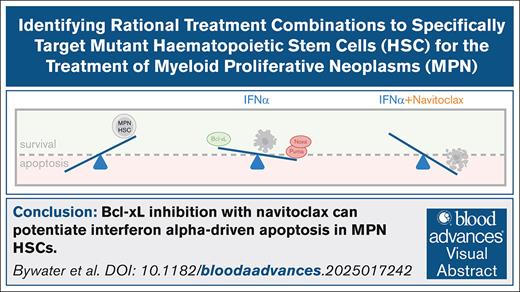
Visual Abstract
TO THE EDITOR:
Classical BCR::ABL-negative myeloproliferative neoplasms (MPNs) are progressive blood cancers characterized by the overproduction of mature, functional myeloid elements, including red blood cells in polycythemia vera (PV), platelets in essential thrombocythemia, and a fibrocellular megakaryocytic proliferation in myelofibrosis. MPNs are driven by mutations in JAK2, calreticulin (CALR), or the thrombopoietin receptor (MPL), which consistently result in the constitutive activation of the JAK/STAT signaling pathway in hematopoietic stem cells (HSCs). Patients with PV and essential thrombocythemia typically undergo cytoreductive therapy with hydroxycarbamide or increasingly with noncytotoxic agents such as pegylated interferon alfa (pegIFNα).1,2 To date, pegIFNα is the only therapy used in the management of MPN, outside of allogeneic stem cell transplantation, and it has been demonstrated to act at the level of the disease-initiating HSC population and to have a durable long-term effect on the reduction of MPN-driver mutational burden, especially in JAK2V617F-driven disease.3,4 However, IFN-α shows only subtle preferential effects in MPN-mutant HSCs over normal HSCs.5,6 Here, we demonstrate that the preferential effects of pegIFNα on Jak2V617F HSCs can be augmented with a rational combination of pegIFNα with navitoclax, broadening the therapeutic window of pegIFNα in the management of chronic-phase MPN.
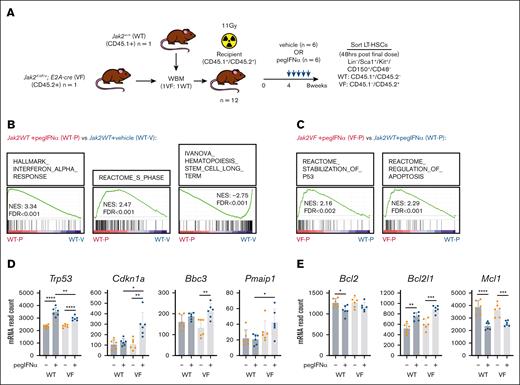
To resolve the preferential and unique effects of IFN-α on Jak2V617F long-term HSCs (LT-HSCs) that could be exploited to increase selectivity, we performed RNA sequencing on purified Jak2fl-V617F/+, Tg(E2A-cre) (VF) and Jak2+/+, Tg(E2A-cre) (WT) LT-HSCs isolated from chimeric mice after 4 weeks of treatment with a vehicle or pegIFNα (Figure 1A). As observed in response to unmodified recombinant murine IFN-α,6,7 gene set enrichment analysis determined that pegIFNα drives gene expression changes related to the IFN-α response, S-phase progression, and a reduction in stemness (Figure 1B). Gene set enrichment analysis also revealed that compared to WT LT-HSCs treated with pegIFNα, VF LT-HSCs treated with pegIFNα demonstrate a greater stabilization of p53 and induction of apoptosis (Figure 1C). Although Trp53 transcript expression appeared to be induced to a greater extent in WT LT-HSCs in response to pegIFNα, p53 target genes Cdkn1a, Bbc3, and Pmaip1 appear to only be induced in response to pegIFNα in VF LT-HSCs (Figure 1D). This p53 transcriptional response is also accompanied by an increase in the expression of Bcl2l1 (encoding the antiapoptotic BH3-only protein Bcl-xL) but not Bcl2 and Mcl1 (Figure 1E). These results suggest that the Bcl-xL pathway is preferentially activated after pegIFNα therapy, raising the hypothesis that the coadministration of a Bcl-xL–targeting BH3-mimetic, such as navitoclax,8 may be effective in combination with pegIFNα in targeting VF LT-HSCs.
Chronic administration of pegIFNα preferentially activates p53 and apoptotic transcriptional programs in Jak2V617F LT-HSCs compared to WT. (A) Experimental schematic for determining the effect of chronic pegIFNα administration (600ng, weekly) on transcript expression in Jak2fl-V617F/+;Tg(E2A-cre) (VF, CD45.1–/CD45.2+) or Jak2+/+ (WT, CD45.1+/CD45.2–) LT-HSCs isolated from the bone marrow (BM) of chimeric mice treated with either vehicle (V, n = 6) or pegIFNα (P, n = 6) (B-E). (B) Gene set enrichment analysis (GSEA) comparing transcript expression in WT LT-HSCs isolated from chimeric mice treated with pegIFNα (WT-P, n = 6) with that in WT LT-HSCs treated with vehicle (WT-V, n = 6). (C) GSEA comparing transcript expression in WT LT-HSCs (WT-P, n = 6) vs VF LT-HSCs (VF-P, n = 6) isolated from chimeric mice treated with pegIFNα. (D) Normalized read count for Trp53 and the p53 target genes, Cdkn1a (p21), Bbc3 (Puma), and Pmaip1 (Noxa), as determined by RNA sequencing of WT or VF LT-HSCs isolated from chimeric mice treated with either vehicle (V) or pegIFNα (P)(n = 6/group). (E) Normalized read count for transcripts encoding prosurvival proteins, Bcl2, Bcl-xL (Bcl2l1), and Mcl-1, as determined by RNA sequencing of WT or VF LT-HSCs isolated from chimeric mice treated with either V or pegIFNα (P)(n = 6/group). Individual data points represent data generated from independent recipient mice. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001∗∗∗; ∗∗∗∗P < .0001. FDR, false discovery rate; mRNA, messenger RNA; NES, normalized enrichment score; WBM, whole bone marrow.
Chronic administration of pegIFNα preferentially activates p53 and apoptotic transcriptional programs in Jak2V617F LT-HSCs compared to WT. (A) Experimental schematic for determining the effect of chronic pegIFNα administration (600ng, weekly) on transcript expression in Jak2fl-V617F/+;Tg(E2A-cre) (VF, CD45.1–/CD45.2+) or Jak2+/+ (WT, CD45.1+/CD45.2–) LT-HSCs isolated from the bone marrow (BM) of chimeric mice treated with either vehicle (V, n = 6) or pegIFNα (P, n = 6) (B-E). (B) Gene set enrichment analysis (GSEA) comparing transcript expression in WT LT-HSCs isolated from chimeric mice treated with pegIFNα (WT-P, n = 6) with that in WT LT-HSCs treated with vehicle (WT-V, n = 6). (C) GSEA comparing transcript expression in WT LT-HSCs (WT-P, n = 6) vs VF LT-HSCs (VF-P, n = 6) isolated from chimeric mice treated with pegIFNα. (D) Normalized read count for Trp53 and the p53 target genes, Cdkn1a (p21), Bbc3 (Puma), and Pmaip1 (Noxa), as determined by RNA sequencing of WT or VF LT-HSCs isolated from chimeric mice treated with either vehicle (V) or pegIFNα (P)(n = 6/group). (E) Normalized read count for transcripts encoding prosurvival proteins, Bcl2, Bcl-xL (Bcl2l1), and Mcl-1, as determined by RNA sequencing of WT or VF LT-HSCs isolated from chimeric mice treated with either V or pegIFNα (P)(n = 6/group). Individual data points represent data generated from independent recipient mice. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001∗∗∗; ∗∗∗∗P < .0001. FDR, false discovery rate; mRNA, messenger RNA; NES, normalized enrichment score; WBM, whole bone marrow.
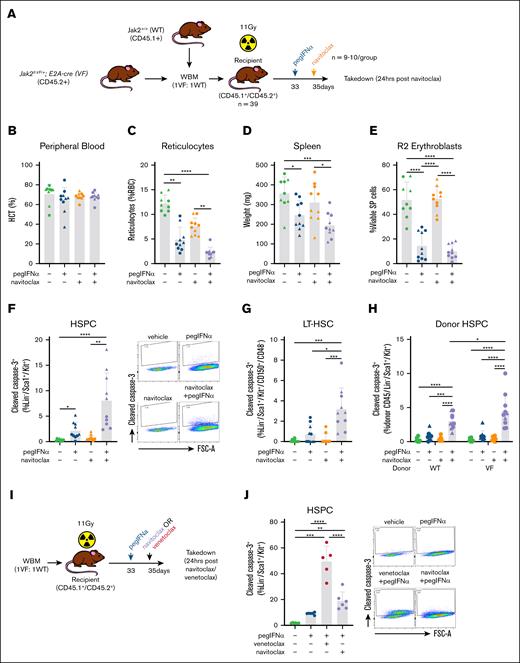
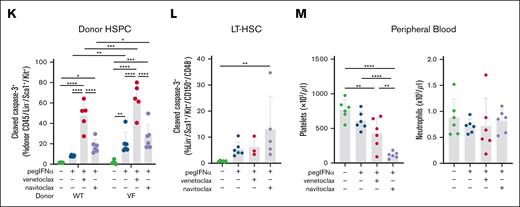
To test this hypothesis, we examined a chimeric model of Jak2V617F-driven MPN, using congenic recipient mice transplanted with VF (CD45.2+) and WT (CD45.1+) bone marrow at a 1:1 ratio. After 4 weeks to allow engraftment, an elevated hematocrit in chimeric recipients was confirmed, and the mice were stratified into groups based on donor chimerism and treated with either a single dose of the vehicle or pegIFNα. After 48 hours, the mice were treated with a single dose of either the vehicle or navitoclax and examined after a further 24 hours (Figure 2A). Although this short-term treatment did not reduce hematocrit (Figure 2B), a single dose of pegIFNα or navitoclax was sufficient to reduce the percentage of the more immature reticulocytes in the peripheral blood. This effect was even greater with the combination (Figure 2C), suggesting that the cytoreduction of hematocrit could be a longer-term consequence of either agent alone or in combination. Furthermore, the reduced frequency of immature erythroid precursors was also observed in the spleen, correlating with robust reduction in splenomegaly and greatest in magnitude in response to the combination therapy (Figure 2D-E). Within the less mature hematopoietic stem and progenitor cell (HSPC) compartment, we found that pegIFNα, but not navitoclax alone, induces apoptosis (as determined by an increased percentage of cells with cleaved caspase-3) in both HSPCs and more specifically LT-HSCs, (Figure 2F-G). However, consistent with our hypothesis, the combined administration of navitoclax increased the magnitude of the pegIFNα-induced apoptotic response in both HSPCs and LT-HSCs (Figure 2F-G), and this effect appears to be greater in VF HSPCs than in WT HSPCs (Figure 2H). The potentiation of apoptosis was not limited to Bcl-xL inhibition, with striking potentiation also mediated by Bcl-2–specific BH3-mimetic venetoclax (Figure 2I-K). However, when restricted to effects within the LT-HSC population, only navitoclax with pegIFNα was able to induce apoptosis (Figure 2L). Consistent with the results observed in clinical trials, thrombocytopenia was most dramatic with the administration of navitoclax and occurred in the absence of neutrophilic leukocytosis (Figure 2M).
Combined administration of navitoclax is able to maintain the efficacy of pegIFNα in reducing MPN-associated hematological disease parameters, in addition to potentiating apoptosis in Jak2V617F HSPCs. (A) Experimental schematic for determining the effect of single doses of pegIFNα (600 ng) and navitoclax (100 mg/kg) in Jak2VF chimeric BM mice (B-H, n = 9-10/group). (B) The percentage of hematocrit (HCT) of the chimeric mice. (C) The percentage of reticulocytes in the peripheral blood of chimeric mice. (D) The spleen weight of the chimeric mice. (E) The percentage of R2 erythroblasts (CD71+/Ter119+) in the spleen of the chimeric mice. (F) Bar graph (left) and example flow cytometry data (right) demonstrating the percentage of cleaved caspase-3+ HSPCs (Lin–/Sca1+/Kit+) in the BM of the chimeric mice. (G) The percentage of cleaved caspase-3+ LT-HSCs in the BM of the chimeric mice. (H) The percentage of cleaved caspase-3+ WT and VF HSPCs in the BM of the chimeric mice. (I) Experimental schematic for determining the effect of single doses of pegIFNα (600 ng) and navitoclax (100 mg/kg) or venetoclax (100 mg/kg) in Jak2VF chimeric BM mice (J-L, n = 5-6/group). (J) Bar graph (left) and example flow cytometry data (right) demonstrating the percentage of cleaved caspase-3+ HSPCs in the BM of the chimeric mice. (K) The percentage of cleaved caspase-3+ WT and VF HSPCs in the BM of the chimeric mice. (L) The percentage of cleaved caspase 3+ LT-HSCs in the BM of the chimeric mice. (M) Platelet and neutrophil numbers in the peripheral blood of chimeric mice. Individual data points represent data generated from independent recipient mice. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001. Different symbols for data points on the same plot denote different cohorts. SP, spleen; WBM, whole bone marrow.
Combined administration of navitoclax is able to maintain the efficacy of pegIFNα in reducing MPN-associated hematological disease parameters, in addition to potentiating apoptosis in Jak2V617F HSPCs. (A) Experimental schematic for determining the effect of single doses of pegIFNα (600 ng) and navitoclax (100 mg/kg) in Jak2VF chimeric BM mice (B-H, n = 9-10/group). (B) The percentage of hematocrit (HCT) of the chimeric mice. (C) The percentage of reticulocytes in the peripheral blood of chimeric mice. (D) The spleen weight of the chimeric mice. (E) The percentage of R2 erythroblasts (CD71+/Ter119+) in the spleen of the chimeric mice. (F) Bar graph (left) and example flow cytometry data (right) demonstrating the percentage of cleaved caspase-3+ HSPCs (Lin–/Sca1+/Kit+) in the BM of the chimeric mice. (G) The percentage of cleaved caspase-3+ LT-HSCs in the BM of the chimeric mice. (H) The percentage of cleaved caspase-3+ WT and VF HSPCs in the BM of the chimeric mice. (I) Experimental schematic for determining the effect of single doses of pegIFNα (600 ng) and navitoclax (100 mg/kg) or venetoclax (100 mg/kg) in Jak2VF chimeric BM mice (J-L, n = 5-6/group). (J) Bar graph (left) and example flow cytometry data (right) demonstrating the percentage of cleaved caspase-3+ HSPCs in the BM of the chimeric mice. (K) The percentage of cleaved caspase-3+ WT and VF HSPCs in the BM of the chimeric mice. (L) The percentage of cleaved caspase 3+ LT-HSCs in the BM of the chimeric mice. (M) Platelet and neutrophil numbers in the peripheral blood of chimeric mice. Individual data points represent data generated from independent recipient mice. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001. Different symbols for data points on the same plot denote different cohorts. SP, spleen; WBM, whole bone marrow.
Collectively, our data indicate that the inhibition of Bcl-xL is an effective method to target MPN disease-initiating HSCs via potentiation of the proapoptotic effect of pegIFNα. Furthermore, the short-term combined administration of navitoclax and pegIFNα also appears more effective at cytoreduction, specifically within the erythroid lineage, suggesting that this combination may also be more effective at achieving hematological responses in patients with chronic-phase MPN, particularly those with PV. Of note, clinical trials combining JAK inhibitors with navitoclax have demonstrated feasibility and some activity in more advanced MPN. However, further clinical development has been impeded by commercial factors and restrictions posed by somewhat arbitrary clinical trial end points.9 We propose that the effectiveness of both Bcl-xL and Bcl-2 inhibition demonstrated in this study warrants clinical research to determine the utility of pegIFNα in combination with BH3-mimetics to achieve deeper and more durable molecular and hematological responses in the management of chronic-phase MPNs.
Existing literature supports the conclusion that the proapoptotic effect of pegIFNα in HSCs is linked to cell cycle entry.10 We have previously observed HSC cell cycle entry at 48 hours after pegIFNα therapy,7 which returned to baseline by 7 days (data not shown). For future clinical studies, we propose a short duration of navitoclax that is administered immediately after pegIFNα, which is designed to reduce the predictable toxicity (thrombocytopenia) but ensure that maximum efficacy is retained.
Acknowledgments: The authors are grateful for the assistance of facility staff in using the QIMR Berghofer animal house and flow cytometry and sequencing facilities and for the helpful comments from members of the Lane laboratory. The authors acknowledge the support of Neil Herron and the Herron Family Trust and the Gordon and Jessie Gilmour Family Trust as dedicated supporters of leukemia research in Queensland. The authors acknowledge the contribution of the Myeloproliferative Neoplasms Alliance Australia for the valuable patient perspective that they provided during this study. S.W.L. was funded by an National Health and Medical Research Council (NHMRC) Investigator Grant (1195987). J.S. was funded by a Cancer Council Queensland (CCQ) fellowship (2025829).
Contribution: M.J.B. and S.W.L. conceptualized and designed the experiments and analyzed and interpreted the data; M.J.B. wrote the manuscript; Y.J., R.H., E.C., L.C., S.H.N.C., and N.H. performed the experiments and collected data; J.S. performed bioinformatic analysis; V.L. contributed to data interpretation and/or intellectual input and supervised to the study; and all authors contributed to and edited the manuscript.
Conflict-of-interest disclosure: PharmaEssentia provided the murine pegIFNα used in this study at no cost. M.J.B. has received research funding from Bristol Myers Squibb and Cylene Pharmaceuticals for unrelated projects. S.W.L. has received research funding from Bristol Myers Squibb; and has consulted for AbbVie, Novartis, Astellas, and GlaxoSmithKline for unrelated projects. The remaining authors declare no competing financial interests.
Correspondence: Megan J. Bywater, Cancer Program, QIMR Berghofer, 300 Herston Rd, Brisbane, QLD 4006, Australia; email: megan.bywater@qimrb.edu.au; and Steven W. Lane; Cancer Program, QIMR Berghofer, 300 Herston Rd, Brisbane, QLD 4006, Australia; email: steven.lane@qimrb.edu.au.
References
Author notes
The RNA sequencing data sets generated in this study are available from the NCBI Gene Expression Omnibus: GSE303462.
Detailed methodology has been included as supplemental Methods.
The full-text version of this article contains a data supplement.