R. received honoraria for scientific talks from Siemens Healthineers and Johnson & Johnson. S.T. received honoraria for scientific talks from AbbVie and Thermo Fisher Scientific. S.J.H. is an employee of The Binding Site Ltd. S.Y.K. received research funding from Amgen and Celgene; and is on the independent data monitoring committee of Janssen. The remaining authors declare no competing financial interests.
Correspondence: Jón Þórir Óskarsson, University of Iceland, Sturlugata 8, 101 Reykjavík, Iceland; email: jto@hi.is.
References
Author notes
Deidentified data are available on reasonable request from the corresponding author, Jón Þórir Óskarsson (jto@hi.is) subject to approval by the Iceland Screens, Treats, or Prevents Multiple Myeloma investigators and the Icelandic National Bioethics Committee.