Key points
Early flow-based MRD dynamics are independently associated with RFS in patients with AML undergoing frontline therapy.
Patients with intermediate-risk AML who achieved early MRD negativity still benefit from allogeneic SCT.
Visual Abstract
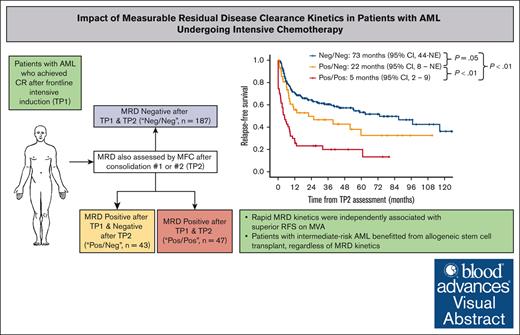
The prognostic impact of measurable residual disease (MRD) in acute myeloid leukemia (AML) is unequivocal; however, the optimal time point for achieving undetectable MRD is unclear. We retrospectively studied patients with newly diagnosed (ND) AML who achieved remission with frontline intensive chemotherapy and had MRD assessed by flow cytometry after induction (time point 1 [TP1]) and after cycles 2 or 3 (TP2). Cases were grouped into MRD negative (Neg)/Neg, positive (Pos)/Neg, or Pos/Pos at TP1 and TP2, respectively. Of 1980 patients with ND AML, 277 met the inclusion criteria and were included in this analysis. The median relapse-free survival (RFS) was 73 months, 22 months, and 5 months for the MRD Neg/Neg, Pos/Neg, and Pos/Pos groups, respectively (P < .01). There was a significant difference between the Neg/Neg and Pos/Neg groups (P = .05), suggesting benefit to early MRD negativity. The median overall survival (OS) was 81 months, 40 months, and 9 months, respectively (P < .01), but the difference between Neg/Neg and Pos/Neg was not statistically significant (P = .19). Landmark analysis demonstrated the benefit of stem cell transplant (SCT), particularly in Neg/Neg intermediate-risk AML (median RFS, not reached vs 15 months; P < .01). On multivariable analysis, MRD Pos/Neg was independently associated with a worse RFS than Neg/Neg (hazard ratio, 1.73; 95% confidence interval, 1.09-2.75; P = .02) but not for OS (P = .15). In conclusion, undetectable flow MRD after induction is associated with better RFS than undetectable MRD achieved later during consolidation. SCT benefited patients with intermediate-risk AML, regardless of MRD kinetics.
Introduction
Acute myeloid leukemia (AML) is a hematologic malignancy characterized by the proliferation of malignant myeloid blasts and the suppression of normal hematopoiesis. Patients are risk stratified at diagnosis by the presence of characteristic molecular and cytogenetic aberrations.1,2 Curative-intent treatment with intensive chemotherapy (IC) is the standard of care for fit patients,3 with complete remission (CR) achieved in 60% to 70%.4 Despite this, relapses are common, and 5-year outcomes remain suboptimal with standard treatments.5
Measurable residual disease (MRD) detection via multiparameter flow cytometry (MFC) or molecular assays is routinely assessed in AML and is predictive of overall survival (OS) and disease-free survival.6 Increasingly, MRD has been used to identify patients at low risk of relapse who may be cured without additional toxic therapies and those who may benefit from treatment intensification.7 Molecular assays for monitoring MRD in AML with fms related receptor tyrosine kinase 3 (FLT3) and nucleophosmin (NPM1) mutations and core-binding factor (CBF) AML have been developed and validated and are used to guide management in patients with access to molecular monitoring.8-10 However, most cases of AML lack genomic aberrations suitable for molecular MRD monitoring by current techniques.11
MRD in AML is commonly assessed by MFC, particularly for patients without a molecular MRD marker. Decisions based on MRD assessed by MFC can be challenging due to interobserver variability, lack of standardization, and uncertainty about the optimal time points at which undetectable MRD is optimally prognostic.12-14 Attainment of undetectable MRD by MFC has been shown to be predictive of reduced relapse risk and survival.15 Analysis of the UK AML17 trial demonstrated that positive (Pos) MRD detected by MFC at the end of both the first and second cycles of IC were predictive of poorer OS.16 However, it is unclear whether the timing at which undetectable MRD is achieved is independently predictive of outcomes, and guidance from the European LeukemiaNet (ELN) suggests MRD evaluation after the second course of treatment.12 In this study, we aim to address this question by studying the prognostic impact of the timing of MRD clearance assessed by MFC in intensively treated patients.
Methods
This was a retrospective study of patients with newly diagnosed AML treated with IC at The University of Texas MD Anderson Cancer Center (MDACC) between 2010 and 2021. Patients were included if they were in CR or CR with incomplete hematologic recovery (CRi) at the end of 1 cycle of induction (time point 1 [TP1]) and had flow MRD assessed with sufficient quality and interpretable data at both TP1 and after cycles 2 or 3 (TP2). At our institution, we treat most fit patients with a regimen consisting of idarubicin and dose-intensified cytarabine, with or without the addition of a purine analog and/or venetoclax (VEN).17-19 Consolidation cycles use the same drugs as in induction, with or without idarubicin, administered at lower doses.
Patients with CBF AML and acute promyelocytic leukemia were excluded because MRD for these subtypes is assessed via real-time quantitative polymerase chain reaction at our institution. Treated secondary AML (tsAML; AML arising from a myelodysplastic syndrome or chronic myelomonocytic leukemia with prior exposure to hypomethylating agents and/or chemotherapy) was excluded because these patients have very poor outcomes with a clinical course similar to that of relapsed or refractory AML.20,21
All patients had a bone marrow assessment at diagnosis. Cytogenetics were assessed via standard metaphase karyotype analysis, whereas a next-generation sequencing platform was used to identify gene mutations. Because the study period covered over a decade, the next-generation sequencing panel differed between cases. A 28-gene panel was used before 2017, with an 81-gene panel introduced thereafter, as previously reported.22 Cytomolecular risk was assigned per the 2022 ELN recommendations.1FLT3 mutations (internal tandem duplication [ITD] and tyrosine kinase domain) were assessed using polymerase chain reaction followed by capillary electrophoresis.
A comprehensive MFC panel was performed in all cases, comprising CD34, CD38, CD117, CD33, CD13, CD123, HLA-DR, myeloperoxidase, CD56, ±CD133, and CD54, monocytic markers (CD4 and CD64m ± CD36), and lymphoid antigens (CD2, CD5, CD7, and CD25).23 Our MFC MRD panel used between 8 and 12 colors over the 11-year study period. Antigen expression was evaluated qualitatively by experienced hematopathologists. MRD status was assessed by the integration of 2 methods: leukemia-associated immunophenotype (LAIP) and different from normal (DfN), with a validated sensitivity of 10–3 to 10–4, depending on sample quality and LAIP. Samples were labeled as indeterminate (Ind) if the sample was negative (Neg) for blasts with the LAIP but had CD34+ myeloid progenitors, which were DfN but not sufficient for a definitive diagnosis of AML MRD. These included alterations in the intensity of expression of CD34, CD13, CD117, CD38, CD54, HLA-DR, an abnormal CD13 vs CD33 expression pattern, and/or dim/partial expression of CD5, CD7, and/or CD56. Cases with insufficient events (<200 000 nucleated events and <200 CD34+ myeloid progenitors) for analysis were considered inadequate for MRD determination. MRD results at the end of induction (TP1) and after cycles 2 or 3, whichever was done first (TP2), were coded as Neg, Pos, or Ind. Patients with Neg or Pos MRD assessments at both time points were included in the outcomes analysis and categorized into 1 of 3 groups: Neg/Neg (MRD undetectable TP1 and TP2), Pos/Neg (MRD-Pos TP1 and undetectable TP2), or Pos/Pos (MRD-Pos TP1 and TP2).
Relapse-free survival (RFS) was defined as the time from TP2 bone marrow assessment to relapse or death from any cause. OS was defined as the time from TP2 bone marrow assessment to death from any cause. Statistical analysis was done with R: a language and environment for statistical computing, version 4.3.1 (R Core Team, R Foundation for Statistical Computing, Vienna, Austria). Categorical data were compared with the χ2 test. Continuous data were compared with the Kruskal-Wallis test. Survival curves were plotted using the Kaplan-Meier method and compared with the log-rank test. Multivariable analysis was done using a Cox proportional hazards model with time-dependent covariates.
Ethics approval for this study was obtained from the MDACC Institutional Review Board, and the study was conducted in accordance with the Declaration of Helsinki.
Results
From April 2010 to August 2021, a total of 1980 patients with newly diagnosed AML, excluding those with CBF, acute promyelocytic leukemia, or tsAML, were treated at MDACC (supplemental Figure 1). Of these, 850 were treated with IC, and 580 achieved CR/CRi after induction. Three hundred ninety-eight patients had flow MRD assessed at both TP1 and TP2. Overall, a total of 277 cases were classified as Neg/Neg (n = 187 [68%]), Pos/Neg (n = 43 [16%]), or Pos/Pos (n = 47 [17%]), with the remainder being patients with at least 1 inadequate/Ind MRD assessment (n = 105) or patients who relapsed at TP2 after initially attaining undetectable MRD (n = 16). In 184 patients (66%), TP2 was based on MRD assessment after the first consolidation cycle, whereas for the other 93 patients (34%), TP2 was based on MRD after the second consolidation.
Patient characteristics
The median age of the cohort was 53 years (range, 19-82), of whom 57 (21%) were aged ≥60 years. Forty-two (15%), 139 (50%), and 96 patients (35%) were classified as ELN favorable, intermediate, and adverse risk, respectively. Of the favorable-risk patients, 37 (88%) had NPM1mut AML; the remaining patients had CEBPA biallelic or basic leucine zipper domain mutations (CEBPAbi/BZIP). Among the adverse-risk patients, 47 (49%) had complex cytogenetics and/or TP53mut. Fifty-three patients (19%) were treated with IC+VEN; the remainder were treated with regimens without VEN. Purine analogues were administered in 217 patients (78%), typically as part of the cladribine, cytarabine, and idarubicin (CLIA) or fludarabine, cytarabine, filgrastim, and idarubicin (FLAG-IDA) regimens. No patients received “7+3” chemotherapy. Two hundred thirty-two patients (84%) were treated on clinical trials. Five patients in the Pos/Pos group received maintenance therapy after the completion of IC, 4 of whom were still MRD Pos at the time of initiating maintenance therapy.
Baseline characteristics stratified by MRD status are shown in Table 1. Pos/Pos patients were more likely to be aged ≥60 years (38% vs 18% and 12% for Neg/Neg and Pos/Neg, respectively) and have adverse-risk disease (68% vs 25% and 42% for Neg/Neg and Pos/Neg, respectively). They were also more likely to have TP53mut (23%) and ASXL1mut (17%). Neg/Neg patients were more likely to have NPM1mut (36% vs 21% and 13% for Pos/Neg and Pos/Pos, respectively) and FLT3ITD (28% vs 19% and 17% for Pos/Neg and Pos/Pos, respectively).
Baseline characteristics
| . | Overall (N = 277) . | Neg/Neg (n = 187) . | Pos/Neg (n = 43) . | Pos/Pos (n = 47) . | P value . |
|---|---|---|---|---|---|
| Age, y | |||||
| Median (Min, Max) | 53 (19, 82) | 51 (19, 78) | 51 (27, 72) | 57 (20, 82) | <.01 |
| ≥60 | 57 (21%) | 34 (18%) | 5 (12%) | 18 (38%) | <.01 |
| ELN 2022 risk | |||||
| Favorable | 42 (15%) | 31 (17%) | 8 (19%) | 3 (6%) | <.01 |
| Intermediate | 139 (50%) | 110 (59%) | 17 (40%) | 12 (26%) | |
| Adverse | 96 (35%) | 46 (25%) | 18 (42%) | 32 (68%) | |
| Treatment | |||||
| IC | 224 (81%) | 153 (82%) | 30 (70%) | 41 (87%) | .19 |
| IC+VEN | 53 (19%) | 34 (18%) | 13 (30%) | 6 (13%) | |
| Cytogenetics | |||||
| Diploid | 142 (51%) | 106 (57%) | 22 (51%) | 14 (30%) | <.01 |
| Adverse (non-complex) | 19 (7%) | 7 (4%) | 4 (9%) | 8 (17%) | |
| Complex | 43 (16%) | 22 (12%) | 7 (16%) | 14 (30%) | |
| Others | 73 (26%) | 52 (28%) | 10 (23%) | 11 (23%) | |
| Mutations | |||||
| ≥1 adverse∗ | 64 (23%) | 29 (16%) | 12 (28%) | 23 (49%) | <.01 |
| ASXL1 | 17 (6%) | 6 (3%) | 3 (7%) | 8 (17%) | .02 |
| CEBPAbi/BZIP | 9 (3%) | 7 (4%) | 1 (2%) | 1 (2%) | .01 |
| FLT3ITD | 68 (25%) | 52 (28%) | 8 (19%) | 8 (17%) | <.01 |
| IDH1 | 17 (6%) | 10 (5%) | 4 (9%) | 3 (6%) | .80 |
| IDH2 | 38 (14%) | 31 (17%) | 5 (12%) | 2 (4%) | .13 |
| NPM1 | 83 (30%) | 68 (36%) | 9 (21%) | 6 (13%) | <.01 |
| NRAS | 30 (11%) | 20 (11%) | 8 (19%) | 2 (4%) | .18 |
| PTPN11 | 20 (7%) | 10 (5%) | 6 (14%) | 4 (9%) | .18 |
| RUNX1 | 19 (7%) | 11 (6%) | 2 (5%) | 6 (13%) | .60 |
| TP53 | 21 (8%) | 7 (4%) | 3 (7%) | 11 (23%) | <.01 |
| . | Overall (N = 277) . | Neg/Neg (n = 187) . | Pos/Neg (n = 43) . | Pos/Pos (n = 47) . | P value . |
|---|---|---|---|---|---|
| Age, y | |||||
| Median (Min, Max) | 53 (19, 82) | 51 (19, 78) | 51 (27, 72) | 57 (20, 82) | <.01 |
| ≥60 | 57 (21%) | 34 (18%) | 5 (12%) | 18 (38%) | <.01 |
| ELN 2022 risk | |||||
| Favorable | 42 (15%) | 31 (17%) | 8 (19%) | 3 (6%) | <.01 |
| Intermediate | 139 (50%) | 110 (59%) | 17 (40%) | 12 (26%) | |
| Adverse | 96 (35%) | 46 (25%) | 18 (42%) | 32 (68%) | |
| Treatment | |||||
| IC | 224 (81%) | 153 (82%) | 30 (70%) | 41 (87%) | .19 |
| IC+VEN | 53 (19%) | 34 (18%) | 13 (30%) | 6 (13%) | |
| Cytogenetics | |||||
| Diploid | 142 (51%) | 106 (57%) | 22 (51%) | 14 (30%) | <.01 |
| Adverse (non-complex) | 19 (7%) | 7 (4%) | 4 (9%) | 8 (17%) | |
| Complex | 43 (16%) | 22 (12%) | 7 (16%) | 14 (30%) | |
| Others | 73 (26%) | 52 (28%) | 10 (23%) | 11 (23%) | |
| Mutations | |||||
| ≥1 adverse∗ | 64 (23%) | 29 (16%) | 12 (28%) | 23 (49%) | <.01 |
| ASXL1 | 17 (6%) | 6 (3%) | 3 (7%) | 8 (17%) | .02 |
| CEBPAbi/BZIP | 9 (3%) | 7 (4%) | 1 (2%) | 1 (2%) | .01 |
| FLT3ITD | 68 (25%) | 52 (28%) | 8 (19%) | 8 (17%) | <.01 |
| IDH1 | 17 (6%) | 10 (5%) | 4 (9%) | 3 (6%) | .80 |
| IDH2 | 38 (14%) | 31 (17%) | 5 (12%) | 2 (4%) | .13 |
| NPM1 | 83 (30%) | 68 (36%) | 9 (21%) | 6 (13%) | <.01 |
| NRAS | 30 (11%) | 20 (11%) | 8 (19%) | 2 (4%) | .18 |
| PTPN11 | 20 (7%) | 10 (5%) | 6 (14%) | 4 (9%) | .18 |
| RUNX1 | 19 (7%) | 11 (6%) | 2 (5%) | 6 (13%) | .60 |
| TP53 | 21 (8%) | 7 (4%) | 3 (7%) | 11 (23%) | <.01 |
Max, maximum; Min, minimum.
One or more adverse mutation as defined by the ELN2022 criteria.1
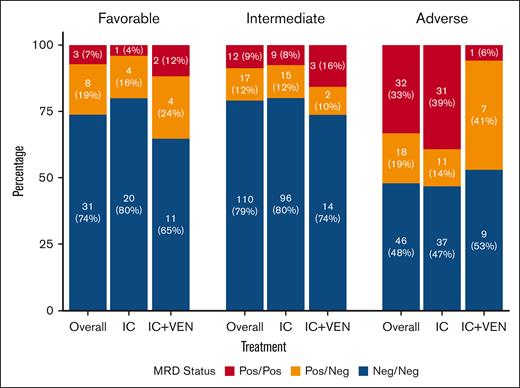
Favorable- and intermediate-risk patients were more likely to be MRD Neg/Neg, whereas adverse-risk patients were more likely to be Pos/Pos. Rates of MRD Neg/Neg for favorable-, intermediate-, and adverse-risk patients were 74%, 79%, and 48%, respectively. Thirty-three percent of adverse-risk patients were Pos/Pos vs 7% and 9% for favorable- and intermediate-risk patients, respectively. MRD responses by ELN risk and treatment type are shown in Figure 1. In the adverse-risk category, 50 (52%) were MRD Pos after induction, and 18 (19%) became MRD Neg by TP2. The proportion of patients who achieved MRD negativity by TP2 was higher in those treated with IC+VEN (7/8 [88%]) vs IC (11/42 [26%]; P < .01).
MRD responses by subgroups. MRD responses stratified by ELN2022 risk category and treatment. ELN2022, ELN 2022 classification.
MRD responses by subgroups. MRD responses stratified by ELN2022 risk category and treatment. ELN2022, ELN 2022 classification.
RFS and OS
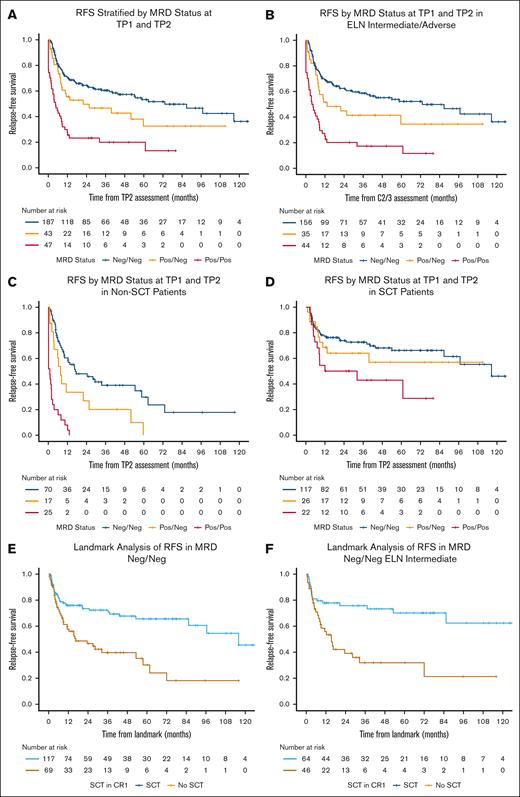
The median follow-up time in surviving patients was 46 months (95% confidence interval [CI], 42-58). Patients in the Neg/Neg, Pos/Neg, and Pos/Pos groups had a median RFS of 73 months (95% CI, 44 to not estimable [NE]), 22 months (95% CI, 8 to NE), and 5 months (95% CI, 2-9), respectively (P < .01; Figure 2A). Pairwise log-rank tests showed a significant difference in RFS between the Neg/Neg and Pos/Neg (P = .05), Neg/Neg and Pos/Pos (P < .01), and Pos/Neg and Pos/Pos (P < .01) groups. The 5-year RFS rates were 53% (95% CI, 45-63), 33% (95% CI, 19-56), and 13% (95% CI, 5-36) for the Neg/Neg, Pos/Neg, and Pos/Pos groups, respectively.
RFS. (A-F) RFS curves are shown for all patients, stratified by MRD status at TP1 and TP2 (A); ELN intermediate or adverse risk, stratified by MRD status at TP1 and TP2 (B); non-SCT recipients, stratified by MRD status at TP1 and TP2 (C); SCT recipients, stratified by MRD status at TP1 and TP2 (D); landmark analysis of RFS in MRD Neg/Neg patients, stratified by SCT in CR1 (E); and landmark analysis of RFS in ELN intermediate-risk patients who were MRD Neg/Neg, stratified by SCT in CR1 (F).
RFS. (A-F) RFS curves are shown for all patients, stratified by MRD status at TP1 and TP2 (A); ELN intermediate or adverse risk, stratified by MRD status at TP1 and TP2 (B); non-SCT recipients, stratified by MRD status at TP1 and TP2 (C); SCT recipients, stratified by MRD status at TP1 and TP2 (D); landmark analysis of RFS in MRD Neg/Neg patients, stratified by SCT in CR1 (E); and landmark analysis of RFS in ELN intermediate-risk patients who were MRD Neg/Neg, stratified by SCT in CR1 (F).
The median OS was 81 months (95% CI, 54 to NE), 40 months (95% CI, 22 to NE), and 9 months (95% CI, 6-30) for Neg/Neg, Pos/Neg, and Pos/Pos patients, respectively (P < .01; supplemental Figure 2A). The difference between Neg/Neg and Pos/Neg was not significant (P = .19) but was significant between Neg/Neg and Pos/Pos (P < .01) and Pos/Neg and Pos/Pos (P < .01). The 5-year OS was 54% (95% CI, 46-64), 48% (95% CI, 34-68), and 28% (95% CI, 17-46) for the Neg/Neg, Pos/Neg, and Pos/Pos groups, respectively.
Impact of MRD clearance kinetics in subgroups analyses
Age
Early attainment of undetectable MRD was discriminatory for RFS in patients aged <60 years but not in patients aged ≥60 years (supplemental Figure 2A). In patients aged <60 years, the median RFS was 97 months (95% CI, 54 to NE), 40 months (95% CI, 8 to NE), and 4 months (95% CI, 2-12; P < .01) for Neg/Neg, Pos/Neg, and Pos/Pos cases, respectively. RFS differed significantly between the Neg/Neg and Pos/Neg (P = .04), Neg/Neg and Pos/Pos (P < .01), and Pos/Neg and Pos/Pos groups (P = .01). In patients aged ≥60 years, the median RFS was 24 months (95% CI, 6 to NE), 22 months (95% CI, 8 to NE), and 5 months (95% CI, 2-13) for Neg/Neg, Pos/Neg, and Pos/Pos cases, respectively (P = .02), but there was no significant difference between Neg/Neg and Pos/Neg groups (P = .71).
Cytomolecular risk
MRD dynamics influenced RFS in intermediate- and adverse-risk AML (Figure 2B). For intermediate-risk patients, the median RFS was 87 months (95% CI, 33 to NE) for Neg/Neg, 11 months (95% CI, 6 to NE) for Pos/Neg, and 6 months (95% CI, 2 to NE) for Pos/Pos groups (P < .01). For adverse-risk patients, the median RFS was 59 months (95% CI, 21 to NE), 13 months (95% CI, 8 to NE), and 4 months (95% CI, 2-9) for Neg/Neg, Pos/Neg, and Pos/Pos groups, respectively (P < .01). In contrast, MRD dynamics had no influence on RFS for favorable-risk patients (P = .52).
For patients with TP53mut or complex cytogenetics, the median RFS in Neg/Neg patients (which accounted for 24/47 [51%] of responders) was 38 months (95% CI, 9 to NE), compared with 8 months (95% CI, 2 to NE) and 2 months (95% CI, 0-8) for Pos/Neg and Pos/Pos patients, respectively (P < .01; supplemental Figure 2C). The pairwise log-rank test showed no statistically significant difference between Neg/Neg and Pos/Neg groups (P = .16).
Type of induction therapy
MRD clearance dynamics were predictive of RFS in patients treated with IC+VEN but not with IC alone (supplemental Figure 2B). In those treated with IC+VEN, the median RFS was not reached (NR) for the Neg/Neg and Pos/Neg groups and 18 months (95% CI, 7 to NE) in the Pos/Pos group (P = .03). There was a significant difference between the Neg/Neg and Pos/Neg groups who received IC+VEN (P = .03). For patients treated with IC without VEN, the median RFS was 59 months (95% CI, 29 to NE), 13 months (95% CI, 9 to NE), and 4 months (95% CI, 2-9) for Neg/Neg, Pos/Neg, and Pos/Pos groups, respectively (P < .01), but the difference between the Neg/Neg and Pos/Neg groups was not statistically significant (P = .09). Neg/Neg patients treated with IC+VEN had particularly favorable outcomes and a significantly longer RFS than those treated with IC (2-year RFS, 88% [95% CI, 76-100] vs 60% [95% CI, 53-69]; P = .02).
Allogeneic SCT
The proportion of patients undergoing allogeneic stem cell transplant (SCT) in first CR was similar between the MRD groups (63%, 61%, and 47% in the Neg/Neg, Pos/Neg, and Pos/Pos groups, respectively; P = .27). MRD dynamics were predictive of RFS in patients who did not undergo transplant but not in patients who received transplant. In patients who did not undergo transplant, the median RFS was 17 months (95% CI, 10-59), 8 months (95% CI, 4 to NE), and 1 month (95% CI, 0-3) for Neg/Neg, Pos/Neg, and Pos/Pos groups, respectively (P < .01; Figure 2C). Among patients who did not undergo transplant, there was a significant difference between Neg/Neg and Pos/Neg (P = .02), Neg/Neg and Pos/Pos (P < .01), and Pos/Neg and Pos/Pos groups (P < .01). In patients who received transplant, the median RFS was 117 months (95% CI, 87 to NE), NR (95% CI, 13 to NE), and 22 months (95% CI, 8 to NE) for the Neg/Neg, Pos/Neg, and Pos/Pos groups, respectively (P = .03; Figure 2D). However, the difference between the Neg/Neg and Pos/Neg groups was not statistically significant (P = .41).
OS subgroup analyses
OS curves stratified by MRD responses and age, ELN risk, and treatment are shown in supplemental Figure 3. MRD was discriminatory of outcomes in patients aged <60 years (P < .01; supplemental Figure 3B), intermediate- or adverse-risk AML (P < .01; supplemental Figure 3C), and patients who received either frontline IC (P < .01) or IC+VEN (P = .05; supplemental Figure 3D). There was no difference in OS between Neg/Neg and Pos/Neg groups in any subgroup, except in those treated with IC+VEN (P = .04).
Impact of allogeneic SCT
To further clarify the potential benefit of allogeneic SCT in different subgroups, we performed a landmark analysis comparing patients who received transplant vs those who did not. The landmark time was the median time from TP2 to SCT (1.07 months). Landmark analysis demonstrated an RFS benefit of SCT in first remission across all analyzed subgroups, regardless of MRD dynamics. In the entire cohort, the median RFS was 116 months (95% CI, 85 to NE) for patients who received transplant vs 11 months (95% CI, 8-25) for patients who did not receive transplant (P < .01; supplemental Figure 4A). Patients who were MRD Neg/Neg also benefited from SCT (P < .01; Figure 2E), even when ELN favorable-risk AML was excluded (P < .01; supplemental Figure 4B). The benefit of SCT was also observed when the analysis was limited only to Neg/Neg intermediate-risk patients, a group in which SCT decisions are often individualized (median RFS, NR [95% CI, 85 to NE] for SCT vs 15 months [95% CI, 9 to NE] for no SCT; P < .01; Figure 2F).
Allogeneic SCT was also associated with better OS in the entire cohort (median OS, 98 months [95% CI, 59 to NE] for patients who received transplant vs 25 months [95% CI, 15-45] for patients who did not receive transplant; P < .01; supplemental Figure 4C) and intermediate- or adverse-risk patients (P < .01). When restricted to only MRD Neg/Neg intermediate- or adverse-risk patients, SCT was still beneficial (P < .01; supplemental Figure 4D). MRD Neg/Neg ELN intermediate-risk patients also benefited from SCT (median OS, NR [95% CI, 85 to NE] for SCT vs 28 months [95% CI, 20 to NE] for non-SCT; P < .01).
Multivariable analysis
For the multivariable analysis, the adverse cytomolecular risk group was separated into those with adverse-risk disease but without TP53mut or complex cytogenetics and those who had TP53mut and/or complex cytogenetics, because the latter group of patients have particularly poor outcomes.24-26 The univariable and multivariable analyses for RFS are shown in Table 2. On multivariable analysis, early attainment of undetectable MRD was independently associated with improved RFS. Relative to the Neg/Neg group, the hazard ratio (HR) for relapse or death was 1.73 (95% CI, 1.09-2.75; P = .02) for the Pos/Neg group and 3.67 (95% CI, 2.35-5.76; P < .01) for the Pos/Pos group. Other variables that influenced RFS were SCT in first remission (HR, 0.41; 95% CI, 0.28-0.60; P < .01), treatment with IC+VEN (HR, 0.47; 95% CI, 0.24-0.90; P = .02), and the presence of TP53mut and/or complex cytogenetics (HR, 2.58; 95% CI, 1.34-4.98; P < .01).
Univariable and multivariable analysis for RFS
| Characteristic . | Univariable . | Multivariable . | ||||||
|---|---|---|---|---|---|---|---|---|
| N . | Event, n . | HR . | 95% CI . | P value . | HR . | 95% CI . | P value . | |
| Age, y | 277 | 140 | ||||||
| <60 | Ref | Ref | ||||||
| ≥60 | 1.82 | 1.24-2.65 | <.01 | 1.17 | 0.77-1.77 | .45 | ||
| ELN 2022 | 277 | 140 | ||||||
| Favorable | Ref | Ref | ||||||
| Intermediate | 1.30 | 0.74-2.28 | .36 | 0.98 | 0.50-1.90 | .95 | ||
| Adverse (non-complex, TP53WT) | 1.35 | 0.70-2.60 | .37 | 1.00 | 0.50-2.02 | 1.00 | ||
| Adverse (complex and/or TP53mut) | 2.81 | 1.54-5.14 | <.01 | 2.58 | 1.34-4.98 | <.01 | ||
| Treatment | 277 | 140 | ||||||
| IC | Ref | Ref | ||||||
| IC+VEN | 0.37 | 0.20-0.71 | <.01 | 0.47 | 0.24-0.90 | .02 | ||
| MRD status | 277 | 140 | ||||||
| Neg/Neg | Ref | Ref | ||||||
| Pos/Neg | 1.56 | 0.98-2.46 | .058 | 1.73 | 1.09-2.75 | .02 | ||
| Pos/Pos | 3.65 | 2.46-5.40 | <.01 | 3.67 | 2.35-5.76 | <.01 | ||
| SCT in first remission | 277 | 140 | 0.46 | 0.32-0.66 | <.01 | 0.41 | 0.28-0.60 | <.01 |
| Characteristic . | Univariable . | Multivariable . | ||||||
|---|---|---|---|---|---|---|---|---|
| N . | Event, n . | HR . | 95% CI . | P value . | HR . | 95% CI . | P value . | |
| Age, y | 277 | 140 | ||||||
| <60 | Ref | Ref | ||||||
| ≥60 | 1.82 | 1.24-2.65 | <.01 | 1.17 | 0.77-1.77 | .45 | ||
| ELN 2022 | 277 | 140 | ||||||
| Favorable | Ref | Ref | ||||||
| Intermediate | 1.30 | 0.74-2.28 | .36 | 0.98 | 0.50-1.90 | .95 | ||
| Adverse (non-complex, TP53WT) | 1.35 | 0.70-2.60 | .37 | 1.00 | 0.50-2.02 | 1.00 | ||
| Adverse (complex and/or TP53mut) | 2.81 | 1.54-5.14 | <.01 | 2.58 | 1.34-4.98 | <.01 | ||
| Treatment | 277 | 140 | ||||||
| IC | Ref | Ref | ||||||
| IC+VEN | 0.37 | 0.20-0.71 | <.01 | 0.47 | 0.24-0.90 | .02 | ||
| MRD status | 277 | 140 | ||||||
| Neg/Neg | Ref | Ref | ||||||
| Pos/Neg | 1.56 | 0.98-2.46 | .058 | 1.73 | 1.09-2.75 | .02 | ||
| Pos/Pos | 3.65 | 2.46-5.40 | <.01 | 3.67 | 2.35-5.76 | <.01 | ||
| SCT in first remission | 277 | 140 | 0.46 | 0.32-0.66 | <.01 | 0.41 | 0.28-0.60 | <.01 |
Ref, reference; TP53mut, mutated TP53; TP53WT, wildtype TP53.
Boldface indicates P values < 0.05 are considered significant.
For OS, the HR for death was 1.45 (95% CI, 0.88-2.38; P = .15) for the Pos/Neg group and 2.46 (95% CI, 1.54-3.92; P < .01) for the Pos/Pos group (supplemental Table 5). Other variables that influenced OS were SCT in first remission (HR, 0.55; 95% CI, 0.37-0.80; P < .01), age (HR, 1.63; 95% CI, 1.08-2.46; P = .02), and the presence of TP53mut and/or complex cytogenetics (HR, 2.95; 95% CI, 1.47-5.92; P < .01).
Similar results were obtained when the multivariable analysis was repeated but included only patients who had an MRD assessment after the first consolidation cycle (n = 184). Using only data from induction and the end of the first consolidation cycle, early attainment of undetectable MRD remained significant for RFS (HR, 2.08; 95% CI, 1.20-3.63; P = .01) but not for OS (HR, 1.51; 95% CI, 0.84-2.72; P = .17).
Discussion
In this study, we demonstrate that in intensively treated patients in first remission, early attainment of undetectable MRD by MFC at the end of induction is independently associated with improved RFS. Patients with delayed achievement of undetectable MRD after 1 to 2 cycles of consolidation had worse RFS than patients who were MRD undetectable after induction. Neg/Neg MRD status was most discriminatory in patients aged <60 years, those treated with IC+VEN, and those who did not undergo allogeneic SCT in first remission. Although Neg/Neg MRD status was associated with better RFS, it was not sufficient to identify which patients should proceed to SCT in first remission, because patients with intermediate-risk AML who achieved undetectable MRD after induction still benefited from consolidative SCT.
Our results are consistent with other studies that demonstrated improved outcomes with the attainment of early undetectable MRD. In the Dutch-Belgian Hemato-Oncology Cooperative Group/Swiss Group for Clinical Cancer Research AML 42A study, centralized, prospective assessment of MRD by MFC (sensitivity, 0.1%) was performed.27 Exploratory analysis in 89 patients showed that patients who were MRD Neg after cycle 1 and cycle 2 had the lowest cumulative incidence of relapse, compared with those who achieved MRD negativity later or not at all (P < .001). An analysis of 85 patients treated at the Fred Hutchinson has also shown that undetectable MRD by MFC at the end of induction is associated with a lower cumulative incidence of relapse.28 For this study, any measurable MRD was considered Pos. Similarly, although not a direct comparison, a multivariable analysis of 120 patients treated with fludarabine, cytarabine, and idarubicin demonstrated that undetectable MRD by MFC TP1 was the strongest predictor of relapse.29 MRD status after cycle 2 (ie, the first cycle of consolidation) was not significant when TP1 status was included, but it should be noted that the threshold for MRD in this study was 25 clustered leukemic cells per 105 events (0.025%).
Attainment of undetectable MRD in AML has been consistently associated with improved outcomes in patients treated both intensively and nonintensively.6,12,30,31 However, the optimal time point at which MRD is prognostic, especially for AML subtypes in which there is no molecular marker to track MRD, is not clearly defined. Consensus recommendations suggest that MRD, when assessed by MFC, should be assessed after 2 courses of treatment.12 This was based, in part, on a retrospective analysis of 100 intensively treated patients enrolled on the European Organisation for Research and Treatment of Cancer/Gruppo Italiano Malattie EMatologiche dell’Adulto AML-10, AML-12, and AML-13 trials.32 The study found that MRD status by MFC after consolidation (using a cutoff of ≥3.5 × 10–4 leukemic cells) was the most significant predictor of outcome, regardless of MRD status after induction. Differences from our study include the following: (1) the immunophenotypic LAIP was assessed by 3- or 4-color MFC; (2) a lower proportion of patients with undetectable MRD at the end of induction (35% vs 68% in our study); (3) a higher proportion of patients with undetectable MRD at the end of induction who subsequently became positive after consolidation (25% vs only 4% in our study); and (4) a higher proportion of patients undergoing autologous SCT instead of allogeneic SCT (whereas all patients who received a transplant in our study underwent allogeneic SCT). Our MFC MRD panel used between 8 and 12 colors over the 11-year study period.23 This likely improved detection limits and LAIP specificity and may explain the finding of discrepant MRD results at various time points.
In our analysis, patients who achieved MRD negativity after induction had significantly longer RFS than the Pos/Neg and Pos/Pos groups. This was independent of other risk factors and treatments on multivariable analysis. This indicates that early attainment of undetectable MRD is an important biomarker for prognostication in AML. Biologically, this may reflect disease that is more chemotherapy sensitive, especially in patients treated with more intensive IC+VEN regimens. Such cases may attain more rapid, deeper responses with frontline treatments, which is associated with better outcomes.26 However, although the achievement of MRD negativity was associated with superior OS, the early MRD clearance kinetics did not significantly affect OS in our analysis. This suggests that selected patients with poor MRD clearance kinetics may be successfully salvaged, reducing the magnitude of the OS benefit.
The poor outcomes of patients in the Pos/Neg and Pos/Pos groups suggests that this group should be targeted for treatment intensification, and that intensification should be prioritized early in the treatment course. For patients who are SCT candidates, we showed that SCT can overcome the poor prognostic effect of MRD clearance kinetics. Such patients should proceed to SCT as quickly as possible. For patients who are not candidates for SCT, delayed MRD clearance is predictive of poorer RFS. Available evidence suggests that changes in treatments have the potential to overcome adverse effects of MRD. In the UK AML18 trial, intensification with FLAG-IDA or daunorubicin, cytarabine, and cladribine was associated with a significant OS benefit over a second course of standard 3+7 in patients who were MRD Pos at the end of induction.7 MRD may also be targeted by novel agents33-36; these in combination with chemotherapy may represent a future strategy to improve outcomes for MRD-Pos patients, especially those who are ineligible for SCT or may not be able to tolerate treatment intensification.
This is one of the largest studies, to our knowledge, of the kinetics of MRD responses in AML. However, there are some limitations. We included only patients in CR/CRi after induction. The degree to which our results can be extrapolated to patients with suboptimal responses such as partial response or morphologic leukemia-free state, and to subgroups that were excluded from this study, such as tsAML and CBF AML, is uncertain. In addition, we selected only patients with clear Pos/Neg MRD status for analysis. The outcomes of patients with Ind MRD (including patients with a DfN immunophenotype without a clear LAIP) and the kinetics of such responses should be explored in further studies. Lastly, most favorable-risk patients had NPM1mut AML. MFC MRD monitoring may not be as discriminatory as molecular monitoring of NPM1, which is highly predictive of outcomes in this group of patients.37 Over the period this study was conducted, high sensitivity molecular NPM1 or FLT3 MRD was not routinely available at our institution, limiting our ability to interrogate its utility with respect to MFC. Lastly, because relatively few patients had MRD assessment performed after both the first (n = 184) and second (n = 93) cycles of consolidation, we combined these for analysis (TP2). We were, therefore, unable to differentiate the impact of attaining MRD negativity after the first vs second consolidation or after induction and the first consolidation.
In conclusion, we demonstrate that the kinetics of MRD responses in intensively treated patients with AML are prognostic of RFS, independent of baseline cytomolecular risk profile, addition of VEN, and use of consolidative SCT. Early MRD kinetics did not significantly affect OS, which was driven primarily by age, cytomolecular risk, and SCT consolidation. However, despite the prognostic value of contemporary MFC-based MRD, it is not sufficiently sensitive to guide further SCT decisions, because patients benefited from SCT regardless of MRD kinetics. MRD dynamics may instead be a signal of when treatment intensification is warranted. Further research is warranted to develop more sensitive markers of low-level disease in AML, which could be used to identify patients who may be cured without SCT.
Acknowledgment
This research is supported in part by MD Anderson Cancer Center Leukemia Specialized Programs of Research Excellence grant CA100632.
Authorship
Contribution: N.J.S. and W.-Y.J. designed the study; S.P. and C.N. collected the data; W.-Y.J., K.S., and N.J.S. analyzed the data; W.-Y.J. drafted the manuscript; N.J.S., C.D.D., S.L., S.A.W., W.W., H.A.A., and F.R. revised the manuscript; and all authors reviewed and approved the final version of the manuscript.
Conflict-of-interest disclosure: S.L. has received research funding from Amgen and Astellas; consulting fees from Guidepoint, QualWorld, Gerson Lehrman Group, AbbVie, Daiichi Sankyo, Blueprint Medicine, Caris Diagnostics, Recordati, AlphaSights, Arima, Qiagen, and Cogent Biosciences; and has stock in AbbVie. N.J.S. has received consulting fees from Pfizer Inc, GlaxoSmithKline, Nkarta, Autolus, and Sanofi; research funding from Takeda Oncology, Astellas Pharma Inc, Xencor, Stemline Therapeutics, and NextCure; and honoraria from Adaptive Biotechnologies, Novartis, Amgen, Takeda Oncology, Pfizer Inc, Astellas Pharma Inc, Sanofi, and BeiGene. The remaining authors declare no competing financial interests.
Correspondence: Nicholas J. Short, Department of Leukemia, The University of Texas MD Anderson Cancer Center, 1515 Holcombe Blvd, Houston, TX 77030; email: nshort@mdanderson.org.
References
Author notes
The study data are not publicly available to respect participant confidentiality. Requests for sharing of deidentified data should be directed to the corresponding author, Nicholas J. Short (nshort@mdanderson.org).
The full-text version of this article contains a data supplement.