Key Points
RAPID-CRISPR assay provides swift diagnosis of APL that facilitates timely therapeutic intervention minimizing the risk of early mortality.
Designed for resource-limited settings, this assay features an easy-to-read format with 100% sensitivity and 100% specificity.
Visual Abstract
Acute promyelocytic leukemia (APL), distinguished by the presence of PML::RARA fusion transcript, is a medical emergency because of its high early death rate, which is preventable when diagnosed early. Current diagnostic methods are precise and reliable but are time intensive, require sophisticated instruments, and analytical expertise. This study has redefined APL identification by CRISPR system (RAPID-CRISPR) to rapidly (<3 hours) detect PML::RARA. APL cell lines (NB4 and UF-1) and bone marrow/peripheral blood samples from 74 patients with APL (66/8, retrospective/prospective) and 48 controls were included in the study. We used a DETECTR (DNA endonuclease-targeted CRISPR transreporter) assay to identify the bcr1, bcr2, and bcr3 PML::RARA isoforms. To ensure high specificity, we used PML::RARA–specific loop-mediated isothermal amplification (LAMP) primers, synthetic protospacer-adjacent motif sites, and isoform-specific CRISPR RNAs. RAPID-CRISPR recognized APL with 100% sensitivity and 100% specificity in an ambispective cohort of patient samples. Furthermore, our blinded validation approach to detect PML::RARA in an unbiased manner provides an additional layer in the diagnostic precision of APL. RAPID-CRISPR demonstrated superior sensitivity, detecting as few as 1 copy of PML::RARA compared with 10 copies by the gold-standard reverse transcriptase qualitative and quantitative polymerase chain reaction. The nucleic acid extraction–free protocol combined with the 1-step reverse transcriptase LAMP–based DETECTR followed by lateral flow readout makes the RAPID-CRISPR assay suitable for diagnosing APL in point-of-care settings. This simple, cost-effective tool, with its easy-to-read format, is particularly valuable in underresourced regions. The assay facilitates timely diagnosis and prompt administration of lifesaving therapies such as all-trans retinoic acid and arsenic trioxide in APL.
Introduction
Acute promyelocytic leukemia (APL) is characterized by reciprocal translocation between PML on chromosome 15 and RARA on chromosome 17 resulting in PML::RARA fusion, which is present in >95% of APL cases. The 3 isoforms of PML::RARA that is, bcr1 (∼55%), bcr2 (∼10%), and bcr3 (∼35%) derive from PML breakpoints in intron 6, exon 6, and intron 3 and are referred to as long isoform, variable, and short isoform, respectively.1-3 Clinically, APL is linked to a tendency for excessive bleeding due to increased fibrinolysis leading to early deaths (EDs) in ∼30% patients. The genetic confirmation of PML::RARA is mandatory for therapeutic intervention to minimize the risk of EDs.4,5
APL is deemed as a medical emergency because of high death rate if not diagnosed and treated early. Current diagnostic methods such as fluorescence in situ hybridization, promyelocytic leukemia protein-nuclear bodies (PML-NBs) immunofluorescence, reverse transcriptase qualitative and quantitative polymerase chain reaction (RT/RQ-PCR) for APL offer sensitivity and accuracy, but they are time-consuming and require sophisticated instruments along with analytical and technical expertise.1 Therefore, there is a need to develop methods that are less complex, time and cost effective, without compromising sensitivity. With the advent of CRISPR-associated protein 12 (Cas12) and Cas13 enzymes, the CRISPR-Cas system has emerged as a potent tool for molecular diagnosis of infectious diseases and certain cancers.6-8 In addition, nucleic acid extraction–free methods combined with isothermal amplification and lateral-flow–based visual readouts have enabled point-of-care testing. The specific high-sensitivity enzymatic reporter unlocking (SHERLOCK) system uses Cas13, whereas the DNA endonuclease-targeted CRISPR transreporter (DETECTR) assay uses Cas12, both facilitating the detection of viral infections and genetic aberrations associated with cancer.6,9 In this study, we developed a loop-mediated isothermal amplification (LAMP)–mediated CRISPR/LbCas12a–based sensitive DETECTR assay to facilitate the rapid detection of PML-RARA. This proof-of-concept study has “redefined APL identification” (RAPID) by CRISPR system (RAPID-CRISPR) to detect bcr1, bcr2, and bcr3 isoforms of PML::RARA. The implementation of a lateral flow (LF) strip-based readout enabled the diagnosis of APL within 3 hours after the collection of blood samples, making it suitable for outpatient or onsite testing with minimal instrumentation. The RAPID-CRISPR assay demonstrated 100% sensitivity and specificity in differentiating APL from non-APL cases, allowing for immediate initiation of lifesaving therapy. This assay would be particularly beneficial in resource-limited peripheral hospitals, in which timely diagnosis and treatment of APL could significantly reduce the risk of early mortality.
Methods
Cell lines and clinical samples
NB4 (PML::RARA, bcr1 isoform), UF-1 (PML::RARA, bcr3 isoform), and non-APL acute myeloid leukemia cells (HL60 and MOLM13) were grown in RPMI 1640 with 1% antibiotics and 10% fetal bovine serum. A total of 122 samples were analyzed in this study, including 109 retrospective samples (66 cases and 43 controls) and 13 prospective samples (8 cases and 5 controls). The 66 patients with APL (54 peripheral blood [PB] and 12 bone marrow [BM] samples) were molecularly confirmed, with the following PML::RARA isoforms: bcr1 (n = 32), bcr2 (n = 3), and bcr3 (n = 31; Table 1). The control group consisted of 43 individuals (33 with non-APL leukemias and 10 healthy individuals; supplemental Figure 1). Additionally, 6 postinduction PML::RARA–positive (PML::RARA+) (minimal residual disease positive [MRD+], n = 6) and PML::RARA–negative (PML::RARA-) (MRD-negative [MRD−], n = 6) patients were included for RAPID-CRISPR analysis (Table 2). All patients and donors provided written informed consent and the ethics committee of Tata Memorial Centre, Mumbai (TMC-IEC III) approved the study (approval reference number, 900824/2021).
Clinicobiological parameters of 48 patients with APL included in the study for validation of RAPID-CRISPR assay
| Sample ID . | PML::RARA isoform . | PML::RARA isoform by RAPID-CRISPR . | Age/sex . | Hemoglobin (g/dL) . | Risk group . | Abnormal promyelocytes (%) . |
|---|---|---|---|---|---|---|
| UPN1 | BCR3 | BCR3 | 23/F | 5.1 | High | 98 |
| UPN2 | BCR3 | BCR3 | 30/M | 9.6 | High | 90 |
| UPN3 | BCR3 | BCR3 | 37/M | 8.0 | High | 79 |
| UPN4 | BCR3 | BCR3 | 47/F | 5.9 | High | 98 |
| UPN5 | BCR3 | BCR3 | 32/M | 8.3 | Low | 32 |
| UPN6 | BCR3 | BCR3 | 32/M | 10.6 | Intermediate | 70 |
| UPN7 | BCR3 | BCR3 | 25/F | 5.2 | High | 90 |
| UPN8 | BCR3 | BCR3 | 53/M | 10.2 | Low | 32 |
| UPN9 | BCR3 | BCR3 | 28/M | 8.5 | High | 92 |
| UPN10 | BCR3 | BCR3 | 18/M | 5.3 | High | 80 |
| UPN11 | BCR3 | BCR3 | 45/M | 7.3 | Intermediate | 70 |
| UPN12 | BCR3 | BCR3 | 18/M | 5.9 | Intermediate | 15 |
| UPN13 | BCR3 | BCR3 | 41/M | 6.6 | Low | 44 |
| UPN14 | BCR3 | BCR3 | 35/M | 11.2 | High | 84 |
| UPN15 | BCR3 | BCR3 | 26/M | 5.9 | High | 91 |
| UPN16 | BCR1 | BCR1 | 44/M | 7.7 | Intermediate | 65 |
| UPN17 | BCR1 | BCR1 | 22/M | 8.0 | Intermediate | 40 |
| UPN18 | BCR1 | BCR1 | 17/M | 9.7 | Low | 90 |
| UPN19 | BCR1 | BCR1 | 31/F | 5.2 | Intermediate | 78 |
| UPN20 | BCR1 | BCR1 | 19/F | 8.6 | Low | 85 |
| UPN21 | BCR1 | BCR1 | 55/F | 10.7 | Low | 87 |
| UPN22 | BCR1 | BCR1 | 33/M | 8.1 | Intermediate | 59 |
| UPN23 | BCR1 | BCR1 | 45/M | 8.9 | Intermediate | 20 |
| UPN24 | BCR1 | BCR1 | 27/F | 6.8 | Intermediate | 90 |
| UPN25 | BCR3 | BCR3 | 12/M | 8.5 | Low | 79 |
| UPN26 | BCR1 | BCR1 | 14/F | 6.5 | High | 86 |
| UPN27 | BCR1 | BCR1 | 21/M | 7.5 | High | 84 |
| UPN28 | BCR1 | BCR1 | 13/F | 9.7 | Intermediate | 70 |
| UPN29 | BCR1 | BCR1 | 56/F | 9.4 | Intermediate | 85 |
| UPN30 | BCR1 | BCR1 | 26/M | 8.4 | Intermediate | 70 |
| UPN31 | BCR1 | BCR1 | 15/M | 8.4 | Low | 90 |
| UPN32 | BCR1 | BCR1 | 28/F | 8.6 | Intermediate | 94 |
| UPN33 | BCR1 | BCR1 | 51/M | 8.7 | Low | 75 |
| UPN34 | BCR1 | BCR1 | 47/F | 8.7 | Low | 43 |
| UPN35 | BCR1 | BCR1 | 6/F | 12.2 | Low | 35 |
| UPN36 | BCR1 | BCR1 | 37/M | 7.1 | Intermediate | 91 |
| UPN37 | BCR1 | BCR1 | 22/M | 6.7 | Low | 55 |
| UPN38 | BCR1 | BCR1 | 9/M | 6.5 | Low | 91 |
| UPN39 | BCR1 | BCR1 | 51/F | 9.5 | Intermediate | 86 |
| UPN40 | BCR3 | BCR3 | 20/M | 9.1 | Intermediate | 80 |
| UPN41 | BCR3 | BCR3 | 36/F | 9.5 | High | 77 |
| UPN42 | BCR3 | BCR3 | 20/F | 7.3 | High | 91 |
| UPN43 | BCR3 | BCR3 | 20/M | 9.3 | High | 84 |
| UPN44 | BCR3 | BCR3 | 23/F | 5.1 | High | 98 |
| UPN45 | BCR3 | BCR3 | 9/M | 9.0 | Intermediate | 70 |
| UPN46 | BCR2 | BCR2 | 24/F | 9.6 | High | 90 |
| UPN47 | BCR2 | BCR2 | 48/F | 10.1 | Low | 83 |
| UPN48 | BCR2 | BCR2 | 41/M | 9.1 | High | 85 |
| UPN49 | BCR1 | BCR1 | 43/M | 7.4 | Intermediate | 69 |
| UPN50 | BCR1 | BCR1 | 11/M | 7.7 | High | 37 |
| UPN51 | BCR1 | BCR1 | 47/F | 7.3 | Intermediate | 85 |
| UPN52 | BCR3 | BCR3 | 46/F | 8.6 | Intermediate | 62 |
| UPN53 | BCR3 | BCR3 | 32/F | 6.4 | High | 95 |
| UPN54 | BCR1 | BCR1 | 11/M | 6.7 | High | 85 |
| UPN55 | BCR3 | BCR3 | 7/F | 9.4 | Low | 80 |
| UPN56 | BCR3 | BCR3 | 67/M | 9.9 | High | 93 |
| UPN57 | BCR3 | BCR3 | 9/M | 9 | Intermediate | 34 |
| UPN58 | BCR3 | BCR3 | 40/M | 9.4 | High | 68 |
| UPN59 | BCR1 | BCR1 | 16/M | 9.3 | Intermediate | 34 |
| UPN60 | BCR1 | BCR1 | 25/M | 6.5 | Low | 83 |
| UPN61 | BCR1 | BCR1 | 38/M | 8.9 | High | 71 |
| UPN62 | BCR3 | BCR3 | 32/M | 8.1 | Intermediate | 86 |
| UPN63 | BCR1 | BCR1 | 11/M | 6.7 | High | 85 |
| UPN64 | BCR3 | BCR3 | 37/M | 9.5 | Intermediate | 77 |
| UPN65 | BCR3 | BCR3 | 43/F | 8.7 | High | 85 |
| UPN66 | BCR1 | BCR1 | 25/M | 6.5 | Low | 83 |
| Sample ID . | PML::RARA isoform . | PML::RARA isoform by RAPID-CRISPR . | Age/sex . | Hemoglobin (g/dL) . | Risk group . | Abnormal promyelocytes (%) . |
|---|---|---|---|---|---|---|
| UPN1 | BCR3 | BCR3 | 23/F | 5.1 | High | 98 |
| UPN2 | BCR3 | BCR3 | 30/M | 9.6 | High | 90 |
| UPN3 | BCR3 | BCR3 | 37/M | 8.0 | High | 79 |
| UPN4 | BCR3 | BCR3 | 47/F | 5.9 | High | 98 |
| UPN5 | BCR3 | BCR3 | 32/M | 8.3 | Low | 32 |
| UPN6 | BCR3 | BCR3 | 32/M | 10.6 | Intermediate | 70 |
| UPN7 | BCR3 | BCR3 | 25/F | 5.2 | High | 90 |
| UPN8 | BCR3 | BCR3 | 53/M | 10.2 | Low | 32 |
| UPN9 | BCR3 | BCR3 | 28/M | 8.5 | High | 92 |
| UPN10 | BCR3 | BCR3 | 18/M | 5.3 | High | 80 |
| UPN11 | BCR3 | BCR3 | 45/M | 7.3 | Intermediate | 70 |
| UPN12 | BCR3 | BCR3 | 18/M | 5.9 | Intermediate | 15 |
| UPN13 | BCR3 | BCR3 | 41/M | 6.6 | Low | 44 |
| UPN14 | BCR3 | BCR3 | 35/M | 11.2 | High | 84 |
| UPN15 | BCR3 | BCR3 | 26/M | 5.9 | High | 91 |
| UPN16 | BCR1 | BCR1 | 44/M | 7.7 | Intermediate | 65 |
| UPN17 | BCR1 | BCR1 | 22/M | 8.0 | Intermediate | 40 |
| UPN18 | BCR1 | BCR1 | 17/M | 9.7 | Low | 90 |
| UPN19 | BCR1 | BCR1 | 31/F | 5.2 | Intermediate | 78 |
| UPN20 | BCR1 | BCR1 | 19/F | 8.6 | Low | 85 |
| UPN21 | BCR1 | BCR1 | 55/F | 10.7 | Low | 87 |
| UPN22 | BCR1 | BCR1 | 33/M | 8.1 | Intermediate | 59 |
| UPN23 | BCR1 | BCR1 | 45/M | 8.9 | Intermediate | 20 |
| UPN24 | BCR1 | BCR1 | 27/F | 6.8 | Intermediate | 90 |
| UPN25 | BCR3 | BCR3 | 12/M | 8.5 | Low | 79 |
| UPN26 | BCR1 | BCR1 | 14/F | 6.5 | High | 86 |
| UPN27 | BCR1 | BCR1 | 21/M | 7.5 | High | 84 |
| UPN28 | BCR1 | BCR1 | 13/F | 9.7 | Intermediate | 70 |
| UPN29 | BCR1 | BCR1 | 56/F | 9.4 | Intermediate | 85 |
| UPN30 | BCR1 | BCR1 | 26/M | 8.4 | Intermediate | 70 |
| UPN31 | BCR1 | BCR1 | 15/M | 8.4 | Low | 90 |
| UPN32 | BCR1 | BCR1 | 28/F | 8.6 | Intermediate | 94 |
| UPN33 | BCR1 | BCR1 | 51/M | 8.7 | Low | 75 |
| UPN34 | BCR1 | BCR1 | 47/F | 8.7 | Low | 43 |
| UPN35 | BCR1 | BCR1 | 6/F | 12.2 | Low | 35 |
| UPN36 | BCR1 | BCR1 | 37/M | 7.1 | Intermediate | 91 |
| UPN37 | BCR1 | BCR1 | 22/M | 6.7 | Low | 55 |
| UPN38 | BCR1 | BCR1 | 9/M | 6.5 | Low | 91 |
| UPN39 | BCR1 | BCR1 | 51/F | 9.5 | Intermediate | 86 |
| UPN40 | BCR3 | BCR3 | 20/M | 9.1 | Intermediate | 80 |
| UPN41 | BCR3 | BCR3 | 36/F | 9.5 | High | 77 |
| UPN42 | BCR3 | BCR3 | 20/F | 7.3 | High | 91 |
| UPN43 | BCR3 | BCR3 | 20/M | 9.3 | High | 84 |
| UPN44 | BCR3 | BCR3 | 23/F | 5.1 | High | 98 |
| UPN45 | BCR3 | BCR3 | 9/M | 9.0 | Intermediate | 70 |
| UPN46 | BCR2 | BCR2 | 24/F | 9.6 | High | 90 |
| UPN47 | BCR2 | BCR2 | 48/F | 10.1 | Low | 83 |
| UPN48 | BCR2 | BCR2 | 41/M | 9.1 | High | 85 |
| UPN49 | BCR1 | BCR1 | 43/M | 7.4 | Intermediate | 69 |
| UPN50 | BCR1 | BCR1 | 11/M | 7.7 | High | 37 |
| UPN51 | BCR1 | BCR1 | 47/F | 7.3 | Intermediate | 85 |
| UPN52 | BCR3 | BCR3 | 46/F | 8.6 | Intermediate | 62 |
| UPN53 | BCR3 | BCR3 | 32/F | 6.4 | High | 95 |
| UPN54 | BCR1 | BCR1 | 11/M | 6.7 | High | 85 |
| UPN55 | BCR3 | BCR3 | 7/F | 9.4 | Low | 80 |
| UPN56 | BCR3 | BCR3 | 67/M | 9.9 | High | 93 |
| UPN57 | BCR3 | BCR3 | 9/M | 9 | Intermediate | 34 |
| UPN58 | BCR3 | BCR3 | 40/M | 9.4 | High | 68 |
| UPN59 | BCR1 | BCR1 | 16/M | 9.3 | Intermediate | 34 |
| UPN60 | BCR1 | BCR1 | 25/M | 6.5 | Low | 83 |
| UPN61 | BCR1 | BCR1 | 38/M | 8.9 | High | 71 |
| UPN62 | BCR3 | BCR3 | 32/M | 8.1 | Intermediate | 86 |
| UPN63 | BCR1 | BCR1 | 11/M | 6.7 | High | 85 |
| UPN64 | BCR3 | BCR3 | 37/M | 9.5 | Intermediate | 77 |
| UPN65 | BCR3 | BCR3 | 43/F | 8.7 | High | 85 |
| UPN66 | BCR1 | BCR1 | 25/M | 6.5 | Low | 83 |
F, female; M, male.
Postinduction APL samples for RAPID-CRISPR analysis
| Sample ID . | PML::RARA isoform . | Detected by RAPID-CRISPR . | Copy number of PML::RARA . | Fluorescence value by RAPID-CRISPR . |
|---|---|---|---|---|
| UPN61 | BCR1 | Yes | 25 | 1451 |
| UPN62 | BCR3 | Yes | 44 | 1354 |
| UPN63 | BCR1 | Yes | 36 | 2655 |
| UPN64 | BCR3 | Yes | 30 | 2662 |
| UPN65 | BCR3 | Yes | 71 | 1476 |
| UPN66 | BCR1 | Yes | 61 | 1471 |
| UPN67 | BCR1 | No | 0 | 490 |
| UPN68 | BCR1 | No | 0 | 302 |
| UPN69 | BCR1 | No | 0 | 240 |
| UPN70 | BCR3 | No | 0 | 724 |
| UPN71 | BCR3 | No | 0 | 203 |
| UPN72 | BCR3 | No | 0 | 455 |
| Sample ID . | PML::RARA isoform . | Detected by RAPID-CRISPR . | Copy number of PML::RARA . | Fluorescence value by RAPID-CRISPR . |
|---|---|---|---|---|
| UPN61 | BCR1 | Yes | 25 | 1451 |
| UPN62 | BCR3 | Yes | 44 | 1354 |
| UPN63 | BCR1 | Yes | 36 | 2655 |
| UPN64 | BCR3 | Yes | 30 | 2662 |
| UPN65 | BCR3 | Yes | 71 | 1476 |
| UPN66 | BCR1 | Yes | 61 | 1471 |
| UPN67 | BCR1 | No | 0 | 490 |
| UPN68 | BCR1 | No | 0 | 302 |
| UPN69 | BCR1 | No | 0 | 240 |
| UPN70 | BCR3 | No | 0 | 724 |
| UPN71 | BCR3 | No | 0 | 203 |
| UPN72 | BCR3 | No | 0 | 455 |
Total cellular RNA was extracted from mononuclear cells using either the TRIzol (Invitrogen, Waltham, MA) method or RNeasy method (Qiagen, Hilden, Germany) as per the manufacturer’s instructions. The 1 μg of total RNA was converted into complementary DNA (cDNA) using a high-capacity cDNA reverse transcription kit (Thermo Scientific, Waltham, MA). Using a primer/probe set created as a part of the Europe Against Cancer program, RQ-PCR for PML::RARA detection was carried out.10 The LightCycler (Roche, Basel, Switzerland) along with dedicated software was used for the quantification of PML::RARA copy number relative to control gene ABL1.
LAMP primers and crRNA design
Four core primers were designed (assigned as F1c-F2 [forward internal primer], B1c-B2 [backward internal primer (BIP)], F3, and B3) to specifically target the 3 isoforms of PML::RARA (supplemental Table 1). All the primers were designed using the PrimerExplorer version 4 (https://primerexplorer.jp/elamp4.0.0/). The BIP sequence was modified to create a synthetic protospacer-adjacent motif site (TTTV) for LbCas12a recognition close to the PML::RARA junction for bcr1, bcr2, and bcr3 isoforms. PML::RARA’s junctional sequence was used to design the CRISPR RNA (crRNA) for bcr3, whereas the RARA region closest to the PML::RARA junction was used to create a shared crRNA for bcr1 and bcr2 (details in supplemental Materials and methods). All the primers and crRNAs were synthesized by Integrated DNA Technologies (IDT, Coralville, IA) and sequences are provided in supplemental Table 1.
DETECTR assay with fluorescence and LF-based readout
The cDNA was subjected to LAMP reaction as per New England Biolabs (NEB, Ipswich, MA) LAMP protocol for 40 minutes at 68°C (details in supplemental Materials and methods; supplemental Figure 2A). CRISPR/LbCas12a–based PML::RARA detection coupled with a fluorescence readout was performed. The procedure involved a 30-minute incubation of equimolar concentrations (500 nM) of crRNA and LbCas12a with 1× r2.1 buffer at 37°C for the ribonucleoprotein (RNP) complex formation. The RNPs were incubated with 5 μL of LAMP product and 500 nM of fluorescent DNA reporter (DNaseAlert substrate) (IDT, Coralville, IA) at 37°C for 60 minutes for target-specific LbCas12a cleavage reaction. After the incubation, the intensity of fluorescence was monitored using BioTek Cytation 5 (Agilent, Santa Clara, CA) cell imaging multimode reader (details in supplemental Materials and methods; supplemental Figure 2B).
The DNaseAlert substrate was replaced with 200 nM LF reporter molecule (5’6-carboxyfluorescein [6-FAM]-TTATTATT-BIO3′) compatible with the antibodies in the Milenia HybriDetect 1 LF strip (Milenia Biotec, Giessen, Germany). The reaction mixture was mixed with 75 μL of HybriDetect assay buffer after it had been incubated for 60 minutes at 37°C. The LF strip was subsequently immersed into the reaction tube and results were observed within 5 minutes. A negative result was determined by the presence of a single band on the control line, whereas a positive result was indicated by 2 bands on both the control and test lines or a single band on the test line.
Serial dilution of cDNA
cDNA from NB4 and UF-1 cell lines was diluted up to 7 times (1:10 series) with HL60 cDNA to use in RQ-PCR and RAPID-CRISPR assays for sensitivity analyses. The cDNA from a patient with APL was serially diluted 6 times (1:10 series) with a background of healthy donor cDNA to assess the limit of detection (LOD) for PML::RARA transcript.
Modified HUDSON for extraction-free RT-LAMP assay
PB/BM (200 μL) from APL and controls were collected in EDTA tubes. Red blood cell lysis was performed by adding 1 mL of red blood cell lysis buffer (Qiagen, Hilden, Germany) and incubating for 10 minutes at room temperature. After incubation, the lysed blood was washed twice with 1× phosphate-buffered saline at 2500g for 5 minutes each. The mononuclear cell pellet was resuspended in 100 mM tris(2-carboxyethyl)phosphine hydrochloride and incubated for 5 minutes at 50°C, followed by the addition of 1 mM EDTA and further incubation for 5 minutes at 64°C as per heating unextracted diagnostic samples to obliterate nucleases (HUDSON) protocol.11 The enriched RNA was directly used as a template for the RT-LAMP assay, which has RT WarmStart reverse transcriptase (NEB, Ipswich, MA), eliminating a separate cDNA synthesis step. The reaction mixture was incubated at 68°C for 40 minutes, as mentioned in the previous LAMP assay.
Statistics and data analysis
The raw fluorescence data were analyzed by GraphPad 8.4.2 and IBM SPSS (version 25). The Shapiro-Wilk normality test was used to determine the normality of the data. The Student t test/nonparametric Mann-Whitney U test was applied to measure the differences in mean/median relative fluorescence units (RFUs) between the groups. A Kruskal-Wallis test followed by a post hoc test (multiple comparisons) was used to compare median fluorescence values among 3 isoforms of PML::RARA. RAPID-CRISPR’s diagnostic accuracy was analyzed using area under the receiver operating characteristic (ROC) curve (area under the curve) method and Youden index was used to estimate the optimal cutoff.
Results
Specific and sensitive detection of PML::RARA by RAPID-CRISPR assay
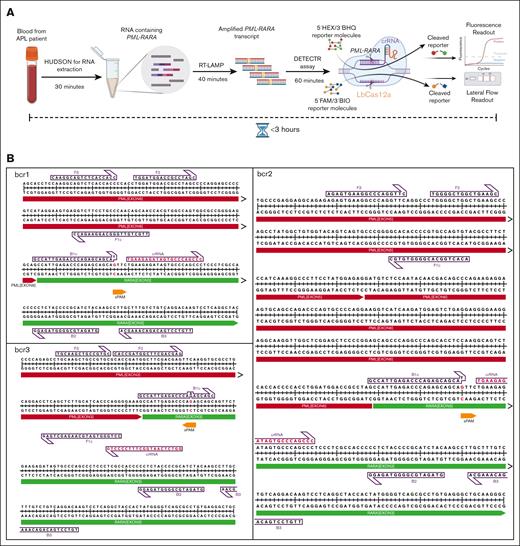
The comprehensive workflow of the assay is depicted in Figure 1A and LAMP primers, crRNA, and PML::RARA junctional sequences are provided in supplemental Tables 1 and 2, respectively. Identification of PML::RARA transcript isoforms is crucial for diagnosing and essential for effective management of APL. To detect PML::RARA fusion transcript isoforms with high precision, we included 3 specific features in the RAPID-CRISPR assay: (1) PML::RARA fusion–specific LAMP primers provide selective amplification of bcr1, bcr2, and bcr3; (2) a single nucleotide change in BIP primers creates a synthetic protospacer-adjacent motif site for LbCas12a recognition adjacent to PML::RARA junctional sequences during the LAMP reaction (Figure 1B); and (3) the binding of isoform-specific crRNAs occurs only when successful PML::RARA amplification by LAMP generates the crRNA recognition site for the cleavage by LbCas12a. This dual-layer specificity enables the detection of bcr1, bcr2, and bcr3 isoforms of the fusion transcript, eliminating off-target effects in the RAPID-CRISPR assay.
Design of RAPID-CRISPR assay. (A) Experimental workflow and timeline of RAPID-CRISPR assay for PML::RARA fusion transcript. (B) Illustration of LAMP primers and crRNA position on bcr1, bcr2, and bcr3 isoforms. sPAM, synthetic protospacer-adjacent motif.
Design of RAPID-CRISPR assay. (A) Experimental workflow and timeline of RAPID-CRISPR assay for PML::RARA fusion transcript. (B) Illustration of LAMP primers and crRNA position on bcr1, bcr2, and bcr3 isoforms. sPAM, synthetic protospacer-adjacent motif.
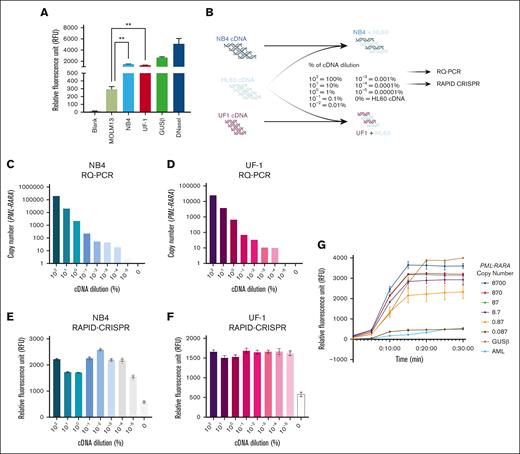
The LAMP amplicons from NB4 (bcr1 isoform), UF-1 (bcr3 isoform), and MOLM13 (PML::RARA negative) cells were mixed with isoform-specific RNP complex (crRNA and LbCas12a), and a fluorescent DNA reporter. The collateral cleavage activity of activated LbCas12a, upon binding to its target (PML::RARA fusion) in NB4 and UF-1 cells, resulted in the highest fluorescence release measured as RFU (NB4: median RFU = 1486; UF-1: median RFU = 1274). In contrast, only a background signal was observed in the control (MOLM13: median RFU = 294), which was significantly lower than NB4 and UF-1 (P < .001; Figure 2A).
RAPID-CRISPR for the specific detection of PML::RARA fusion transcript. (A) Isoform-specific RAPID-CRISPR assay in APL and non-APL cell lines. The median with interquartile range is represented; P < .001. (B) Experimental workflow of cDNA dilutions for RAPID-CRISPR and RQ-PCR. (C-D) Quantification of PML::RARA copy number by RQ-PCR in NB4 (blue) and UF-1 (pink) cDNA dilutions. (E-F) Quantification of fluorescence by RAPID-CRISPR using cDNA dilutions in NB4 (blue) and UF-1 (pink). (G) LOD of PML::RARA using cDNA from an APL case.
RAPID-CRISPR for the specific detection of PML::RARA fusion transcript. (A) Isoform-specific RAPID-CRISPR assay in APL and non-APL cell lines. The median with interquartile range is represented; P < .001. (B) Experimental workflow of cDNA dilutions for RAPID-CRISPR and RQ-PCR. (C-D) Quantification of PML::RARA copy number by RQ-PCR in NB4 (blue) and UF-1 (pink) cDNA dilutions. (E-F) Quantification of fluorescence by RAPID-CRISPR using cDNA dilutions in NB4 (blue) and UF-1 (pink). (G) LOD of PML::RARA using cDNA from an APL case.
Next, we assessed the sensitivity of the RAPID-CRISPR assay compared with the RQ-PCR in detecting PML::RARA. Serial dilutions of PML::RARA–positive cDNA from NB4 and UF-1 cells were prepared by spiking with PML::RARA–negative cDNA from HL60 cells (Figure 2B). Both approaches used these serially diluted cDNA as templates. As anticipated with RQ-PCR, cDNA concentrations were directly proportional to the copy numbers of PML::RARA (Spearman R2 = 1.00 and 0.996, respectively; P < .0001; Figure 2C-D). However, the RAPID-CRISPR assay plateaued, showing no correlation between cDNA concentrations and fluorescence signal intensity (R2 = 0.467 and 0.067, respectively; P > .05; Figure 2E-F). RAPID-CRISPR assay was able to detect PML::RARA down to a 10−5 dilution, whereas RQ-PCR could detect it only up to a 10−4 dilution level (Figure 2C-F).
To determine the LOD, we used a cDNA harboring 87 000 copies of PML::RARA. Six 10-fold serial dilutions (starting with 8700 copies) of this cDNA sample were subjected to the RAPID-CRISPR assay. The results revealed that the RAPID-CRISPR assay could detect as few as 0.87 to 1 copy of PML::RARA (Figure 2G).
Clinical validation of the RAPID-CRISPR assay
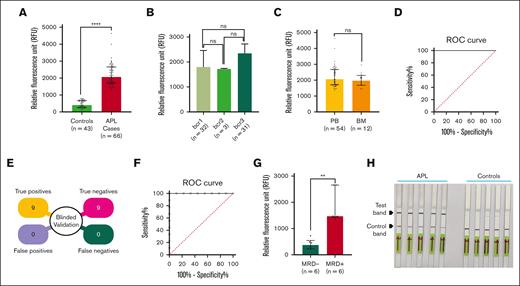
Quantitative measurement of the fluorescence revealed a fivefold increase in signal intensity in patients with APL (n = 66; median RFU = 2063) compared with controls (n = 43; median RFU = 407). The Mann-Whitney U test of significance showed that the relative fluorescence in PML::RARA+ samples was significantly higher than in controls (P < .0001; Figure 3A). The PML::RARA isoform-specific RAPID-CRISPR assay showed no significant difference in signal intensity among the 3 fusion transcript isoforms (median RFU: bcr1 = 1800, bcr2 = 1717, bcr3 = 2348; P = not significant), demonstrating the assay’s ability to detect all isoform variants with equal efficiency (Figure 3B). Furthermore, there was no significant difference in fluorescence levels between PB (n = 54, median RFU = 2063) and BM (n = 12; median RFU = 1969.5) APL cases (P = not significant) (Figure 3C), indicating that the assay is equally effective in detecting PML::RARA regardless of the sample type. These results corroborate with the APL cell line data and confirm that the RAPID-CRISPR assay is highly efficient in detecting 3 PML::RARA isoforms.
Validation of RAPID-CRISPR assay using clinical samples. (A) Measurement of fluorescence using isoform-specific crRNA in APL cases (n = 66) and controls (n = 43) by RAPID-CRISPR. The result is represented as a median with an interquartile range; P < .0001. (B) Fluorescence data of bcr1, bcr2, and bcr3 isoforms of PML::RARA by RAPID-CRISPR. A median with an interquartile range represents the data P = ns (nonsignificant). (C) Fluorescence data of APL cases from the PB (n = 54) and BM (n = 12). The data are represented as a median with interquartile range; P = ns. (D) ROC curve to estimate the clinical sensitivity and specificity of the RAPID-CRISPR assay with APL (n = 66) and control (n = 43) cases. Area under the curve (AUC) = 1.000; 95% confidence interval (CI), 1.000-1.000. (E) Illustration of blinded validation of RAPID-CRISPR in APL (n = 9) and control (n = 9) cases. (F) ROC curve to estimate the clinical sensitivity and specificity of the RAPID-CRISPR assay in a blinded validation experiment. AUC = 1.000; 95% CI, 1.000-1.000. (G) Measurement of fluorescence between MRD− (n = 6) and MRD+ (n = 6) APL cases by RAPID-CRISPR assay. The data are represented as a median with interquartile range; P < .01. (H) LF-based readouts of RAPID-CRISPR assay for PML::RARA detection using 5 APL cases and 5 controls.
Validation of RAPID-CRISPR assay using clinical samples. (A) Measurement of fluorescence using isoform-specific crRNA in APL cases (n = 66) and controls (n = 43) by RAPID-CRISPR. The result is represented as a median with an interquartile range; P < .0001. (B) Fluorescence data of bcr1, bcr2, and bcr3 isoforms of PML::RARA by RAPID-CRISPR. A median with an interquartile range represents the data P = ns (nonsignificant). (C) Fluorescence data of APL cases from the PB (n = 54) and BM (n = 12). The data are represented as a median with interquartile range; P = ns. (D) ROC curve to estimate the clinical sensitivity and specificity of the RAPID-CRISPR assay with APL (n = 66) and control (n = 43) cases. Area under the curve (AUC) = 1.000; 95% confidence interval (CI), 1.000-1.000. (E) Illustration of blinded validation of RAPID-CRISPR in APL (n = 9) and control (n = 9) cases. (F) ROC curve to estimate the clinical sensitivity and specificity of the RAPID-CRISPR assay in a blinded validation experiment. AUC = 1.000; 95% CI, 1.000-1.000. (G) Measurement of fluorescence between MRD− (n = 6) and MRD+ (n = 6) APL cases by RAPID-CRISPR assay. The data are represented as a median with interquartile range; P < .01. (H) LF-based readouts of RAPID-CRISPR assay for PML::RARA detection using 5 APL cases and 5 controls.
The RAPID-CRISPR assay was assessed for its diagnostic accuracy through ROC curve analysis on 66 PML::RARA+ samples and 43 PML::RARA- controls. The area under the curve for the RAPID-CRISPR assay was 1.000 (95% confidence interval, 1.000-1.000; P < .0001). A fluorescence cutoff of 1071 was found to discriminate PML::RARA–positive cases from the controls (supplemental Table 3), achieving 100% sensitivity and 100% specificity (Figure 3D). Considering that any conscious or unconscious bias could affect the reproducibility of the assay, we conducted the blinded validation of the RAPID-CRISPR assay in a cohort of 18 samples (9 APL and 9 controls). The validation set was blinded by an independent statistician and the case/controls status remained undisclosed until prediction based on RFU values were submitted. Based on a cutoff of RFU value of 1071, the assay distinguished all 9 APL cases from controls (Figure 3E). The ROC analysis of the blinded validation showed 100% concordance between RAPID-CRISPR and RQ-PCR assays in detecting PML::RARA (Figure 3F).
To assess the ability of the RAPID-CRISPR assay to detect MRD, we observed significantly elevated RFU levels (MRD+ median RFU = 1473.5 vs MRD− median RFU = 378.5; P < .01) in all 6 postinduction samples analyzed, for which RQ-PCR monitoring indicated low PML::RARA copy numbers. These findings confirm that the RAPID-CRISPR assay is able to detect fewer copies of PML::RARA in follow-up samples (Figure 3G; Table 2; supplemental Figure 3).
LF strip–based readout
An APL diagnostic model without the need of complex instruments was developed by incorporating a LF reporter molecule with 6-FAM at 5′ and biotin at 3′ into the LbCas12a reaction setup. The crRNA-LbCas12a reaction mixture was incubated with the dual reporter for 60 minutes and a LF strip with predefined test and control lines was dipped into the reaction mix.12 In the absence of the PML::RARA transcript, the intact biotin of the dual reporter is bound to the streptavidin-coated control band. In the presence of the PML::RARA transcript, activated LbCas12a cleaves the reporter causing migration of the gold nanoparticle–conjugated 6-FAM to the test band containing anti-species 6-FAM antibody. The appearance of an intense band at the test line indicates a positive test for APL. We tested 5 APL and 5 controls using the LF assay. We found a strong band in the test line and a faint band in the control line for all the APL cases, whereas all the controls displayed a strong band in the control line and a faint band in the test line (Figure 3H). This LF readout accurately distinguished APL cases from controls, demonstrating its applicability in the diagnosis under resource-limited conditions.
RT-LAMP coupled with DETECTR assay to expedite turnaround time of RAPID-CRISPR
RNA extraction using TRIzol or kit-based columns from blood or BM and subsequent cDNA synthesis have well-established protocols. However, both steps are time-consuming and require specialized equipment, which limits the development of a rapid point-of-care assay suitable for field settings. To address this, we first optimized RT-LAMP reaction using a modified HUDSON protocol by using PB samples from 2 healthy individuals (controls) followed by DETECTR assay for GUSβ (Figure 4A-B). We then used diagnostic blood samples from 8 patients with APL and 5 controls to conduct RT-LAMP followed by RAPID-CRISPR assay (Figure 4C). We found that APL cases (n = 8; mean RFU = 1727) exhibited significantly higher signal intensity than controls (n = 5; mean RFU = 528; Figure 4C, P < .0001).
PML::RARA detection by RAPID-CRISPR assay using RT-LAMP to expedite the turnaround time of APL diagnosis. (A) Optimization of RT-LAMP using PB of 2 healthy individuals (H1 and H2) served as a control (no template control [NTC]). (B) Quantification of fluorescence of GUSβ by RAPID-CRISPR assay using 1-step RT-LAMP in control samples. (C) Quantification of fluorescence of PML::RARA by RAPID-CRISPR assay using RT-LAMP in APL cases (n = 8) and control (n = 5). The results are represented as a mean ± standard deviation; P < .0001. (D) LF-based readouts of 4 APL cases and 4 controls by RAPID-CRISPR assay using RT-LAMP.
PML::RARA detection by RAPID-CRISPR assay using RT-LAMP to expedite the turnaround time of APL diagnosis. (A) Optimization of RT-LAMP using PB of 2 healthy individuals (H1 and H2) served as a control (no template control [NTC]). (B) Quantification of fluorescence of GUSβ by RAPID-CRISPR assay using 1-step RT-LAMP in control samples. (C) Quantification of fluorescence of PML::RARA by RAPID-CRISPR assay using RT-LAMP in APL cases (n = 8) and control (n = 5). The results are represented as a mean ± standard deviation; P < .0001. (D) LF-based readouts of 4 APL cases and 4 controls by RAPID-CRISPR assay using RT-LAMP.
Additionally, we assessed the effectiveness of the RT-LAMP–coupled DETECTR assay with a LF strip readout. Using the RT-LAMP product from 4 APL cases and 4 controls, we performed the LbCas12a-coupled LF reporter cleavage reaction. The data revealed that LF readouts distinguished APL cases from controls (Figure 4D). These findings corroborate the data of the LAMP-coupled DETECTR obtained from APL cases collected retrospectively (Figure 3A,F). The nucleic acid extraction–free protocol combined with the 1-step RT-LAMP–based DETECTR followed by LF readout makes the RAPID-CRISPR assay suitable for diagnosing APL in point-of-care settings.
Discussion
The presence of PML::RARA is a diagnostic hallmark of APL. A diagnostic delay is highly fatal for patients with APL because of coagulopathy, leading to fatal hemorrhages and thromboses, with a reported ED rate of up to 30%.5,13 Early detection of PML::RARA may help to reduce ED rates, the last major barrier to successful targeted treatment of APL.5,14 Real-world data indicating high ED rates suggest that some patients in peripheral hospitals may succumb before reaching specialized cancer centers for diagnostic confirmation of PML::RARA. To address this, we have developed a highly sensitive RAPID-CRISPR assay to diagnose APL with bcr1, bcr2, or bcr3 isoforms with 100% sensitivity and specificity, suitable for use in smaller hospitals with limited resources. Furthermore, our blinded validation approach to detect PML::RARA in an unbiased manner provides an additional layer in the diagnostic precision of APL. The LOD for PML::RARA using the RAPID-CRISPR assay was 1 copy, whereas at least 10 copies are needed for detection by gold-standard RQ-PCR. This indicates that the RAPID-CRISPR assay is 10-times more sensitive than RQ-PCR.10,15
The molecular diagnosis using the CRISPR-Cas system has proven to be highly effective for infectious diseases, and its role in cancer diagnosis is rapidly emerging.7,8,16,17 Since the flexibility associated with the synthetic design of the target sequence, it has also been used to detect disease biomarkers such as transforming growth factor β1 and prostate-specific antigen.18,19
Recently CRISPR-based RNA-fusion transcript detection assay using SHERLOCK for diagnosing chronic myeloid leukemia, and epidermal growth factor receptor oncogenic alleles have been reported. The study demonstrated the utility of SHERLOCK in rapid, sensitive, and variant-specific detection of BCR::ABL1 and epidermal growth factor receptor alterations.20 The potential of SHERLOCK in cancer diagnosis was further validated using clinical samples presenting with PML::RARA or BCR::ABL1. The combination of isothermal fusion-specific reverse transcription-recombinase polymerase amplification and junction-specific crRNA-guided Cas13 detection in SHERLOCK achieves high target sensitivity and specificity.21 Wang et al22 have shown that CRISPR/LbCas12a activation via inter–G-quadruplex structures enhances the concentration of nuclease to efficiently cleave the synthetic bcr1 isoform of PML::RARA, which can be detected by spectrometry. As with SHERLOCK, our DETECTR-based RAPID-CRISPR assay has produced similar results, illustrating the versatility of the CRISPR-Cas system for the molecular diagnosis of cancer with fusion oncogenes. With recent advancements in activation time and steady-state trans cleavage rates of Cas12a, the performance of the diagnostic assays is expected to improve further in future studies.23
APL represents 1 of the best paradigms of successful targeted therapy using all-trans retinoic acid and arsenic trioxide, providing the first example of a chemotherapy-free cure for acute leukemia.24 Although ∼90% of APL cases harbor the bcr1 and bcr3 isoforms, identification of all 3 isoforms (bcr1, bcr2, and bcr3) at diagnosis is essential to predict any impending relapse by RQ-PCR during follow-up. After the first course of consolidation therapy, patients with molecular relapse confirmed in 2 consecutive samples will invariably relapse (hematologic) until additional therapy is given. Early intervention during molecular relapse improves both relapse-free survival and overall survival compared with treatment during hematologic relapse.25 This is, to our knowledge, the first study to report the use of CRISPR/Lbcas12–based DETECTR system for rapid and accurate identification of APL isoforms in an outpatient setting.
Acknowledgments
The authors thank all patients and healthy donors who were involved in this study, and central instrumentation facilities.
This study was funded by the Department of Atomic Energy, Government of India (Basic & Translational Research in Cancer; reference number 1/3(7)/2020/TMC/R&D-II/ 8823).
Authorship
Contribution: A.M., A.S., and R.K. performed the experiments and analyzed the data; J.V. performed reverse transcriptase qualitative and quantitative polymerase chain reaction; J.G. helped in statistical analyses; H.J., B.B., P.G.S., M.S., N.K., and N.P. recruited patients and provided clinical samples; S.K.H. and A.M. wrote the manuscript with inputs from A.S. and R.K.; S.K.H. designed and supervised the study, analyzed the data, and acquired funding; and all authors discussed the results and implications, and reviewed the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Syed K. Hasan, Advanced Centre for Treatment Research and Education in Cancer, Tata Memorial Centre, Department of Biopathology, Sector 22, Kharghar, Navi, Mumbai 410210; email: shasan@actrec.gov.in.
References
Author notes
A.M., A.S., and R.K. contributed equally to this study.
All the original data are available on request from the corresponding author, Syed K. Hasan (shasan@actrec.gov.in).
The full-text version of this article contains a data supplement.


![PML::RARA detection by RAPID-CRISPR assay using RT-LAMP to expedite the turnaround time of APL diagnosis. (A) Optimization of RT-LAMP using PB of 2 healthy individuals (H1 and H2) served as a control (no template control [NTC]). (B) Quantification of fluorescence of GUSβ by RAPID-CRISPR assay using 1-step RT-LAMP in control samples. (C) Quantification of fluorescence of PML::RARA by RAPID-CRISPR assay using RT-LAMP in APL cases (n = 8) and control (n = 5). The results are represented as a mean ± standard deviation; P < .0001. (D) LF-based readouts of 4 APL cases and 4 controls by RAPID-CRISPR assay using RT-LAMP.](https://ash.silverchair-cdn.com/ash/content_public/journal/bloodadvances/9/3/10.1182_bloodadvances.2024014539/4/m_blooda_adv-2024-014539-gr4.jpeg?Expires=1763460698&Signature=0OnGf5GcPWr8mgQ4ZEbQDHr6Er0PIS0wcfwGlj~Ipsww-lJa7N6LwGiLMVxgcLN79Ugg8Lkm-pM8mYeLGy4~edXPxZZtLYwsmIpJ2ZviEeX9RKx6saWKHOrK3NNruQSejFJaUkjak5VaMkjaF3Yx4iwphW~Bj3GiEAa~ug4-~weiJRPkUQ7vb7uRL6YzbZsj5YwjW7EKz0hLqnYmUMQKTbq2SksIvewHAGI4UwBFmdPMNiBXRx5ez~XXtz7INH7~yAY0x7JeFoEEr~Lbon7Ns9Tk2Kkw09BC~h-aanrDI-H9PdwgLqiOaeVn1bbMpMwB0u8Ba2dz5ZzRSD8yuo9z0A__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)