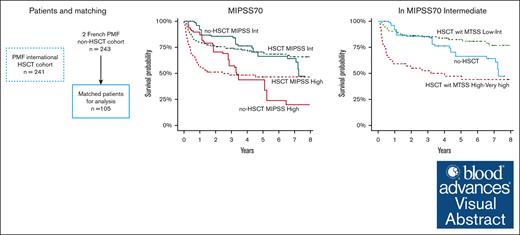
Key Points
Patients with high MIPSS70 scores benefit from transplantation.
Patients with intermediate MIPSS70 scores may benefit from transplantation if their MTSS score is low or intermediate.
Visual Abstract
The aim of our study was to analyze the potential survival benefit associated with hematopoietic stem cell transplantation (HSCT) according to clinicobiological scores, which incorporate mutation-enhanced international prognostic score system (MIPSS) to facilitate decision-making in this context. One transplant (n = 241) and 1 nontransplant cohort (n = 239) were used to test the hypothesis that patients with primary myelofibrosis with higher risk molecular score benefit from HSCT. A weighted propensity score was applied to balance confounding factors with the transplanted cohort as reference. Weighted Cox proportional hazard models and logistic regression analyses were performed. Overall, 105 patients who did not receive transplant could be matched to the 239 patients who did receive transplants. HSCT was associated with a higher 6-year overall survival rate in intermediate-2 (60.1% vs 41.5%) and high-risk DIPSS patients (44.4% vs 6.55%), high-risk MIPSS70 (46.5% vs 23.9%), high-risk (73.2% vs 39.7%) or very high-risk MIPSS70+V2 (51.8% vs 24%). Patients with intermediate MIPSS70 scores have an advantage of survival with HSCT only when their myelofibrosis transplant scoring system (MTSS) were low or intermediate. Patients who received transplant had an increased mortality risk the first year, but a significant benefit with HSCT after the 1-year landmark was observed in higher risk patients. This study confirms that, similar to DIPSS, MIPSS70 and MIPSS70+V2 risk score in addition to MTSS can be used to determine which patients with primary myelofibrosis have survival benefit from HSCT over non-HSCT strategies.
Introduction
Primary myelofibrosis (PMF) is a chronic hematologic malignancy characterized by progressive marrow fibrosis, myeloproliferation, and myeloid metaplasia leading to constitutional symptoms, hepatomegaly, splenomegaly, and cytopenia. Several classifications have been developed over time to predict overall survival (OS) and the risk of transformation into acute myeloid leukemia.1-5 The Dynamic International Prognostic Scoring System (DIPSS) and DIPSS Plus, which includes cytogenetics, have been largely used and validated.1,2
The recent advances in molecular biology has clarified that PMF is characterized by frequent exclusive driver mutations (JAK2V617, MPL, and CALR) involved in the pathophysiology of the disease as well as other subclonal pathogenic mutations.6,7 Knowledge of these driver mutations has led to the development of a new therapeutic class based on JAK inhibition, with ruxolitinib being the first approved for PMF.8
More recently, the scores have included additional mutations and are increasingly used to stratify patients and select treatment accordingly.3-5 Indeed, this is the balance between treatment risk and disease risk, which helps physicians make therapeutic decisions. Allogeneic hematopoietic stem cell transplantation (HSCT) is the only curative therapy for patients with PMF, but the risk of nonrelapse mortality limits its use to the more fit patients and the younger patients whereas PMF generally occurs in older patients aged >60 years (median age at diagnosis: 65 years). Main risk factors for posttransplantation mortality are patient age, type of donor (HLA mismatched), the physical performance status before transplantation, comorbidities, and disease-related risks.9-11 Specific scores have been developed to estimate posttransplant outcome in patients with PMF including main pre-HSCT risk factors, and, among them, the myelofibrosis transplant scoring system (MTSS) is able to classify patients in several prognostic categories.9,10,12 Severe acute graft-versus-host disease and nonengraftment or rejection are major risk factors but are not clearly predictable before HSCT.13 More than half of transplant recipients with PMF can be cured, depending on their risk factors.9,10,14-16 However, knowing whom and when to transplant remains controversial. Efforts have previously been made to determinate which DIPSS scores may benefit from transplantation.17 Based on registry data, it has been reported that patients who are at low and intermediate-1 (int-1) risk by DIPSS do not achieve a survival benefit from transplantation whereas those at intermediate-2 (int-2) and high risk, do.18 Of note, these patients were aged <65 years. Another international study confirmed the previous findings in a population who was older and treated with ruxolitinib (30% in nontransplant recipients).19 In contrast, a meta-analysis of 9 retrospective studies using Markov models concluded that there was a benefit of HSCT in all categories, depending on the timing.20 To benefit from transplantation, lower-risk patients may have received transplantation later than patients with higher risk. On the basis of this literature, international recommendations are in favor of transplantation in fit patients who are DIPSS int-2 or high.21,22 In our current study, we have taken the opportunity of a French nontransplant cohort23,24 and an international transplant25 cohort, all with molecular annotations, to investigate the role of high molecular risk (HMR) in transplant decision.
Method
Patient selections
Data for consecutive patients who received transplantation between 2001 and 2022 were collected by means of a global multicenter effort (appendix in supplemental Data). Patients were selected based on molecular data availability. The nontransplant cohort comprised cases from the Hôpital Saint-Louis and from the French Intergroup of Myeloproliferative Disease.23,24 Inclusion criteria were as follows: (1) age of <72 years because patients received transplantation up to this age; and (2) Eastern Cooperative Oncology Group performance status score of <2. To limit mortality bias from patient selection, patients who did not undergo transplantation who died within 3 months after the date of evaluation were excluded, and patients who received HSCT >5 years after diagnosis were also excluded. Overall, 243 patients who did not undergo transplantation and 239 patients who underwent transplantation were included in the study (supplemental Figure 1). The study was conducted in accordance with the Declaration of Helsinki.
Molecular data and prognostic score systems
DIPSS, MIPSS70 and MIPSS70+V2 scores were available at time of transplant in the transplant cohort. Center for International Blood and Marrow Transplant Research myelofibrosis pretransplant10 and MTSS9 scores were calculated according to previous publications.1,3,4,9,10 In the patients who did not undergo transplantation, DIPSS, MIPSS70, and MIPSS70 V2 scores were calculated at diagnosis and at all time points available during the follow-up using clinical data of follow-up and somatic mutations screened at diagnosis. The molecular evaluation was performed by next-generation sequencing at the time of transplantation in the transplant cohort and at the time of diagnosis in the nontransplant cohort. Only pathogenic or likely pathogenic mutations were retained, with an allele burden threshold of 2%. We retained analyses on genes covered in all patients: JAK2, MPL, CALR, ASXL1, CBL, DNMT3A, EZH2, IDH1, IDH2, KRAS, NRAS, SF3B1, SRSF2, U2AF1, TET2, and TP53.
The HMR status was defined based on genes included in theMIPSS70 (ASXL1, SRSF2, EZH2, IDH1, and IDH2) and extended HMR according to MIPSS70+V2 (including also U2AF1) with the addition of KRAS, NRAS, CBL, and TP53 pathogenic variants.24
Statistical analysis
For descriptive analysis, quantitative variables were mean and standard deviation, and qualitative variables were reported using effectives and proportions. Comparisons were performed using Mann-Whitney U tests for quantitative variables, and Fisher exact test for qualitative variables.
Although no patient received HSCT at diagnosis and because scores were those performed at time of transplantation, we used a time-point assessment for the patients who did not undergo transplantation during follow-up, when available (n = 175). In more detail, we derived DIPSS at each visit available in our databases and we considered the higher DIPSS even if it was then downstaged. In case of several visits with the same DIPSS, we selected those closer to the median time of transplantation in the transplant cohort (ie, 12.7 months). With this method, time from diagnosis to assessment was better distributed between patients in the transplant (median, 12.7 months [IQR, 0.46-59.4]) and nontransplant cohorts (median, 9.8 months [IQR, 0-60]) and limits mortality bias in patients who did not undergo transplantation (supplemental Figure 2).
Because of the heterogeneity between the 2 cohorts (Table 1), including after stratification by score subgroups (data not shown), a nearest neighbor matching propensity score based on Mahalanobis distance was performed (R package 10.18637/jss.v042.i08). This method aimed to balance potential confounding factors between both cohorts with the transplant cohort set as reference. Here, we aimed to match patients in the transplant cohort with similar patients in the nontransplant cohort (with possible replacement) for the following variables associated with transplantation and prognosis: age, sex at birth, hemoglobin, platelet and leukocyte levels, constitutional symptoms, and circulating blasts (supplemental Figure 3). Of note, pretransplant exposure to ruxolitinib was not associated with survival, (supplemental Figure 4) and ruxolitinib was not used for matching.
Patient characteristics before and after matching with weighted variable
| Characteristics . | Transplant cohort . | Before matching . | P value∗ . | After matching . | P value† . |
|---|---|---|---|---|---|
| Nontransplant cohort . | Nontransplant cohort . | ||||
| n = 239 . | n = 243 . | n = 105 . | |||
| Patient sex, Female | 33.1% | 32.9% | 1 | 34.3% | 1 |
| Age, y | 55.5 (9.49) | 60.9 (10.6) | <.001 | 57.9 (9.36) | .052 |
| Hemoglobin (g/dL) | 9.58 (1.88) | 10.8 (2.22) | <.001 | 9.63 (1.74) | .816 |
| Platelet (×109/L) | 188 (270) | 270 (291) | <.001 | 168.9 (237.3) | .510 |
| White blood cells (×109/L) | 13.1 (17.3) | 12.9 (13.7) | .863 | 11.7 (13.22) | .942 |
| Circulating blasts ≥1% | 54% | 43.2% | .023 | 66% | .099 |
| Constitutional symptoms | 55.2% | 28.8% | <.001 | 54.4% | 1 |
| DIPSS | |||||
| Low | 7.53% | 17.6% | .001 | 7.9% | 1 |
| Int-1 | 30.1% | 37.1% | 29.3% | ||
| Int-2 | 47.7% | 37.6% | 46.9% | ||
| High | 14.6% | 7.7% | 15.9% | ||
| Driver mutations | |||||
| Calreticuline | 17.6% | 29.8% | <.001 | 31.4% | .005 |
| JAK2 V617F | 53.6% | 56.3% | 57.7% | ||
| MPL | 6.3% | 6.5% | 4.2% | ||
| Triple negative | 22.6% | 7.3% | 6.7% | ||
| HMR | 42.3% | 50.6% | .08 | 64.0% | .006 |
| MIPSS70 | |||||
| Low | 6.3% | 34.7% | <.001 | 19.7% | .003 |
| Intermediate | 60.3% | 46.1% | 43.5% | ||
| High | 33.5% | 19.2% | 36.8% | ||
| Mutations | |||||
| ASXL1 | 31.4% | 42.4% | .016 | 51.9% | .003 |
| EZH2 | 4.2% | 6.1% | .436 | 7.9% | .292 |
| IDH1 | 1.7% | 1.6% | 1 | 4.1% | .254 |
| IDH2 | 3.4% | 4.1% | .838 | 13.4% | .249 |
| SRSF2 | 11.5% | 11.4% | 1 | 15.9 % | .548 |
| U2AF1 | 6.7% | 12.2% | .050 | 15.5% | .021 |
| TP53 | 10.9% | 4.9% | .024 | 5.9% | .306 |
| DNMT3A | 5.9% | 8.6% | .316 | 1.7% | .362 |
| TET2 | 15.5% | 29.4% | <.001 | 25.9% | .058 |
| Characteristics . | Transplant cohort . | Before matching . | P value∗ . | After matching . | P value† . |
|---|---|---|---|---|---|
| Nontransplant cohort . | Nontransplant cohort . | ||||
| n = 239 . | n = 243 . | n = 105 . | |||
| Patient sex, Female | 33.1% | 32.9% | 1 | 34.3% | 1 |
| Age, y | 55.5 (9.49) | 60.9 (10.6) | <.001 | 57.9 (9.36) | .052 |
| Hemoglobin (g/dL) | 9.58 (1.88) | 10.8 (2.22) | <.001 | 9.63 (1.74) | .816 |
| Platelet (×109/L) | 188 (270) | 270 (291) | <.001 | 168.9 (237.3) | .510 |
| White blood cells (×109/L) | 13.1 (17.3) | 12.9 (13.7) | .863 | 11.7 (13.22) | .942 |
| Circulating blasts ≥1% | 54% | 43.2% | .023 | 66% | .099 |
| Constitutional symptoms | 55.2% | 28.8% | <.001 | 54.4% | 1 |
| DIPSS | |||||
| Low | 7.53% | 17.6% | .001 | 7.9% | 1 |
| Int-1 | 30.1% | 37.1% | 29.3% | ||
| Int-2 | 47.7% | 37.6% | 46.9% | ||
| High | 14.6% | 7.7% | 15.9% | ||
| Driver mutations | |||||
| Calreticuline | 17.6% | 29.8% | <.001 | 31.4% | .005 |
| JAK2 V617F | 53.6% | 56.3% | 57.7% | ||
| MPL | 6.3% | 6.5% | 4.2% | ||
| Triple negative | 22.6% | 7.3% | 6.7% | ||
| HMR | 42.3% | 50.6% | .08 | 64.0% | .006 |
| MIPSS70 | |||||
| Low | 6.3% | 34.7% | <.001 | 19.7% | .003 |
| Intermediate | 60.3% | 46.1% | 43.5% | ||
| High | 33.5% | 19.2% | 36.8% | ||
| Mutations | |||||
| ASXL1 | 31.4% | 42.4% | .016 | 51.9% | .003 |
| EZH2 | 4.2% | 6.1% | .436 | 7.9% | .292 |
| IDH1 | 1.7% | 1.6% | 1 | 4.1% | .254 |
| IDH2 | 3.4% | 4.1% | .838 | 13.4% | .249 |
| SRSF2 | 11.5% | 11.4% | 1 | 15.9 % | .548 |
| U2AF1 | 6.7% | 12.2% | .050 | 15.5% | .021 |
| TP53 | 10.9% | 4.9% | .024 | 5.9% | .306 |
| DNMT3A | 5.9% | 8.6% | .316 | 1.7% | .362 |
| TET2 | 15.5% | 29.4% | <.001 | 25.9% | .058 |
Quantitative variables are reported as mean and standard deviation and qualitative variables as frequencies.
P value comparing nontransplant to transplant before matching.
P value comparing nontransplant to transplant after matching.
For survival analysis, the baseline time was defined as date of HSCT for the transplant cohort and as the date of assessment for the nontransplant cohort (see above). A weighted Cox proportional hazard model, which aimed to explain the OS after HSCT within the first 8 years was performed. Weighting was defined using the nearest neighbor matching propensity score results for all variables used for matching. Proportional hazard assumption was checked graphically; if this assumption was not verified, a landmark approach was applied. Results of the models are summarized with hazard ratio, and 95% confidence intervals (CIs) and P values computed in the model. Kaplan-Meier curves with number of patients at risk at different time points were provided. Then, analyses were stratified according to the risk categories defined by the prognostic scoring systems as well as molecular risk categories. The performance of each prognostic scoring system was assessed through area under the curve (AUC) for predicting OS at 6 years. The benefit of the transplantation for each group of prognosis scoring systems was evaluated by 6-year survival and reported with odds ratio (OR) and 95% CIs. Prediction models were fit with logistic regression and an outcome of OS at 6 years, and these were used to generate receiver operating characteristic curves and summarized with the area under the receiver operating characteristic curves. Multiple testing correction based of the Bonferroni-Holm method was performed to control the family-wise error rate at 5%.
Results
Patient characteristics
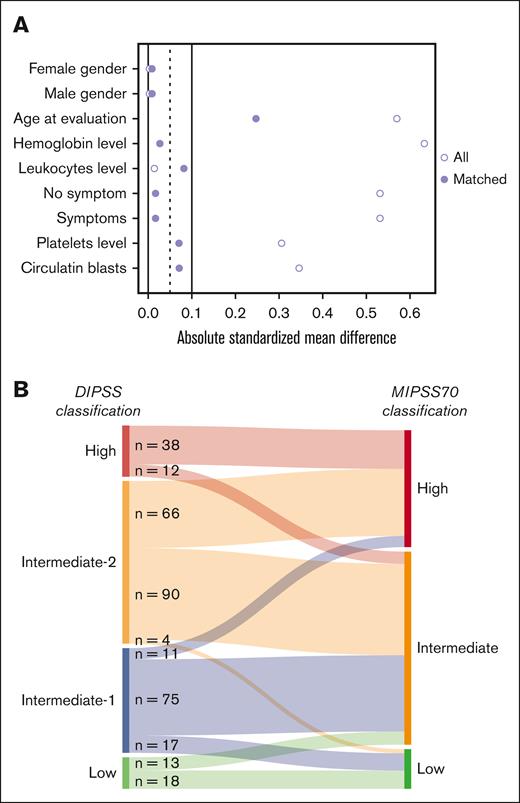
Table 1 shows patient characteristics. In the original population, patients who did not undergo transplantation (n = 243) were significantly older, had higher levels of hemoglobin and platelets, a lower percentage of circulating blood blasts, fewer constitutional symptoms, and were more often at low or int-1 risk compared with patients who received transplantation (n = 239). The proportions of patients treated with ruxolitinib were 31% (73/238) and 29.7% (30/101) in the transplant and nontransplant groups, respectively. In the transplant cohort, the donor was a HLA-mismatched related donor in 4 (1.7%), an HLA-mismatched unrelated donor in 61 (25.5%), a HLA-matched related donor in 65 (27.2%), and a HLA-matched unrelated donor in 109 (45.6%). Center for International Blood and Marrow Transplant Research score before transplantation was low in 91 (38.1%), intermediate in 119 (49.8%), and high in 29 (12.1%) patients. MTSS scores before transplantation was low in 49 (20.5%), intermediate in 95 (39.7%), high in 47 (19.7%), and very high in 48 (20.1%) patients. CALR mutations were more frequent in patients who did not undergo transplantation, whereas triple-negative status was less frequent in this group. Taking patients who received transplantation as the reference, and after matching and weighting for leukocyte count, hemoglobin level, platelet count, circulating blasts, constitutional symptoms, age, and sex, groups were comparable (Figure 1A; Table 1; supplemental Figure 3). Of note, triple negative for driver mutations remained more frequent in patients who received transplantation, and ASXL1 was more frequent in patients who did not undergo transplantation, consequently HMR was more frequent in patients who did not undergo transplantation. Sankey plots show the reclassification of DIPSS to MIPSS70 in the matched population (Figure 1B).
Matching cohorts and reclassification between DIPSS and MIPSS70. (A) Love plot showing how matching largely erased the differences between the 2 groups. (B) Sankey diagram represents the reclassification of DIPSS categories into MIPSS70 scoring system.
Matching cohorts and reclassification between DIPSS and MIPSS70. (A) Love plot showing how matching largely erased the differences between the 2 groups. (B) Sankey diagram represents the reclassification of DIPSS categories into MIPSS70 scoring system.
OS
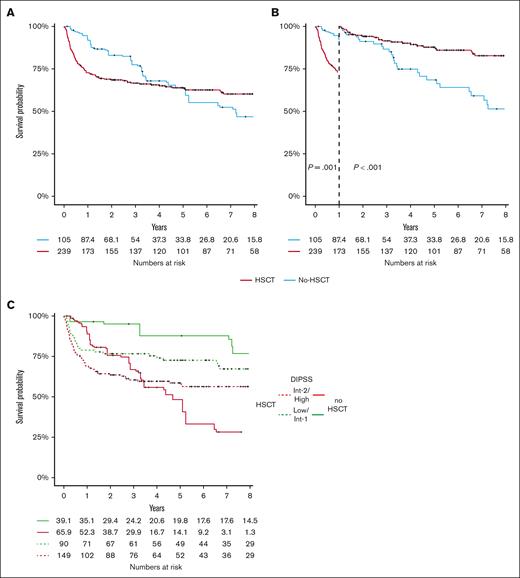
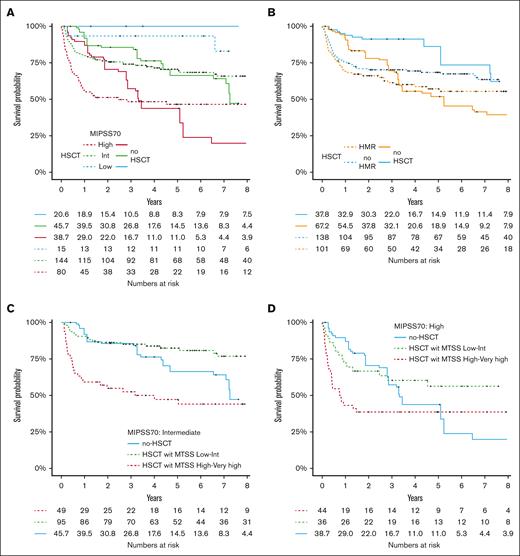
Analyses are restricted to the matched patients who did not undergo transplantation and patients who received transplantation. Median follow-up was 3.2 and 4 years in patients who did not undergo nontransplantation and patients who received HSCT, respectively. Figure 2A shows OS in the transplant and matched nontransplant cohorts. In the first year of follow-up, there is a high mortality in patients who received transplantation, inducing a later crossover in the 2 curves. Figure 2B shows OS with a landmark at 1 year to highlight the 2 different periods and avoid crossover to respect the proportional hazard for statistical tests. We then analyzed the survival by prognostic subgroups: DIPSS, HMR or extended HMR, and MIPSS70. The 6-year OS stratified for the 4 groups of DIPSS (low, int-1, int-2, and high) were 76.9 (95% CI, 59.3-99.7), 71.6 (95% CI, 60.8-83), 60.1 (95% CI, 51.3-70.4), and 44.4% (95% CI, 30.2-65.1) in the transplant group and 93.3 (95% CI, 80.9-100), 86 (95% CI, 68.9-100), 41.5 (95% CI, 22.1-78), and 6.55% (95% CI, 0.7-55) in the nontransplant group (Figure 2C; supplemental Figure 5). The gain of survival with transplantation in DIPSS int-2 and high-risk patients was +18.6% and +37.9%, respectively. The gain of OS without transplanted was +16.4% and 14.4% in DIPSS int-1 and low, respectively. OR for the survival advantage with transplant at 6 years in high and int-2 risk DIPSS was 2.18 (0.97-5.26). OS stratified on MIPSS70 is shown in Figure 3A and supplemental Figure 6. Low-risk MIPSS70 had no event at 6 years (OS at 100%) in the nontransplant group, whereas 6-year OS was 93% (95% CI, 81.5-100) with transplantation. Intermediate-risk MIPSS70 had similar 6-year OS with or without transplantation (68.4% [95% CI, 60.9-76.9] with and 66.3% [95% CI, 48.9-89.9] without transplantation). High-risk MIPSS70 had a better 6-year OS with transplantation: 46.5% (95% CI, 36.6-59.2) vs 23.9% (95% CI, 0.8-69.6). OR for the 6-year OS benefit with transplantation in high-risk MIPSS70 was 1.85 (95% CI, 0.65-6.02). OR for 6-year OS did not show any advantage for transplantation in DIPSS low/int-1 (OR, 0.37 [95% CI: 0.090-1.17]) and MIPSS70 intermediate (OR, 1.09 [95% CI: 0.44-2.64]). For the MIPSS70-low subgroup, there was no event in patients who did not undergo transplantation, resulting in an OR not applicable. Then, we considered the MTSS categories to estimate whether it can change the results. High-risk MIPSS70 patients had an advantage for survival with transplantation whatever the MTSS score (Figure 3D). In contrast, MIPSS70 intermediate-risk patients did benefit from transplantation when they also had a low or intermediate MTSS risk (80.8% vs 66.3%) but not when they had a high or very-high MTSS risk (Figure 3C). Of note, MIPSS70 intermediate-risk patients had the best survival with transplantation from an HLA-matched sibling donor and the worst with an HLA-mismatched unrelated donor; they had similar survival with an HLA-matched unrelated donor or without transplantation (supplemental Figure 6). The group of patients with HMR had 6-year OS of 55.4% (95% CI, 45.9-66.9) with transplantation and 45.4% (95% CI, 28.9-71.3) without transplantation (OR, 1.18; 95% CI, 0.53-2.64; Figure 3B). The group of patients with extended HMR had a 6-year OS of 55.8% (95% CI, 47.5-65.6) with transplantation and 45.5% (95% CI, 29.1-71.1) without transplantation (supplemental Figure 8). The benefit of transplantation seemed more significant in patients with ≥2 HMR with a 6-year OS of 66.7% (95% CI, 49.3-90.2) with transplantation and 34.6% (95% CI, 12.2-98.3) without transplantation (supplemental Figure 9). Finally, when we looked at the impact of each somatic mutations from the extended HMR, the benefit of HSCT was not the same in all mutations, but there was the same trend of advantage with HSCT (supplemental Figure 10). Period had no effect on transplant outcome (supplemental Figure 11), even after adjustment for age, type of donor, and disease risk (data not shown).
OS according to HSCT and DIPSS classification. (A) OS in the global cohort was depicted according to HSCT and (B) with a landmark at 1-year of follow-up. (C) OS was represented according to DIPSS categories and HSCT (supplemental Figure 4 for landmark analysis). The numbers of individuals at risk for the nontransplant cohort are fractions because of the inclusion of weighting in the analysis.
OS according to HSCT and DIPSS classification. (A) OS in the global cohort was depicted according to HSCT and (B) with a landmark at 1-year of follow-up. (C) OS was represented according to DIPSS categories and HSCT (supplemental Figure 4 for landmark analysis). The numbers of individuals at risk for the nontransplant cohort are fractions because of the inclusion of weighting in the analysis.
Impact of HSCT on molecular subtypes. (A) OS was depicted according to HSCT and MIPSS70, and (B) the presence of HMR mutation (landmark analysis in supplemental Figure 5). OS was represented in patients with (C) MIPSS70 intermediate and (D) high according to the MTSS score for patients who received transplantation. The number of individuals at risk for the nontransplanted cohort are fractions because of the inclusion of weighting in the analysis.
Impact of HSCT on molecular subtypes. (A) OS was depicted according to HSCT and MIPSS70, and (B) the presence of HMR mutation (landmark analysis in supplemental Figure 5). OS was represented in patients with (C) MIPSS70 intermediate and (D) high according to the MTSS score for patients who received transplantation. The number of individuals at risk for the nontransplanted cohort are fractions because of the inclusion of weighting in the analysis.
Cox analyses and performance of models
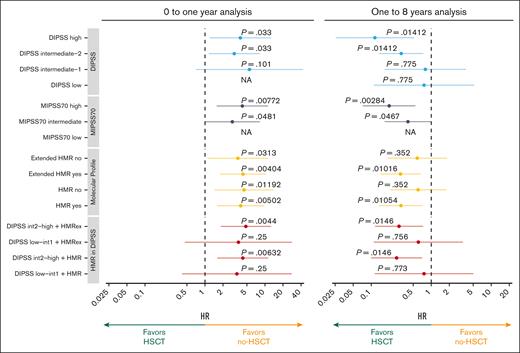
Separate analyses over time were done with respect to the proportional risk in the Cox model at 1 year and with the 1-year landmark to respect proportional hazards due to crossing curves. All groups had a significant mortality increase with transplantation the first year after HSCT (Figure 4). In contrast, some categories of patients had a significantly decreased risk of mortality with transplantation after the 1-year landmark: DIPSS high or int-2, MIPSS70 high, HMR mutations, and extended HMR mutations. Of note, DIPSS low or int-1 with or without HMR (extended or not) did not have a decrease in mortality risk after the 1-year landmark.
Impact of HSCT in every category of prognostic scoring systems and according to the presence of HMR. Forest plot representing the hazard ratio (HR) of HSCT in OS in each category. Analyses were performed in the 2 periods of the landmark analysis: 0 to 1 year (left panel) and 1 to 8 years (right panel). P values were corrected using the Bonferroni-Holm method. For some subgroups, results were not reported (NA) because of an estimation error.
Impact of HSCT in every category of prognostic scoring systems and according to the presence of HMR. Forest plot representing the hazard ratio (HR) of HSCT in OS in each category. Analyses were performed in the 2 periods of the landmark analysis: 0 to 1 year (left panel) and 1 to 8 years (right panel). P values were corrected using the Bonferroni-Holm method. For some subgroups, results were not reported (NA) because of an estimation error.
Finally, a discrimination test using AUC showed that the performance of the model to predict OS at 6 years for the whole cohort was better with the use of DIPSS + HMR (AUC = 0.720) than for DIPSS (AUC = 0.694) or MIPSS70 (AUC = 0.697) or HMR alone (AUC = 0.603). The performance of the models was better in patients who did not undergo transplantation. AUC was 0.837 for DIPSS + HMR, 0.858 for DIPSS, and nonapplicable for MIPSS70 in patients who did not undergo transplantation. AUC was 0.639 for DIPSS + HMR, 0.656 for DIPSS, and 0.713 for MIPSS70.
Stratification on MIPSS70+V2
Finally, we analyzed survival according to MIPSS70+V2 in patients underwent transplantation and those who did not undergo transplantation. A new matched population was created including only the patients with an available karyotype (n = 159 for patients who underwent transplantation and 143 for patients who did not undergo transplantation before matching, and n = 159 and 66, respectively, after matching; supplemental Figure 10). A Sankey plot shows the restratification from DIPSS to MIPSS70+V2 (Figure 5B). Because there were no MIPSS70+V2 very low-risk patients and only 1 low-risk patient in the transplant cohort, this analysis was not performed in these groups. The high and very-high risk groups had a 6-year survival at 39.7 (95% CI, 19.8-79.7) and 24% (95% CI, 9.32-61.8), respectively, without transplantation, and 73.2 (95% CI, 62.2-86.1) and 51.8% (95% CI, 41.8-64.2), respectively, with transplantation. OR for the 6-year OS advantage with transplantation was 5.26 (95% CI, 1.33-27.1) and 2.15 (95% CI, 0.70-8.03) in high and very high risk MIPSS70+V2, respectively. The AUC testing the performance of the model to predict OS at 6 years for MIPSS70+V2 was 0.672.
Subanalysis of patients with available cytogenetics. A new matching was performed in patients with available karyotype. (A) Sankey diagram represents the reclassification of DIPSS categories into MIPSS70+V2 scoring system. (B) OS was depicted according to HSCT and MIPSS70+V2 (supplemental Figure 10 for landmark analysis). The numbers of individuals at risk in the nontransplant cohort are fractions because of the inclusion of weighting in the analysis.
Subanalysis of patients with available cytogenetics. A new matching was performed in patients with available karyotype. (A) Sankey diagram represents the reclassification of DIPSS categories into MIPSS70+V2 scoring system. (B) OS was depicted according to HSCT and MIPSS70+V2 (supplemental Figure 10 for landmark analysis). The numbers of individuals at risk in the nontransplant cohort are fractions because of the inclusion of weighting in the analysis.
Discussion
The indication for transplantation in patients with MF remains challenging. Actually, some scores have been validated to predict survival and acute myeloid leukemia transformation, whereas other scores have been developed to estimate post-HSCT outcome.1-5 Furthermore, although patients with MF with “higher” disease risk have improved survival with transplantation over nontransplant strategies, higher disease risk also affects the post-HSCT outcome.9,13 Indeed, the more severe the disease is, the more comorbidities occur, with huge splenomegaly, liver dysfunction, vascular events, refractoriness to JAK inhibitors, or consequences of massive transfusion with iron overload or anti-HLA immunization. Previous cohort studies comparing patients who underwent transplantation and those who did not undergo transplantation have been based on DIPSS and demonstrate a gain of survival in int-1–risk or high-risk patients, with curves of survival only crossing later in lower risk.18,19 Our international retrospective study could confirm that patients with PMF with an int-2 or high DIPSS risk have a better long-term outcome with transplantation whereas those with low or int-1 DIPSS risk have a better long-term outcome without transplantation. We have no evidence to transfer these findings to secondary myelofibrosis, which were excluded from this study. However, this advantage is mitigated by high early posttransplant mortality. The main point of our work was to show that MIPSS70 score can be used to help with transplant decision. Integration of genetic alterations in transplant decision-making is the major point of this paper. We were able to confirm that patients who have a high-risk MIPSS70 or a high/very high risk MIPSS70+V2 have a benefit of long-term survival with transplantation. Furthermore, we could identify a subgroup of MIPSS70 intermediate-risk patients who could also benefit from transplantation if they were MTSS low or intermediate risk. We also tested HMR, extended HMR, number of mutation, and each somatic mutations separately but it was less performant than scores including mutations. Especially, advantage for transplantation in patients with a single HMR mutation was modest but it was more significant when they had at least 2 HMR mutations. We conclude that a single, poor prognostic mutation is not a sufficient criterion to indicate transplantation. An exception maybe patients with TP53 mutations who all had a very poor prognosis without transplantation. Our transplant group has previously reported that TP53 mutation in patients who received transplantation greatly affects the outcome, especially when this is a multihit TP53 mutation, with survival at 6 years of 25% (multihit) to 56% (single hit).25 Because of small numbers of TP53 in patients who did not undergo transplantation (n = 12 before matching), we did not have the opportunity to test the specific transplant advantage in multihit vs single-hit TP53 mutations.
However, several points should be highlighted regarding nonrelapse mortality. First, early mortality after HSCT, which is in the current study between 25% and 45% at 1 year, alters the potential global advantage of transplantation. In addition, the early mortality is higher in higher risk disease patients, possibly related to the more frequent comorbidities in this category of patients, as previously mentioned. Another explanation for higher early mortality in higher risk disease patients is that, when only an HLA-mismatched donor is available, physicians are waiting longer before performing HSCT for the patients because of the inferior results expected with this type of donor. Consequently, higher risk disease patients have also higher risk MTSS scores, driven, in part, by donor type but also because of the delay to transplant. However, in our study, we failed to observe an effect of the period on posttransplantation mortality; and MTSS score, age, or type of donor were still the most significant variables associated with posttransplant mortality. Early posttransplant mortality may change over time if significant improvements are made, especially in the setting of HLA-mismatched donors, using, for instance, posttransplant cyclophosphamide, cytomegalovirus prophylaxis by letermovir, steroid refractory graft-versus-host disease management, or other beneficial strategies.11,26-37 It is probable that if this mortality could be reduced, it may increase indications for transplantation, including lower-risk disease patients. Scores predicting nonrelapse mortality should probably be shortly updated according to management improvement. Another point in our study to explain why transplantation outcomes were worse in patients at low risk is that the survival in low-risk MIPSS70 patients was close to 100%.
To reduce the bias inherent to the different transplant and nontransplant populations, we made the choice of using a propensity score with weighting to obtain a nontransplant group with features comparable with patients who underwent transplantation. This methodology allows more accurate causal inferences by balancing nonequivalent groups that may result from using a nonrandomized design.38 If the most common confounding factors are considered, then the relationships determined can be considered as causal. However, some confounding factors are not captured in this study, such as the response to nontransplant therapies or donor availability. This analysis will not be as reliable as a prospective randomized trial but the advantage of survival in higher risk patients will not be in favor in such a randomized trial. Finally, assessment at time of transplantation induces a mortality bias in the nontransplant cohort. To limit this bias, patients who did not undergo transplantation and who died within 3 months from diagnosis and patients who received transplantation >5 years after diagnosis were excluded. Furthermore, patients who did not undergo transplantation were also assessed over time, allowing a longer time between diagnosis and assessment, which was closer to the diagnosis–transplantation delay. The limit of these efforts to reduce selection bias is reduction of the sample size, which decreases statistical power.
To conclude, MIPSS70 and MIPSS70+V2 are reliable scores for transplant decision-making for patients with PMF. Furthermore, combination with the MTSS allows a better assessment, especially in patients with intermediate-risk disease. In contrast, 1 HMR should not be taken as the single variable to indicate transplantation because they do not discriminate enough the potential advantage of transplantation.
Acknowledgments
The authors thank the French Clinical and Biological Network of Myeloproliferative Neoplasms (FIMBANK) and the Comprehensive Myeloproliferative neoplasms Center of Saint-Louis Hospital for providing high-quality clinical data and biological resources; and the French Intergroup of Myeloproliferative Neoplasms for scientific discussions.
FIMBANK network was funded by the French Institut National du Cancer (base de données clinico-biologiques 2013 and 2022).
Authorship
Contribution: M.R., D.L.P., J.R., J.-J.K., and N.K. designed the study; D.L.P. and J.R. performed statistical analyses; and all authors were involved in the analysis and interpretation of results, approved the final manuscript, and all authors provided data.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Marie Robin, Service d’hématologie–greffe Hôpital Saint-Louis, Assistance Publique–Hôpitaux de Paris, 1 avenue Claude Vellefaux, 75475 Paris, France; email: marie.robin@aphp.fr.
References
Author notes
J.-J.K., N.K., and M.R. contributed equally as senior authors to this study.
Data are available on request from the corresponding author, Marie Robin (marie.robin@aphp.fr).
The full-text version of this article contains a data supplement.