Key Points
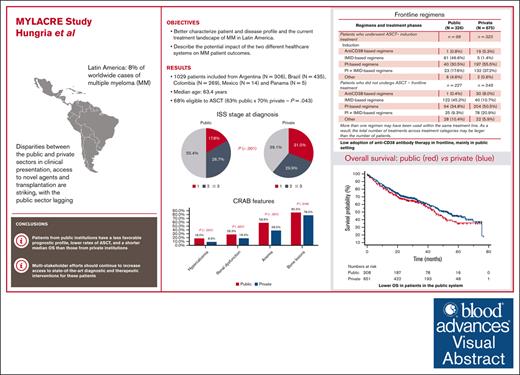
MYLACRE highlights shortcomings in the management of myeloma in Latin America, with differences between public and private health systems.
Visual Abstract
Although systemic therapy for multiple myeloma (MM) has evolved considerably over the past 2 decades, state-of-the-art treatment is not uniformly available in Latin America. In some countries, disparities between the public and private sectors in clinical presentation, access to novel agents, and transplantation are striking, with the public sector lagging. We conducted a multicenter, observational study of patients with MM in 5 Latin American countries (Argentina, Brazil, Colombia, Mexico, and Panama). We enrolled patients aged ≥18 years diagnosed with MM between January 2016 and June 2021, using data collected between May 2019 and June 2022. We categorized institutions as “public” when primarily funded by federal or local government, and “private” when financed mostly or completely by other sources. We analyzed 1029 patients, 1021 of whom could be classified into public (n = 339) and private (n = 682) institutions. These 2 groups differed in many respects, with patients from the latter having better baseline prognostic features (including eligibility to transplantation) and receiving combinations of immunomodulatory drugs and proteasome inhibitors, as well as anti-CD38 antibodies, more frequently than patients from public institutions. Among 960 patients with complete data for this analysis, the median overall survival was 44.6 months in public institutions and 53.3 months in private institutions (hazard ratio, 0.84; 95% confidence interval, 0.67-1.04; P = .109). Our results indicate diagnostic and therapeutic shortcomings in the management of MM in Latin America, with important gaps in patient profile, treatment patterns and long-term outcomes between public and private institutions. This trial was registered at www.clinicaltrials.gov as #NCT03955900.
Introduction
Multiple myeloma (MM) is an incurable disease that accounts for 17% to 19% of hematological malignancies in high-income countries and nearly 14% worldwide.1-4 Latin America has intermediate incidence rates in comparison with the rest of the world and accounts for 8% of the nearly 188 000 new worldwide cases of MM estimated for 2022.5 Nevertheless, systemic therapy for MM has evolved considerably over the past 2 decades, mostly from the introduction of autologous stem cell transplantation (ASCT) and novel classes of anticancer agents, such as immunomodulatory drugs, proteasome inhibitors, and anti-CD38 monoclonal antibodies.3 As a result, patient overall survival (OS) has more than doubled in high-income countries over a period of 2 decades.6,7 With currently available treatment strategies, the expected median OS for patients with newly diagnosed disease has steadily increased and now surpasses 5 years among ASCT-ineligible patients,8-12 and the 5-year OS rate is close to 80% among ASCT-eligible patients.13 Further progress continues, for example, with the more recent introduction of chimeric antigen receptor T-cell therapy and T-cell–redirecting bispecific antibodies, which promise to further improve OS as their use moves to earlier lines of therapy.14-17
Unfortunately, many state-of-the-art treatments and the required diagnostic infrastructure for their implementation are not available to a large proportion of patients with MM in health care settings facing more stringent resource limitations. This is often the case in Latin America, where the quality of health care provided varies in most countries. In many Latin American countries, a dual health care system exists, whereby a proportion of the population receives health care funded exclusively or mostly by the government, whereas a typically smaller proportion has access to supplemental modalities more often made available through private insurance. In some countries, such as Argentina, Brazil, and Mexico, health care for patients with supplemental insurance is usually funded exclusively by nongovernmental entities. In Colombia, for instance, a different model exists, in which the supplemental insurance is used simultaneously with government-funded health care. Regardless of these regional differences, there may be differences in patient profile and in access to state-of-the-art therapy between public and private health care settings; currently, the consequences of such differences are unknown.
Previous studies conducted in Latin America have characterized the patient profile in this region, as well as outcomes in recent decades, mostly before the introduction of the more novel agents.18-21 Likewise, registries have been established in some Latin American countries, such as Argentina (Fundación Argentina de Mieloma), Brazil (Grupo Brasileiro de Mieloma), and Colombia (Mieloma Múltiple, Epidemiología Nacional y territorial). Although disparities in access to diagnostic and therapeutic modalities in MM have been characterized previously,22 there is limited documentation on the prevalence and impact of such shortcomings in Latin America.23 In Brazil, previous studies have shown that treatment patterns for MM vary widely and often differ from those recommended by practice guidelines.24 This practice may have important implications regarding patient outcomes. The MYLACRE (Multiple Myeloma Latin American Cancer Registry) study was designed to better characterize patient and disease profile and the current treatment landscape of MM in Latin America. The current report aims to describe the potential impact of the 2 different health care systems on outcomes of patients with MM.
Methods
Study design and oversight
MYLACRE was a multicenter, noninterventional retrospective registry of patients with MM treated at reference centers in 5 Latin American countries (Argentina, Brazil, Colombia, Mexico, and Panama). As an observational study, it collected demographic and clinical data from diagnosis until the last follow-up date, but treatment and patient evaluation were left to the discretion of the study sites and local standards of care, with no influence by participation in the study. Eligible patients were aged ≥18 years and had to have been diagnosed with MM between 1 January 2016 and 30 June 2021, based on 2014 International Myeloma Working Group criteria.25 Patients with smoldering myeloma or plasma-cell leukemia were excluded from participation. Patients under active participation in clinical trials were not eligible, but those previously participating in a clinical trial at the time of accrual could be enrolled. Informed consent was obtained from all patients; the ethics committee approved a waiver in case collection of informed consent was not possible (deceased patient or loss of follow-up at the treatment center upon study initiation). Patients were to be followed-up for the purpose of the study until death or the end of the study (ie, 30 June 2022). The study was designed by the sponsor, Janssen-Cilag, which provided financial support and oversight of data collection and analysis. All authors vouch for the accuracy of this report.
Study objectives
MYLACRE had the objectives of (1) characterizing the MM population in terms of its demographics and clinical characteristics, (2) describing the landscape of MM treatment in 5 Latin American countries, (3) evaluating clinical outcomes (including OS, time to next treatment [TTNT], and the proportion of patients undergoing assessment of minimal residual disease [MRD]) overall and according to health care setting, which was categorized as public or private. The term “public” denotes health care that is primarily funded by federal or local government, whereas “private” is used to denote health care that is financed mostly or completely by other sources, such as health-insurance companies or out-of-pocket payment. The categorization was always made at the institution level, given the features of health care in Latin America. This report is primarily focused on the evaluation of the impact of the 2 different health care systems on patient outcome.
Data collection and statistical analysis
Study data were collected from patient institutional charts at participating centers using an electronic case report form specific for the study. Importantly, the protocol instructed investigators to enroll all consecutive eligible patients, in order to minimize bias from exclusion of those with a short OS. The sponsor provided monitoring activities to the participating institutions to ensure accuracy, completeness, and reliability of the data vis-à-vis source documents. Data were collected, beginning from the first visit at each center related to MM, on patient demographic characteristics, source of treatment funding, general health status, disease characteristics, eligibility for ASCT as per physician discretion at diagnosis, treatments administered and dates of administration, assessment of MRD, and vital status at the end of follow-up. Specifically, regarding the presence of hypercalcemia, renal dysfunction, anemia, and bone lesions (CRAB) features at diagnosis, the following conventions were used based on the available laboratory or imaging results: serum calcium of >11.0 mg/dL; serum creatinine of >2.0 mg/dL or creatinine clearance of <40 mL/min; hemoglobin of <10.0 g/dL; and any bone lesion.
Given the observational nature of the study, no specific hypotheses were formulated a priori, and the sample size was determined based on feasibility of enrolment and informed by a previous study, the Hemato-Oncology Latin America observational registry.20 Although most analyses were conducted with a descriptive intent, selected comparisons were undertaken using statistical testing in an exploratory manner and 95% confidence intervals (CIs) were computed for selected point estimates. Categorical variables were compared using the χ2 test, whereas numerical variables were compared with t test or the Mann-Whitney U test, depending on the whether they were normally distributed. Nominal P values are presented, with no adjustment for multiplicity. OS and TTNT were assessed using Kaplan-Meier analyses, and selected comparisons made using the log-rank test. In order to adjust the comparison of OS between patients from public and private institutions, a Cox proportional hazards model was fitted using covariates with univariate prognostic significance. OS was defined as the time from initiation of the first line of therapy (LOT1) until death from any cause, with censoring of patients alive or lost to follow-up at the last follow-up date. The TTNT was investigated between the initiation of LOT1 and the second LOT (LOT2), and between the initiation of LOT2 and the third LOT (LOT3). For these analyses, the event of interest was initiation of the next LOT, and patients were censored if the next LOT had not been initiated at the date of last visit for those alive or lost to follow-up, or date of death otherwise. Median follow-up was computed using the “reverse Kaplan-Meier” method.26 Overall attrition rates (ie, regardless of funding type and receipt of ASCT) between LOTs were computed as previously published.27
Results
Patient characteristics
Of 1062 patients initially registered, 1029 fulfilled selection criteria and were analyzed. The baseline characteristics of these patients are summarized in Table 1 according to funding source. Funding source could not be ascertained for 8 patients; of the remaining 1021, 339 and 682 were classified as coming from public and private institutions, respectively. Overall, 306 patients were from Argentina, 435 from Brazil, and 269 from Colombia; only 14 and 5 patients were included from Mexico and Panama, respectively, a reason why their results are presented jointly. Patients from public institutions had a similar age distribution to those from private institutions, but these 2 groups differed in many respects: public institutions had larger proportions of males and whites, as well as more patients with comorbidities, than private institutions. Likewise, patients from public institutions had lower mean hemoglobin, higher mean serum creatinine and calcium, a less favorable International Staging System (ISS) distribution, and were less frequently eligible to ASCT at baseline than their counterparts in private institutions. All CRAB features were more frequent among patients from public than from private institutions. Of note, the numbers of patients with previous participation in a clinical trial were 18 in their first-line treatment, 11 in second line, 4 in third line, 4 in fourth line, and none in subsequent lines.
Baseline patient characteristics, overall and according to funding source
| Characteristic . | Total (N = 1029) . | Public (N = 339) . | Private (N = 682) . | P value . |
|---|---|---|---|---|
| Age (mean ± SD), y | 63.4 ± 11.4 | 63.0 ± 10.9 | 63.5 ± 11.7 | .516 |
| Sex, female | 524/1029 (50.9%) | 156/339 (46.0%) | 363/682 (53.2%) | .030 |
| Country of origin, n (%) | <.001 | |||
| Argentina | 306/1029 (29.7%) | 17/339 (5.0%) | 289/682 (42.4%) | |
| Brazil | 435/1029 (42.3%) | 268/339 (79.1%) | 167/682 (24.5%) | |
| Colombia | 269/1029 (26.1%) | 53/339 (15.6%) | 212/682 (31.1%) | |
| Mexico and Panama | 19/1029 (1.8%) | 1/339 (0.3%) | 14/682 (2.1%) | |
| Race, White | 414/907 (45.6%) | 163/308 (52.9%) | 251/592 (42.4%) | .003 |
| Comorbidities present | 771/985 (78.3%) | 261/318 (82.1%) | 505/682 (76.5%) | .005 |
| Hemoglobin (mean ± SD), g/dL | 10.3 ± 2.39 | 9.6 ± 2.45 | 10.7 ± 2.26 | <.001 |
| Serum creatinine (mean ± SD), mg/dL | 1.80 ± 1.97 | 2.06 ± 2.17 | 1.69 ± 1.87 | .002 |
| Serum calcium (mean ± SD), mg/dL | 9.9 ± 2.91 | 9.9 ± 1.72 | 9.5 ± 1.73 | .146 |
| CRAB features present | ||||
| Hypercalcemia | 87/683 (12.7%) | 43/239 (18.0%) | 39/439 (8.9%) | <.001 |
| Renal dysfunction | 191/866 (22.1%) | 80/283 (28.3%) | 106/575 (18.4%) | .001 |
| Anemia | 414/916 (45.2%) | 173/295 (58.6%) | 237/615 (38.5%) | <.001 |
| Bone lesions | 671/837 (80.2%) | 209/245 (85.3%) | 458/587 (78.0%) | .016 |
| ISS stage | <.001 | |||
| I | 235/870 (27.0%) | 46/258 (17.8%) | 188/606 (31.0%) | |
| II | 252/870 (29.0%) | 69/258 (26.7%) | 181/606 (29.9%) | |
| III | 383/870 (44.0%) | 143/258 (55.4%) | 237/606 (39.1%) | |
| Eligibility to ASCT | .043 | |||
| No | 280/875 (32.0%) | 106/291 (36.4%) | 171/577 (29.6%) | |
| Yes | 595/875 (68.0%) | 185/291 (63.6%) | 406/577 (70.4%) | |
| Age as reason for ineligibility to ASCT∗ | 174/226 (77.0%) | 64/88 (72.7%) | 109/136 (80.1%) | .745 |
| Cytogenetic test performed | 493/830 (59.4%) | 61/252 (24.2%) | 429/570 (75.3%) | <.001 |
| Characteristic . | Total (N = 1029) . | Public (N = 339) . | Private (N = 682) . | P value . |
|---|---|---|---|---|
| Age (mean ± SD), y | 63.4 ± 11.4 | 63.0 ± 10.9 | 63.5 ± 11.7 | .516 |
| Sex, female | 524/1029 (50.9%) | 156/339 (46.0%) | 363/682 (53.2%) | .030 |
| Country of origin, n (%) | <.001 | |||
| Argentina | 306/1029 (29.7%) | 17/339 (5.0%) | 289/682 (42.4%) | |
| Brazil | 435/1029 (42.3%) | 268/339 (79.1%) | 167/682 (24.5%) | |
| Colombia | 269/1029 (26.1%) | 53/339 (15.6%) | 212/682 (31.1%) | |
| Mexico and Panama | 19/1029 (1.8%) | 1/339 (0.3%) | 14/682 (2.1%) | |
| Race, White | 414/907 (45.6%) | 163/308 (52.9%) | 251/592 (42.4%) | .003 |
| Comorbidities present | 771/985 (78.3%) | 261/318 (82.1%) | 505/682 (76.5%) | .005 |
| Hemoglobin (mean ± SD), g/dL | 10.3 ± 2.39 | 9.6 ± 2.45 | 10.7 ± 2.26 | <.001 |
| Serum creatinine (mean ± SD), mg/dL | 1.80 ± 1.97 | 2.06 ± 2.17 | 1.69 ± 1.87 | .002 |
| Serum calcium (mean ± SD), mg/dL | 9.9 ± 2.91 | 9.9 ± 1.72 | 9.5 ± 1.73 | .146 |
| CRAB features present | ||||
| Hypercalcemia | 87/683 (12.7%) | 43/239 (18.0%) | 39/439 (8.9%) | <.001 |
| Renal dysfunction | 191/866 (22.1%) | 80/283 (28.3%) | 106/575 (18.4%) | .001 |
| Anemia | 414/916 (45.2%) | 173/295 (58.6%) | 237/615 (38.5%) | <.001 |
| Bone lesions | 671/837 (80.2%) | 209/245 (85.3%) | 458/587 (78.0%) | .016 |
| ISS stage | <.001 | |||
| I | 235/870 (27.0%) | 46/258 (17.8%) | 188/606 (31.0%) | |
| II | 252/870 (29.0%) | 69/258 (26.7%) | 181/606 (29.9%) | |
| III | 383/870 (44.0%) | 143/258 (55.4%) | 237/606 (39.1%) | |
| Eligibility to ASCT | .043 | |||
| No | 280/875 (32.0%) | 106/291 (36.4%) | 171/577 (29.6%) | |
| Yes | 595/875 (68.0%) | 185/291 (63.6%) | 406/577 (70.4%) | |
| Age as reason for ineligibility to ASCT∗ | 174/226 (77.0%) | 64/88 (72.7%) | 109/136 (80.1%) | .745 |
| Cytogenetic test performed | 493/830 (59.4%) | 61/252 (24.2%) | 429/570 (75.3%) | <.001 |
All numbers and percentages are based on patients with known information. Not all sums of percentages for categories within a variable add up to 100% because of rounding. Funding source could not be ascertained for 8 patients.
SD, standard deviation.
The same patient could have >1 reason, not shown here.
Treatment patterns
At baseline, 595 patients were considered as eligible to ASCT, but 156 of these patients (26.2%) did not undergo the procedure. The main reported reasons for this were an insufficient response, disease progression, or death before ASCT (35.9% of patients not undergoing ASCT); patient refusal (16.0%); and lack of access to the procedure (9.0%). At the beginning of LOT1, a total of 427 patients were eligible and underwent ASCT as part of their LOT1; among these patients, 99 were from public and 327 were from private institutions (for 1 patient, information on funding was not available); thus, 29.8% and 48.2% of patients from public and private institutions with available information, respectively, underwent ASCT in LOT1. Table 2 displays the distribution of LOT1 regimens among patients undergoing or not ASCT in public and in private institutions. There were clear differences in regimens used between these 2 types of institutions. For example, induction therapy for patients undergoing ASCT in public institutions was more often done with either an immunomodulatory drug–based (46.6%) or a proteasome inhibitor–based regimen (30.5%), whereas agents from these 2 classes were combined in 17.6% of patients in public institutions and 37.2% of those in private institutions. Of note, an anti-CD38 monoclonal antibody was used for induction in LOT1 in only 20 patients, 19 of which from the private system. For patients not undergoing ASCT, the most frequent LOT1 regimen in public institutions was also an immunomodulatory drug–based therapy (45.2%) or a proteasome inhibitor–based regimen (34.8%), whereas in private institutions the latter regimen was the 1 used more frequently (50.5%), followed by combined regimens with agents from the 2 classes (20.9%); once again, use of an anti-CD38 monoclonal antibody was infrequent (n = 31) and in all but 1 case used in the private system.
Regimens used in LOT1 according to receipt of ASCT and type of institution
| Regimen and treatment phase . | Public (N = 326∗) . | Private (N = 675∗) . |
|---|---|---|
| Patients who underwent ASCT | n = 98 | n = 325 |
| Induction† | ||
| Anti-CD38–based regimens | 1 (0.8%) | 19 (5.3%) |
| IMID-based regimens | 61 (46.6%) | 5 (1.4%) |
| PI-based regimens | 40 (30.5%) | 197 (55.5%) |
| PI + IMID-based regimens | 23 (17.6%) | 132 (37.2%) |
| Other | 6 (4.6%) | 2 (0.6%) |
| Consolidation†,‡ | ||
| Anti-CD38–based regimens | 1 (5.0%) | 12 (11.4%) |
| IMID-based regimens | 6 (30.0%) | 4 (3.8%) |
| PI-based regimens | 6 (30.0%) | 29 (27.6%) |
| PI + IMID-based regimens | 6 (30.0%) | 60 (57.1%) |
| Other | 1 (5.0%) | 0 (0%) |
| Maintenance†,‡ | ||
| Anti-CD38–based regimens | 0 (0%) | 0 (0%) |
| IMID-based regimens | 45 (69.2%) | 206 (83.4%) |
| PI-based regimens | 19 (29.2%) | 25 (10.1%) |
| PI + IMID-based regimens | 0 (0%) | 11 (4.5%) |
| Other | 1 (1.5%) | 5 (2.0%) |
| Patients who did not undergo ASCT | n = 227 | n = 348 |
| Anti-CD38–based regimens | 1 (0.4%) | 30 (8.0%) |
| IMID-based regimens | 122 (45.2%) | 40 (10.7%) |
| PI-based regimens | 94 (34.8%) | 204 (50.5%) |
| PI + IMID-based regimens | 25 (9.3%) | 78 (20.9%) |
| Other | 28 (10.4%) | 22 (5.9%) |
| Regimen and treatment phase . | Public (N = 326∗) . | Private (N = 675∗) . |
|---|---|---|
| Patients who underwent ASCT | n = 98 | n = 325 |
| Induction† | ||
| Anti-CD38–based regimens | 1 (0.8%) | 19 (5.3%) |
| IMID-based regimens | 61 (46.6%) | 5 (1.4%) |
| PI-based regimens | 40 (30.5%) | 197 (55.5%) |
| PI + IMID-based regimens | 23 (17.6%) | 132 (37.2%) |
| Other | 6 (4.6%) | 2 (0.6%) |
| Consolidation†,‡ | ||
| Anti-CD38–based regimens | 1 (5.0%) | 12 (11.4%) |
| IMID-based regimens | 6 (30.0%) | 4 (3.8%) |
| PI-based regimens | 6 (30.0%) | 29 (27.6%) |
| PI + IMID-based regimens | 6 (30.0%) | 60 (57.1%) |
| Other | 1 (5.0%) | 0 (0%) |
| Maintenance†,‡ | ||
| Anti-CD38–based regimens | 0 (0%) | 0 (0%) |
| IMID-based regimens | 45 (69.2%) | 206 (83.4%) |
| PI-based regimens | 19 (29.2%) | 25 (10.1%) |
| PI + IMID-based regimens | 0 (0%) | 11 (4.5%) |
| Other | 1 (1.5%) | 5 (2.0%) |
| Patients who did not undergo ASCT | n = 227 | n = 348 |
| Anti-CD38–based regimens | 1 (0.4%) | 30 (8.0%) |
| IMID-based regimens | 122 (45.2%) | 40 (10.7%) |
| PI-based regimens | 94 (34.8%) | 204 (50.5%) |
| PI + IMID-based regimens | 25 (9.3%) | 78 (20.9%) |
| Other | 28 (10.4%) | 22 (5.9%) |
IMID, immunomodulatory drug; PI, proteasome inhibitor.
A total of 20 of 1021 patients who could be classified according to funding source had missing information.
More than 1 regimen may have been used within LOT1. As a result, the total number of treatments across treatment categories may be larger than the number of patients.
Not all patients who underwent ASCT received consolidation or maintenance therapy.
The overall attrition rate between LOT1 and LOT2 was 23.4%; however, this rate was considerably different between patients undergoing (6.6%) and not undergoing (35.9%) ASCT. For LOT2, 122 and 282 patients from public and private institutions, respectively, had information on treatment regimens. The greatest difference in treatment choice was in the use of an anti-CD38 monoclonal antibody, reported in only 7.1% of cases in public institutions but in 30.3% in private ones. Once again, a regimen containing a proteasome inhibitor and an immunomodulatory drug was more frequently used in private (26.9%) than in public (19.1%) institutions. The overall attrition rate between LOT2 and LOT3 was 28.9%.
Treatment outcomes
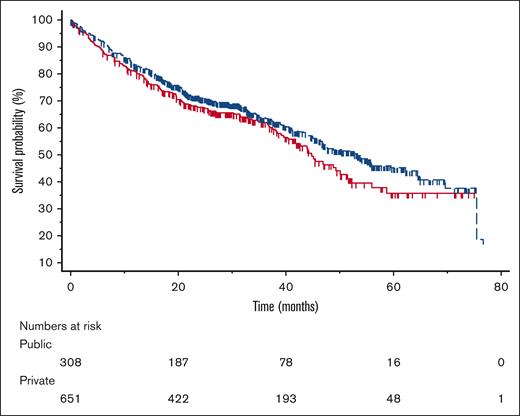
Complete data were available for the analysis of OS in 960 patients, who had a median follow-up of 38.2 months. Overall, the median OS was 48.7 months, with OS estimates of 82.5% at 12 months, 70.1% at 24 months, 63.0% at 36 months, 50.6% at 48 months, and 42.6% at 60 months. As shown in Figure 1, the median OS was 44.6 months for patients from public institutions and 53.3 for patients from private institutions (univariate hazard ratio [HR], 0.84 in favor of private institutions; 95% CI, 0.67-1.04; P = .109). Eligibility to ASCT and ISS stage, the only conventional prognostic factors tested, were significant univariate predictors of OS. The median OS was 37.4 and 75.4 months for ASCT-ineligible and eligible patients, respectively (HR, 0.35 in favor of ASCT-eligible patients; 95% CI, 0.27-0.45; P < .001). The median OS was not reached for stage I, and was 54.0 and 36.7 months for stages II and III, respectively (P < .001). Given the prognostic significance of ASCT eligibility and ISS stage, a multivariable model was fit to adjust the comparison of OS between public and private institutions. The adjusted HR for OS was 1.01 (95% CI, 0.77-1.32; P = .966).
Overall survival. OS among patients from public (red, solid line) and private (blue, dashed line) centers (tick marks represent censoring times).
Overall survival. OS among patients from public (red, solid line) and private (blue, dashed line) centers (tick marks represent censoring times).
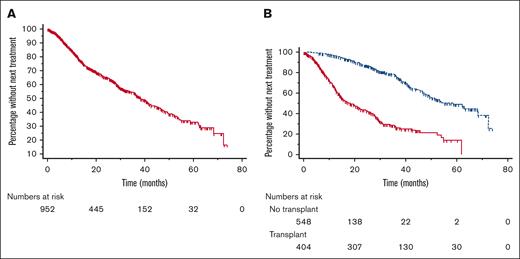
As a result of missing data, 954 of 960 patients analyzed for OS were also analyzed for TNTT between LOT1 and LOT2; 384 of these patients started LOT2, and 570 were censored. The median TTNT from LOT1 to LOT2 was 38.2 months (Figure 2A). When results were analyzed according to receipt of ASCT, the median TTNT was 18.4 months for patients not undergoing ASCT and 58.7 months for those undergoing the procedure (Figure 2B). Among 381 patients with complete data starting LOT2, 156 had an event (ie, started LOT3) and 229 were censored. The median TTNT from LOT2 to LOT3 was 21.15 months.
Time to next treatment from first to second line. TTNT from first to second line, overall (A), and according to receipt of ASCT (B). Red solid line indicates patients not undergoing transplantation, and blue dashed line indicates patients undergoing transplantation.
Time to next treatment from first to second line. TTNT from first to second line, overall (A), and according to receipt of ASCT (B). Red solid line indicates patients not undergoing transplantation, and blue dashed line indicates patients undergoing transplantation.
The assessment of MRD was performed in only 89 patients overall, always in LOT1 and most of them from private institutions.
Discussion
The current study discloses important diagnostic and therapeutic shortcomings in the management of MM in Latin America, with such shortcomings being more pronounced in public than in private health care institutions. The results that lend support to this conclusion can be summarized as follows: (1) regardless of the type of institution, patients with MM in Latin America are typically diagnosed in advanced stages of the disease, as evidenced by the distribution of ISS stages and frequency of CRAB features; (2) patients from public institutions have a less favorable prognostic profile than those from private institutions, including ISS stage and other prognostic features; (3) the median OS of 48.7 months overall is short when compared with current standards, although nearly two-thirds of patients were considered as ASCT eligible; (4) outcomes in public institutions are worse than in private ones; and (5) prognostic stratification is severely constrained, as indicated by the low frequency of use of cytogenetic testing and MRD evaluation, especially in public institutions.
The unfavorable ISS stage distribution of patients with MM in Latin American countries has been highlighted previously. In a first attempt to validate ISS in Brazil, 48.7% and 31.2% of patients diagnosed with MM between 1998 and 2004 had ISS stages II and III, respectively.18 In a subsequent observational study, this time enrolling patients diagnosed between 2005 and 2007 in Argentina, Brazil, Chile, Mexico, and Peru, the corresponding percentages were 36.5% and 34.2%.19 Finally, in the Hemato-Oncology Latin America study of patients from 7 Latin American countries diagnosed between 2006 and 2015, the percentages were 29.4% and 48.8%, very similar to the values found in this study (29.0% and 44.0%; see Table 1). Despite the seemingly low percentages of patients classified in ISS stage I in these Latin American cohorts (20.1% to 29.3%), real-world studies from North America, Europe, and Asia enrolling newly diagnosed patients within the last 15 years have found percentages of ISS stage I ranging from 20.6% to 28.7%.7,28-30 Therefore, the overall ISS stage distribution reported here can be considered typical of observational studies and may not explain the relatively poor outcomes observed. Nevertheless, the fact remains that this distribution is less favorable in public institutions, suggesting later diagnosis than in private institutions as the underlying cause. Although there may be numerous exceptions to this, based on local and country-level characteristics, these findings corroborate the perceived notion that patient profile indeed differs between public and private institutions in Latin America, with the latter displaying advantages in terms of waiting times and other constraints that may eventually affect patient profile and outcomes.
The median OS among all patients in this study (48.7 months) is somewhat short in comparison with other real-world studies, in which median OS among newly diagnosed patients (both eligible and ineligible to ASCT) from other world regions enrolled within the last 15 years ranged from 63.8 to 103.6 months.7,29,31-33 Moreover, results from this study compare unfavorably with previous real-world results from our own group showing a median OS among Latin American patients of 56 months.28 Those patients had been diagnosed with MM between 1998 and 2007; therefore, although the reasons for the lower median OS in this study remain unclear and might be ascribed to selection bias, the lack of improvement in OS over time is reasonable for even greater concern. One caveat in these comparisons is that in some real-world studies OS is measured from diagnosis, whereas here it was measured from treatment initiation. Nevertheless, the median OS times reported here, even if ASCT-eligible and ASCT-ineligible patients are considered separately (medians of 37.4 and 75.4 months, respectively), are considerably lower than those reported in clinical trials of novel agents, in most of which median OS is not reached at the time of analysis.12,13,34,35 Although cross-study comparisons are potentially flawed, especially between registry studies and clinical trials, results from this study raise the hypothesis that either patient- or disease-related characteristics (including genetic profile and the distribution of prognostic factors) or access to care underlie these unsatisfactory long-term outcomes. With regard to a potential influence of racial or genetic background as a determinant of outcomes in MM, an interesting analysis has suggested that, with equal access to treatment, Hispanics have survival similar to that of Non-Hispanic Whites and Non-Hispanic Blacks in the United States.36 Although this remains speculative, we surmise that at least part of our unfavorable results in terms of OS is because of insufficient access to state-of-the-art treatment. Insufficient access can be a result of the absence of a certain resource, such as ASCT or novel agents, or delays in the availability of such resources because of health care infrastructure. Of note, the introduction of novel agents is slow in Latin America even in the setting of privately funded treatments; in the specific case of MM, access to bispecific antibodies and chimeric antigen receptor T-cell therapy is still incipient.
The first point in investigating state-of-the-art management concerns ASCT. The seemingly low frequency of ASCT receipt as part of LOT1 reported here (41.5%) in fact compares favorably with that from other real-world studies. For example, in the Colombian registry, 28.3% of patients were consolidated with ASCT in the first line21; 23.8% of patients with newly diagnosed MM received an ASCT during the first year in the Province of Alberta, Canada,37 and 25% of patients in a US observational study38; 29% in a large study from France,32 and nearly one-third of Nordic patients with newly diagnosed MM underwent ASCT.39 In contrast, it should be considered that the rate of eligibility to ASCT in this study (68.0%) is somewhat high, suggesting a preferential inclusion of ASCT-eligible patients. Indeed, in a previous observational study from Latin America, only 45.9% of patients were eligible to ASCT.19 Likewise, 73.8% of ASCT-eligible patients in this study underwent the procedure, a rate that is high, for example, compared with that reported in the US study mentioned previously (43.7%), which encompasses a slightly longer period (2011-2019).38 Therefore, the relatively high frequency of ASCT eligibility and receipt further highlights the relatively poor median OS reported here, suggesting that it might be even shorter with a more typical distribution of eligibility to, and receipt of, ASCT, considering that the median OS of ASCT-ineligible patients was only 37.4 months. Moreover, our findings indicate that a greater proportion of patients (70.2%) in the public setting than in the private setting (51.8%) did not undergo ASCT in LOT1. This observation, when considered alongside the poor outcomes experienced by ASCT-ineligible patients, adds further evidence to support the hypothesis that long-term results are influenced, to some extent, by the lack of access to state-of-the-art treatment in the public setting. Once again, these findings corroborate the perceived notion that health care in private institutions is, on average, of superior quality in Latin America than that in public institutions.
Nevertheless, our results suggest that treating physicians are doing their best to take patients to ASCT but remain limited by infrastructural and economic constraints.
The second important point regarding state-of-the-art management, already alluded to above, is the relative lack of access to novel agents in Latin America, a world region in which the drug approval and reimbursement processes have historically lagged behind.40 A typical example of this problem is the fact that the vast majority of instances of an immunomodulatory drug in LOT1 (Table 2) actually refer to thalidomide and not lenalidomide, the latter still infrequently available in Latin America. Although we have no direct evidence of a causal link between lack of access and outcomes, the differences between public and private institutions in treatment patterns lend support to such a hypothesis. Twice as many patients from private institutions in this study received frontline combinations of immunomodulatory drugs and proteasome inhibitors than patients from public institutions. Likewise, nearly all treatment with anti-CD38 antibodies was performed in private institutions, even if in these more privileged institutions the frequency of use was low, considering that daratumumab, for example, was approved in the countries represented here between October 2016 and February 2018, overlapping with the study period. In international registry studies, there has been a steady increase in the use of newer agents (carfilzomib, pomalidomide, and anti-CD38 antibodies) after their introduction in different countries.38,41,42
The implication of any observed difference in OS between public and private institutions is that differences in patient profiles and access to treatment exist between these 2 health care systems. In fact, in our study patients from private institutions had a more favorable profile in terms of ISS stage and eligibility to ASCT, and adjustment for these factors eliminated the apparent, albeit not statistically significant, difference in OS. We have not adjusted the analysis for the type of treatment, because this adjustment for postbaseline (and not randomly assigned) factors could introduce bias in the estimated HR. Therefore, the current results corroborate previous indirect evidence based on a survey of hematologists,22 as well as findings from patient-level data specifically among those who were ASCT eligible,23 in terms of important differences between public and private institutions in Latin America. We should emphasize, moreover, that although the unadjusted difference in OS between public and private institutions was not statistically significant, the magnitude of the difference in medians (9 months) is of concern. If such a difference is confirmed in future studies, it will have deep policy implications.
The attrition rate between LOT1 and LOT2 observed in this study was 23.4% overall, 6.6% for patients undergoing ASCT and 35.9% for those not undergoing ASCT. In the study by Fonseca et al, the corresponding attrition rates were 21% for patients undergoing ASCT and 56.9% for those not undergoing ASCT.27 Although the same method for computing attrition rates was used in both studies, the reason for these large discrepancies remains unclear. Of note, however, Fonseca et al speculate that their use of insurance claims “may have led to the overestimation of attrition, because it included patients who were lost to follow-up due to a switch in insurance plans, loss of insurance coverage, or end of the study period.”27
Our study has some limitations, foremost among which its observational nature and our lack of control over the consecutive enrollment of all eligible patients, notwithstanding efforts to maximize this practice. Likewise, the extent to which the enrolled patients are representative of the Latin American patients with MM at large cannot be ascertained. Given the usual budget limitations and slower access to innovation in Latin American countries, and because of the plausibility that these problems plague public institutions more than private ones, we suggest that our findings reflect general trends in this world region. More importantly, data were missing for some variables with a considerable frequency, and the extent to which the estimated proportions of baseline variables are influenced by such missingness is likewise uncertain. Similarly, the median follow-up for OS in this cohort (38.2 months) is somewhat short, in part reflecting the proximity between the eligibility and the enrollment periods. Finally, socioeconomic status of the patients was not analyzed directly; nevertheless, it should be noted that socioeconomic status in Latin America is closely related to access to the 2 health care systems, with lower status being represented by public institutions as a general rule.
In conclusion, the MYLACRE study highlights existing diagnostic and therapeutic shortcomings in the management of MM in Latin America, with important gaps in patient profile, treatment patterns, and long-term outcomes between public and private institutions. More specifically, patients from public institutions have a less favorable prognostic profile, lower rates of ASCT, and a shorter median OS than those from private institutions. It is likely that this problem affects other countries and world regions where treatment can vary substantially according to different types of funding, and indeed there are data from the United States corroborating our findings in Latin America.43 Given these findings, efforts should continue to increase access to state-of-the-art diagnostic and therapeutic interventions for these patients, and such efforts should involve professional societies, patient associations, pharmaceutical companies, and governmental agencies across the region.
Acknowledgment
The authors thank Everardo D. Saad (Dendrix, São Paulo, Brazil) for medical writing and editorial support.
Authorship
Contribution: V.H. contributed to the conception, study design, and interpretation of the results, and performed the research; R.G., K.G., G.R., N.S., R.B., A.M., G.Q., M.S.C., W.M.T.B., C.C.V., E.C., A.I.E., G.C., J.B., F.L.M., J.F., C.L.S.M., and M.L. performed the research; H.P. contributed to the interpretation of the results; M.F. contributed to the conception and study design; J.S. supervised the project; D.C.T. contributed to the conception, study design, and interpretation of the results, and supervised the project; and all authors reviewed, approved, and contributed to the final manuscript.
Conflict-of-interest disclosure: V.H. reports study funding from Janssen; payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing, or educational events from AbbVie, Amgen, Bristol Myers Squibb, GlaxoSmithKline, Janssen, Pfizer, Sanofi, and Takeda; support for attending meetings and/or travel from Janssen; and participation on a data safety monitoring board or advisory board for Janssen. R.G. reports study funding from Janssen; honoraria for lectures from Janssen; support for attending national and international meetings, including travel expenses, from Janssen; and support for attending meetings and/or travel from Takeda and AbbVie. K.G. reports study funding from Janssen. G.R. reports study funding from Janssen; consulting fees from Janssen, Sanofi, and Pfizer; payment or honoraria for lectures, presentations, speakers' bureaus, manuscript writing, or educational events from Janssen and Sanofi; support for attending meetings and/or travel from Janssen and Sanofi; and participation on advisory board for Janssen, Pfizer, and Sanofi. N.S. reports study funding from Janssen; staff training in multiple myeloma from Janssen; payment or honoraria for lectures from Janssen and Sanofi; travel grant from Sanofi and Takeda; and participation on advisory boards for Janssen, Sanofi, and Pfizer. R.B. received study funding from Janssen. A.M. received study funding from Janssen; reports grants or contracts from any entity from CNPq and FAPERJ; reports consulting fees from Janssen, Takeda, Pfizer, Novartis, Sanofi, Amgen, AbbVie, and Bristol Myers Squibb; reports payment or honoraria for lectures, presentations, speakers' bureaus, manuscript writing, or educational events from Janssen, Takeda, Pfizer, Sanofi, Amgen, and Bristol Myers Squibb; reports participation on a data safety monitoring boards or advisory boards for Janssen, Bristol Myers Squibb, and Pfizer; and reports leadership or fiduciary role in other board, society, committee or advocacy group, paid or unpaid, from Associação Brasileira de Hematologia, Hemoterapia e Terapia Celular. G.Q. received study funding from Janssen; reports payment or honoraria for lectures from AstraZeneca and Amgen; received support for attending meetings and/or travel from Janssen and Roche; and reports participation on advisory boards for Janssen, Sanofi, and AstraZeneca. M.S.C. received study funding from Janssen; reports grants or contracts from any entity from Bristol Myers Squibb; received payment or honoraria for lectures, presentations, speakers' bureaus, manuscript writing, or educational events from AbbVie, AstraZeneca, Raffo, and Takeda; received support for attending meetings and/or travel from Takeda, AbbVie, Raffo, Sanofi, and BeiGene; reports participation on a data safety monitoring boards or advisory boards for Raffo and Sanofi; and reports leadership or fiduciary role in other board, society, committee, or advocacy group, paid or unpaid, and membership of the directive board (treasurer) with Argentinian Society of Hematology (unpaid). W.M.T.B. reports study funding from Janssen; received payment or honoraria for lectures, presentations, speakers' bureaus, manuscript writing, or educational events from Janssen, Bristol, and Sanofi; and reports support for attending meetings and/or travel from Janssen and Sanofi. C.C.V. reports study funding from Janssen. E.C. reports study funding from Janssen; research funding from Janssen; and payment or honoraria for lectures, presentations, speakers' bureaus, manuscript writing, or educational events from Janssen, Amgen, Takeda, Bristol Myers Squibb, GlaxoSmithKline, and Alexion. A.I.E. and G.C. report study funding from Janssen. J.B. reports study funding from Janssen. F.L.M. reports study funding from Janssen; is a researcher in clinical trials sponsored by Janssen-Cilag, Takeda, Novartis, and Bristol Myers Squibb; reports academical scientific presentations or products from lecturer in events sponsored by Janssen-Cilag, Takeda, Bristol Myers Squibb, and Amgen; reports consultancy activities for advisory boards sponsored by Janssen-Cilag, Takeda, and Bristol Myers Squibb; reports support for participation in congresses by Takeda, Janssen-Cilag, Bristol Myers Squibb, and Sanofi; reports consulting fees from Janssen-Cilag, Takeda, Bristol Myers Squibb, Amgen, Sanofi, and Pfizer; reports payment or honoraria for lectures, presentations, speakers' bureaus, manuscript writing, or educational events from Janssen-Cilag, Takeda, Bristol Myers Squibb, Amgen, Takeda, Sanofi, and Pfizer; reports payment for expert testimony from Janssen-Cilag, Takeda, Bristol Myers Squibb, Amgen, Takeda, Sanofi, and Pfizer; reports support for attending meetings and/or travel from Janssen-Cilag, Takeda, Bristol Myers Squibb, Amgen, Takeda, Sanofi, and Pfizer; and reports participation on a data safety monitoring boards or advisory boards for Janssen-Cilag, Takeda, Bristol Myers Squibb, Amgen, Takeda, Sanofi, and Pfizer. J.F. reports study funding from Janssen; payment or honoraria for lectures from Janssen, Sanofi, and AbbVie; support for attending meetings from Amgen, AbbVie, and Janssen; and participation on advisory boards for Sanofi and Valentech Pharma. C.L.S.M. reports study funding from Janssen; received grants from Amgen; reports payment or honoraria for lectures, presentations, speakers' bureaus, manuscript writing, or educational events from Pfizer, Amgen, AbbVie, Astellas, Janssen, Takeda, and Novo Nordisk; reports support for attending meetings and/or travel from Amgen, Novartis, and Janssen; reports participation on a data safety monitoring boards or advisory boards for Janssen and Sanofi; and reports leadership or fiduciary role in other board, society, committee, or advocacy group, paid or unpaid, as/for Vice-presidente Association Colombiana de Hemato-Oncología and Vocal del Consejo Colombiano de acreditación y recertificación medica. M.L. reports study funding from Janssen; reports grants or contracts from any entity from Roche, Bayer, AstraZeneca, and Janssen; received consulting fees from Roche, AstraZeneca, and Janssen; received payment or honoraria for lectures, presentations, speakers' bureaus, manuscript writing, or educational events from Roche, Astellas, and Janssen; received payment for expert testimony from Eli Lily, Roche, and Bristol; and received support for attending meetings and/or travel from Amgen, Janssen, Roche, AstraZeneca, and Pfizer. H.P. reports study funding from Janssen; holds stock or stock options in Janssen; and is an employee of, and owns stocks in, Johnson & Johnson. M.F. reports study funding from Janssen and is an employee of Janssen. J.S. reports study funding from, and is an employee of Janssen. D.C.T. reports study funding from, and is an employee of, Janssen, and holds stock or stock options in Johnson & Johnson.
Correspondence: Vania Hungria, Clínica Sao Germano, R. Comendador Miguel Calfat, 217 Vila Nova Conceição, São Paulo, SP 04537-081, Brazil; email: hungria@dialdata.com.br.
References
Author notes
Data are available on request from the corresponding author, Damila Cristina Trufelli (dtrufell@ITS.JNJ.com), and will be evaluated case by case.