TO THE EDITOR:
Allogeneic hematopoietic stem cell transplantation (alloSCT) is a standard therapy for several malignant and nonmalignant hematological and immunological diseases.1 Chronic graft-versus-host disease (cGVHD) occurs in >50% of patients who receive alloSCT and is a main cause of morbidity and nonrelapse mortality after alloSCT.2,3 Sclerodermatous cGVHD (sclGVHD) is a distinct, poorly understood subtype of cGVHD, often refractory to conventional immunosuppressive treatment and leading to severe disability.4
In sclGVHD, aberrant fibroblast-to-myofibroblast transition, induced by profibrotic T helper 2 (Th2)/Th17 cytokines might be a key underlying pathogenic mechanism, as shown for other fibrotic diseases.5-7 Myofibroblasts deposit excessive amounts of extracellular matrix and remodel it by exerting contraction forces, leading to progressive fibrosis and impairment of organ function.6
The US Food and Drug Administration recently approved 3 drugs for the treatment of cGVHD: ibrutinib, an inhibitor of Bruton tyrosine kinase (BTK), the Rho associated coiled-coil–containing protein kinase-2 inhibitor belumosudil, and the JAK1/2 inhibitor ruxolitinib.8-11 Although these therapies led to improved response rates or responses in prior refractory patients, complete responses are very rare, ranging between 5% for belumosudil and 21% for ibrutinib. Moreover, 30% to 40% of patients with cGVHD are refractory to monotherapy with these drugs, and secondary treatment failures occur frequently.8 Combination therapy targeting different pathogenic pathways may provide a therapeutic opportunity for refractory patients.
We previously demonstrated that therapeutic targeting of canonical Wnt signaling at different levels ameliorates experimental sclGVHD.1 Porcupine inhibitors, which target both canonical and noncanonical Wnt signaling, are effective antifibrotic therapies in preclinical settings and are currently evaluated in early clinical trials for idiopathic pulmonary fibrosis.12-14
Here, we aimed to evaluate the efficiency and tolerability profile of Wnt-C59, a porcupine inhibitor, in monotherapy or in combination with ruxolitinib, ibrutinib, or belumosudil, in experimental sclGVHD.
Materials and methods can be found in the supplemental File.
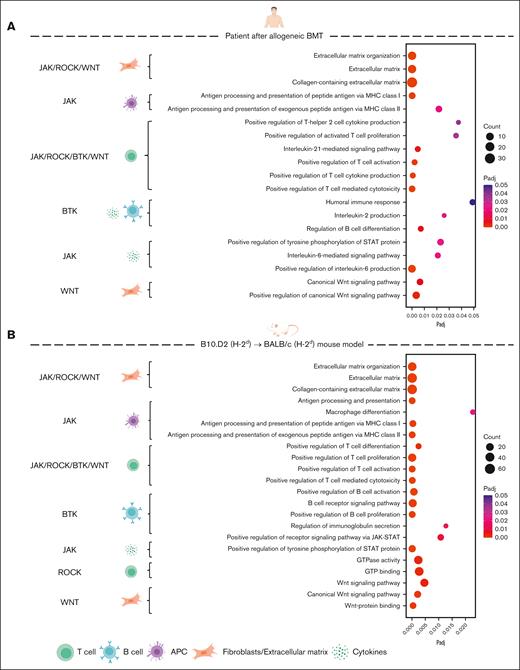
We first analyzed whether the biological processes and signaling pathways modulated by Wnt-C59, ruxolitinib, ibrutinib, or belumosudil are enriched in both human and murine sclGVHD. We data mined a previously published RNA sequencing (RNA-seq) data set from patients with sclGVHD after alloSCT and healthy controls and performed RNA-seq from mice with sclGVHD induced by B10.D2 (H-2d) → BALB/c (H-2d) transplantation and syngeneic controls (synSCT).1 Pathway enrichment analysis showed enrichment of terms related to extracellular matrix deposition, Th2 or Th17 polarization, JAK-STAT signaling, B-cell activation, B-cell receptor signaling, interleukin-2 (IL-2) signaling, IL-21 signaling, and Wnt signaling in human sclGVHD (Figure 1; supplemental Figures 1 and 2). These biological processes and signaling pathways are central modulators of the effects of ruxolitinib, ibrutinib, belumosudil, and Wnt inhibitors in cGVHD.1,15-17 These pathways in human samples were recovered in murine sclGVHD (Figure 1; supplemental Figures 1 and 2). Together, these findings highlight that (1) Wnt, JAK/STAT, BTK, and ROCK signaling are consistently upregulated in sclGVHD and thus provide rational targets for therapeutic intervention and that (2) the B10.D2 (H-2d) → BALB/c (H-2d) sclGVHD model could be predictive for the human sclGVHD with regard to inhibition of Wnt, JAK/STAT, BTK, and ROCK signaling.
Signaling pathways targeted by Wnt-C59, ruxolitinib, ibrutinib, or belumosudil are enriched in both human and murine sclGVHD. (A-B) RNA-seq data were analyzed from 6 patients with sclGVHD and 6 healthy controls (A) as well as from 4 allogeneic (sclGVHD model, vehicle-treated) and 4 syngeneic mice (B). Bubble plots illustrating selected significantly enriched biological processes from Gene Ontology (GO) pathway analysis. The color of each bubble represents the adjusted P value (Padj), whereas the size of the bubble represents the number of differentially expressed genes associated with the respective GO processes. On the left side are marked the signaling pathways directly targeted by Wnt-C59, ruxolitinib, ibrutinib, or belumosudil (WNT–Wnt-C59, BTK–ibrutinib, ROCK–belumosudil, and JAK–ruxolitinib), corresponding to the respective GO processes on the right side. APC, antigen-presenting cell; GTP, guanosine triphosphate.
Signaling pathways targeted by Wnt-C59, ruxolitinib, ibrutinib, or belumosudil are enriched in both human and murine sclGVHD. (A-B) RNA-seq data were analyzed from 6 patients with sclGVHD and 6 healthy controls (A) as well as from 4 allogeneic (sclGVHD model, vehicle-treated) and 4 syngeneic mice (B). Bubble plots illustrating selected significantly enriched biological processes from Gene Ontology (GO) pathway analysis. The color of each bubble represents the adjusted P value (Padj), whereas the size of the bubble represents the number of differentially expressed genes associated with the respective GO processes. On the left side are marked the signaling pathways directly targeted by Wnt-C59, ruxolitinib, ibrutinib, or belumosudil (WNT–Wnt-C59, BTK–ibrutinib, ROCK–belumosudil, and JAK–ruxolitinib), corresponding to the respective GO processes on the right side. APC, antigen-presenting cell; GTP, guanosine triphosphate.
Next, we aimed to evaluate the tolerability and efficacy profile of porcupine inhibition as monotherapy or combined with ruxolitinib, ibrutinib, or belumosudil in B10.D2 (H-2d) → BALB/c (H-2d) model of sclGVHD, administered starting from day 21 after SCT, when clinical signs of cGVHD started to emerge, until day 49 (supplemental Figure 3A).
All mice developed weight loss after irradiation and SCT; alloSCT-untreated mice only partially recovered from day 12 and started to lose weight again from day 18, whereas synSCT mice gained weight continuously from day 4 after SCT (supplemental Figure 3B).
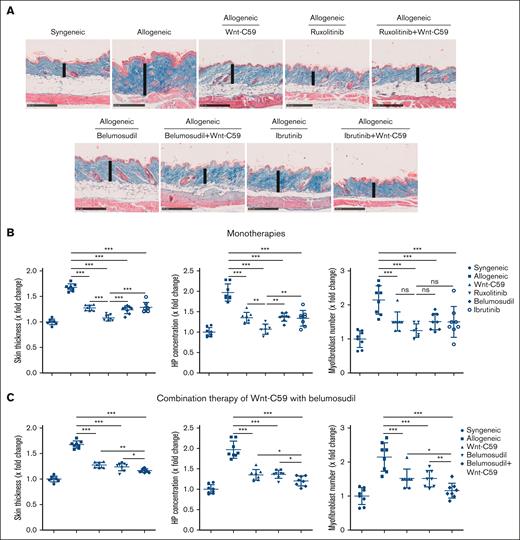
AlloSCT-untreated mice developed systemic fibrotic disease with dermal and peribronchial collagen deposition, cutaneous and pulmonary leukocyte infiltration, atrophy of the subcutaneous fat tissue, an increased dermal thickness, Ashcroft score, hydroxyproline content, and number of myofibroblasts in the dermis and lungs (Figure. 2A-B; supplemental Figure 8A-B).
Wnt-C59 in monotherapy or in combination with ruxolitinib, ibrutinib, or belumosudil ameliorates skin fibrosis in sclGVHD. (A) Representative trichrome staining of the skin of sclGVHD mice at 100-fold magnification (scale bars = 250 μm). The skin thickness is indicated by a vertical line. Quantification of dermal thickening, hydroxyproline content, and number of myofibroblasts in the dermis after monotherapy (B), or Wnt-C59 in combination with belumosudil (C). Data are represented as mean ± standard deviation of n = 7 to 8 independent biological samples per group. P values were determined by 1-way analysis of variance with the Newman-Keuls test. ∗.05 > P > .01; ∗∗.01 > P > .001; ∗∗∗P < .001. HP, hydroxyproline; ns, not significant.
Wnt-C59 in monotherapy or in combination with ruxolitinib, ibrutinib, or belumosudil ameliorates skin fibrosis in sclGVHD. (A) Representative trichrome staining of the skin of sclGVHD mice at 100-fold magnification (scale bars = 250 μm). The skin thickness is indicated by a vertical line. Quantification of dermal thickening, hydroxyproline content, and number of myofibroblasts in the dermis after monotherapy (B), or Wnt-C59 in combination with belumosudil (C). Data are represented as mean ± standard deviation of n = 7 to 8 independent biological samples per group. P values were determined by 1-way analysis of variance with the Newman-Keuls test. ∗.05 > P > .01; ∗∗.01 > P > .001; ∗∗∗P < .001. HP, hydroxyproline; ns, not significant.
Treatment with the porcupine inhibitor Wnt-C59 in monotherapy ameliorated weight loss and clinical signs of cGVHD in alloSCT mice (supplemental Figure 4A). Ruxolitinib prominently reduced weight loss and clinical signs of cGVHD, whereas the effects of ibrutinib or belumosudil were milder and delayed (supplemental Figure 4). Ruxolitinib, belumosudil, or ibrutinib were comparably efficacious in monotherapy or in combination with Wnt-C59 on preventing weight loss and reducing clinical scores (which mainly reflect systemic inflammation) (supplemental Figure 4). All the therapeutic groups showed comparable efficacy in reducing colon inflammation (supplemental Figure 5A-B). Moreover, in contrast to untreated alloSCT mice, animals from all treatment groups had no diarrhea during the treatment period. Consistently, the number of LGR5-positive stem cells in the small intestine was not significantly influenced by these therapies (supplemental Figure 6).
Generally, all treatment groups, including combination therapies, were well tolerated without any clinically noticeable side effects.
In all treated allogeneic groups, improvement in skin and lung fibrosis was observed (Figure 2; supplemental Figures 7-9). Ruxolitinib showed the most potent antifibrotic effects, with almost complete abrogation of myofibroblast differentiation and fibrotic skin and lung remodeling, both in monotherapy and in combination with Wnt-c59 (Figure 2B; supplemental Figures 7A, 8B, and 9A). Wnt-c59, ibrutinib, and belumosudil in monotherapy showed moderate antifibrotic effects (Figure 2B; supplemental Figure 8B). Belumosudil in combination with Wnt-C59 demonstrated additional effects compared with the respective monotherapies, with reduced myofibroblast differentiation and collagen deposition in the skin to levels comparable with the synSCT mice and the mice treated with ruxolitinib (Figure 2C), and a trend toward reduced collagen deposition in the lungs of alloSCT mice (supplemental Figure 8C). Ibrutinib in combination with Wnt-C59 did not show additive effects in reducing myofibroblast differentiation and collagen deposition in the skin and lungs of alloSCT mice compared with the respective monotherapies (supplemental Figures 7B and 9B).
RNA-seq analysis showed profound effects of Wnt-c59 on the skin transcriptome of sclGvHD mice (supplemental Figure 10A-B). Treatment with Wnt-C59 reverted several key pathologic processes related to collagen deposition; production of IL-1, IL-6, and transforming growth factor β; and activation of macrophages, Th17, and B cells (supplemental Figure 10C-D). MAPK pathways, extracellular signal-regulated kinase, JNK, and P38 were de-enriched after Wnt-C59 treatment (supplemental Figure 10C). These pathways may be downstream mediators of the effects of Wnt-C59 on inflammation and fibrosis, in addition to β-catenin. The combination of Wnt-C59 with belumosudil showed additional effects than Wnt-C59 in monotherapy on reverting pathogenetically relevant pathways in sclGvHD, such as JAK-STAT, IL-4, and actin binding, and restoring homeostatic processes, such as hair follicle and epidermis development (supplemental Figure 10E-F). This finding provides a further rationale for the use of Wnt-c59 in combination with belumosudil for sclGVHD.
We demonstrate that the porcupine inhibitor Wnt-C59 as monotherapy or in combination with ibrutinib, ruxolitinib, or belumosudil prevents clinical signs of disease, inflammation, and fibrosis in experimental sclGVHD, without significant toxicity. Wnt-C59 may act synergistically with belumosudil and thus show additive effects in combination with either therapy alone for the treatment of fibrotic tissue remodeling in sclGVHD.
We and others have previously demonstrated that Wnt signaling is upregulated in cGVHD and fibrotic diseases and that inhibition of canonical or noncanonical Wnt signaling prevents fibrotic tissue remodeling.1,18-21 Porcupine inhibitors thus interfere with the core pathways required for fibroblast activation.
Ibrutinib and belumosudil have complementary modes of action to those of porcupine inhibition on preventing fibroblast activation (by targeting B-cell activation, Th2 and Th17 polarization16,17,22), which supports the rationale of using these therapies in combination for fibrotic manifestations. Our data provide proof that Wnt/porcupine inhibition + belumosudil exert synergistic antifibrotic effects and might thus be considered in patients with cGVHD and prominent fibrotic manifestations, whereas Wnt/porcupine inhibition + ibrutinib did not show synergistic antifibrotic effects.
Our data does not support combining a Wnt/porcupine inhibitor to ruxolitinib in clinical use for additional therapeutic benefit, likely due to the potent and broad inhibitory effects of ruxolitinib on multiple pathogenic players in cGVHD such as Th17 cells and fibroblasts, and to a possible cross talk of JAK2 with canonical Wnt signaling.15,23,24
Here, we did not evaluate the effects of Wnt-C59 on graft-versus-leukemia. However, this should be addressed in future studies.
Our study provides proof-of-concept evidence that combination therapies targeting different pathogenetically relevant pathways might be safe and effective for cGVHD. This approach can be extended for novel therapeutic targets.25
Acknowledgments: The authors thank Wolfgang Espach, Vladyslav Fedorchenko, Regina Kleinlein, Lukas Sokolowski, and Christoph Liebel for their excellent technical assistance, and Figdraw for their assistance in creating symbols. Some symbols were used from BioRender. Lowin, T. (2025) https://BioRender.com/a06n595.
This work was supported by the grants DI 1537/14-1, DI 1537/17-1, DI 1537/20-1, DI 1537/22-1, MA 9219/2-1 from the Deutsche Forschungsgemeinschaft (German Research Foundation), SFB TRR 221 project number 324392634 (B04) and project number 52732026 of the Deutsche Forschungsgemeinschaft, grant 2013.056.1 from the Wilhelm-Sander-Foundation, grants 2021_EKEA.03 and 2022_EKMS.02 from the Else Kröner-Fresenius-Foundation, The Edith Busch and World Scleroderma Foundation Research Grant Programme 2022-2023 (A.-E.M), the Research Committee of the Medical Faculty of the Heinrich-Heine University Düsseldorf (Forschungskommission; ID 2022-18, ID 2023-33, and ID 2023-31 to A.-H.G., A.-E.M., and J.H.W.D., respectively), China Scholarship Council, research award of the German Scleroderma Foundation (Deutsche Stiftung Sklerodermie) and Edith Busch Stiftung, and a career support award of Medicine from the Ernst Jung Foundation.
Contribution: A.-E.M. and J.H.W.D. designed the study; X.H., Y.X., L.X., L.S., R.N., V.D., T.T.-M., M.L., A.R.R., Y.Z., Y.-N.L., C.D., A.-E.M., and A.-H.G. performed the experiments and/or analyzed the data; Y.X. performed the bioinformatics analyses; X.H. and A.-E.M. drafted the manuscript; J.H.W.D., D.W., and W.H. revised the manuscript; and all authors approved the final version of the manuscript.
Conflict-of-interest disclosure: J.H.W.D. has acted as a consultant for Actelion, Active Biotech, Anamar, Bayer Pharma, Boehringer Ingelheim, Celgene, Galapagos, GlaxoSmithKline, Inventiva, JB Therapeutics, Medac, Pfizer, Ruiyi, and Union Chimique Belge; has received research funding from Anamar, Active Biotech, Array Biopharma, aTyr Pharma, Bristol Myers Squibb, Bayer Pharma, Boehringer Ingelheim, Celgene, Galapagos, GlaxoSmithKline, Inventiva, Novartis, Sanofi-Aventis, RedX, and Union Chimique Belge; and owns stock in 4D Science. The remaining authors declare no competing financial interests.
Correspondence: Alexandru-Emil Matei, Department of Rheumatology and Hiller Research Center, University Hospital Düsseldorf, Heinrich Heine University, 40225 Düsseldorf, Germany; email: alexandru-emil.matei@med.uni-duesseldorf.de.
References
Author notes
The RNA sequencing data supporting this study have been deposited in the Gene Expression Omnibus database and are publicly accessible under accession number GSE284107.
The full-text version of this article contains a data supplement.