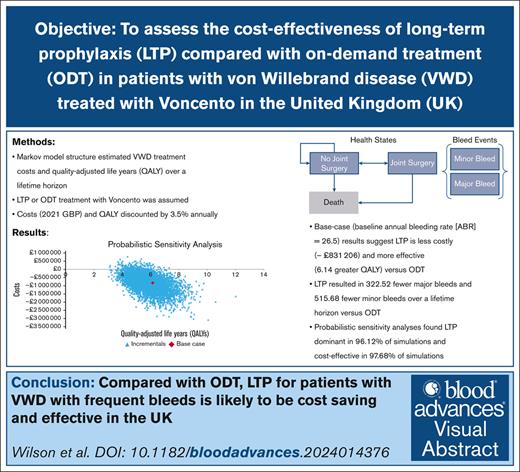
Key Points
The cost-effectiveness of Voncento prophylaxis vs ODT for VWD in the United Kingdom was studied.
Results suggest that prophylaxis with Voncento is more effective and cost saving than ODT in the United Kingdom.
Visual Abstract
von Willebrand factor (VWF) concentrates may be required for on-demand treatment (ODT) or long-term prophylaxis (LTP) in von Willebrand disease (VWD). This study assesses the cost-effectiveness of LTP compared with ODT in patients with VWD treated with Voncento in the United Kingdom. A Markov structure was developed to estimate the quality-adjusted life years (QALYs) and costs of VWD treatment over a lifetime horizon. Treatment options included ODT or LTP. For both options, we assumed plasma-derived VWF/factor VIII 2.4:1 (Voncento) as the VWF product used. Clinical parameters were obtained from published literature and Voncento’s summary characteristics. Utility weights were obtained from published literature. Costs (in 2021 GBP [£]) and outcomes were discounted annually by 3.5%. Sensitivity analyses were conducted. Three baseline annual bleed rate (ABR) scenarios (11, 26.5, and 39.6) were considered. In the base-case analyses, Voncento LTP resulted in lower costs (–£831 206) and greater QALYs (6.14) vs ODT. Savings were primarily due to reductions in product use required (–£529 571) and bleed-related other medical costs (–£301 352). Compared with ODT, LTP also resulted in 322.52 fewer major bleeds and 515.68 fewer minor bleeds over a lifetime horizon. Probabilistic sensitivity analyses showed dominance in 96.12% of simulations and cost-effectiveness in 97.68% of simulations. For the 39.6 ABR scenario also, LTP was dominant compared with ODT. Results suggest that Voncento LTP is more effective and cost saving than ODT in the United Kingdom for patients with VWD with higher ABR. Prophylaxis for patients with frequent bleeds is likely to be a cost-saving and effective strategy.
Introduction
von Willebrand disease (VWD) is the most common inherited bleeding disorder, affecting ∼1 in 1000 individuals.1 The disease is classified in different categories, ranging from type 1 (partial quantitative deficiency of von Willebrand factor [VWF]) to type 3 (virtually complete deficiency of VWF).2 Patients with VWD may experience excessive bleeding events, resulting in morbidity and reduced quality of life; in addition, VWD presents a substantial economic burden to the health care system and patients.3-6
Approaches to the management of VWD include VWF replacement therapy, delivered either as on-demand treatment (ODT) at the time of bleeds or as long-term prophylaxis (LTP) for their prevention. Several studies have suggested that LTP leads to a reduction in bleeds compared with ODT in the management of bleed events.7-10 According to recent treatment guidelines, LTP is recommended in individuals with severe and/or frequent bleeds.11
Although some clinical data for ODT and LTP are available, economic analyses of VWD treatment strategies are sparse. Recent data suggest LTP to be cost-effective compared with ODT in the United States.12 However, to our knowledge, no published studies have estimated the cost-effectiveness of treatment strategies from a UK population. As such, the objective of this study was to assess the cost-effectiveness of LTP vs ODT treatment strategies in patients with VWD with different baseline annual bleed rate (ABR). We conducted the analysis considering a plasma-derived VWF/factor VIII 2.4:1 product (Voncento) in the United Kingdom.
Methods
Model structure
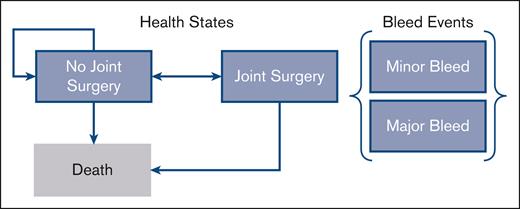
We developed a Markov structure with 6-monthly cycles to estimate the cost-effectiveness of treatment strategies in patients aged ≥12 years diagnosed with VWD and eligible for LTP or ODT with Voncento. Within this model structure, patients incur risk of bleed events (major or minor bleeds) during each cycle, depending on the treatment strategy. Because joint bleeds can lead to progressive joint damage in patients with VWD, the model also considered the development and treatment of arthropathy with joint surgery (Figure 1). The risk of requiring joint surgery was estimated based on patient’s Pettersson score over time; those with a Pettersson score of 28 would require joint surgery, based on a previous study in hemophilia A.13,14 Patients aged >80 years were assumed to forgo surgery even if their Pettersson score reached 28.
Markov structure. The Markov structure includes 3 health states: “no joint surgery,” “joint surgery,” and “death.” Within these health states, patients incur a risk of major and minor bleed events each cycle. The risk of joint surgery is dependent on the number of joint bleeds incurred. Risk of death is a function of age and sex.
Markov structure. The Markov structure includes 3 health states: “no joint surgery,” “joint surgery,” and “death.” Within these health states, patients incur a risk of major and minor bleed events each cycle. The risk of joint surgery is dependent on the number of joint bleeds incurred. Risk of death is a function of age and sex.
Each model health state was associated with a cost and quality-of-life impact. Costs are presented in 2021 GBP (£). The model time horizon was that of a patient’s lifetime with 6-month cycle length, and a national payer perspective was taken. Costs and outcomes were discounted at 3.5%.15
Comparators
The comparison modeled was LTP vs ODT. For the VWF product, we selected Voncento because it represents the majority share of VWD product use in the United Kingdom.16 Dosing details for each strategy are presented in Table 1.
Model parameters
| Parameter . | . | Estimate . | . |
|---|---|---|---|
| Baseline ABR | |||
| Baseline annual number of bleeds | |||
| Low9 | 11.0 | ||
| Medium (base case)8 | 26.5 | ||
| High7 | 39.6 | ||
| LTP treatment-specific inputs | Estimate | ||
| LTP dosing, VWF IU/kg | |||
| Dose strength, VWF IU/kg | 32.5 | ||
| Dose frequency, doses per week | 2 | ||
| Derived relative risk of bleed for LTP8 | 0.04 | ||
| Bleed-related inputs | Minor bleed | Major bleed | |
| Dosing for bleeds, VWF IU/kg | |||
| Dose strength, VWF IU/kg | 30.0 | 45.0 | |
| Daily doses | 2 | 2 | |
| Duration of treatment, d | 1 | 4 | |
| Probability of medical resource use | |||
| Outpatient visit | 25.0% | 50.0% | |
| Inpatient stay for hospitalization | 0.0% | 50.0% | |
| Percentage of bleeds that are joint bleeds17 | 24.0% | 24.0% | |
| Severity of bleeds while receiving ODT8 | 61.84% | 38.16% | |
| Severity of bleeds while receiving prophylaxis treatment8 | 70.00% | 30.00% | |
| Cost inputs | Unit cost | ||
| Voncento list price per IU of VWF18 | ₤0.32 | ||
| Other costs19 | |||
| Outpatient visit | £166.51 | ||
| Inpatient stay for hospitalization | £2992.13 | ||
| Joint surgery costs | £1383.50 | ||
| Weight inputs | Males | Females | |
| Average patient weight, kg20 | |||
| 11-12 y | 46.90 | 47.06 | |
| 13-15 y | 60.99 | 58.29 | |
| 16-24 y | 77.54 | 65.80 | |
| 25-34 y | 85.65 | 72.56 | |
| 35-44 y | 86.82 | 74.47 | |
| 45-54 y | 88.84 | 75.69 | |
| 55-64 y | 87.98 | 73.86 | |
| 65-74 y | 87.45 | 71.73 | |
| 75+ y | 79.98 | 67.34 | |
| Utility inputs | PS 0 | PS 1-27 | Surgery |
| Health state utility weights21-24 | |||
| Age 0-30 y | 0.94 | 0.82 | 0.72 |
| Age 31-40 y | 0.84 | 0.74 | 0.65 |
| Age 41-50 y | 0.86 | 0.69 | 0.61 |
| Age 51-60 y | 0.83 | 0.63 | 0.56 |
| Age 61-100 y | 0.73 | 0.54 | 0.48 |
| Parameter . | . | Estimate . | . |
|---|---|---|---|
| Baseline ABR | |||
| Baseline annual number of bleeds | |||
| Low9 | 11.0 | ||
| Medium (base case)8 | 26.5 | ||
| High7 | 39.6 | ||
| LTP treatment-specific inputs | Estimate | ||
| LTP dosing, VWF IU/kg | |||
| Dose strength, VWF IU/kg | 32.5 | ||
| Dose frequency, doses per week | 2 | ||
| Derived relative risk of bleed for LTP8 | 0.04 | ||
| Bleed-related inputs | Minor bleed | Major bleed | |
| Dosing for bleeds, VWF IU/kg | |||
| Dose strength, VWF IU/kg | 30.0 | 45.0 | |
| Daily doses | 2 | 2 | |
| Duration of treatment, d | 1 | 4 | |
| Probability of medical resource use | |||
| Outpatient visit | 25.0% | 50.0% | |
| Inpatient stay for hospitalization | 0.0% | 50.0% | |
| Percentage of bleeds that are joint bleeds17 | 24.0% | 24.0% | |
| Severity of bleeds while receiving ODT8 | 61.84% | 38.16% | |
| Severity of bleeds while receiving prophylaxis treatment8 | 70.00% | 30.00% | |
| Cost inputs | Unit cost | ||
| Voncento list price per IU of VWF18 | ₤0.32 | ||
| Other costs19 | |||
| Outpatient visit | £166.51 | ||
| Inpatient stay for hospitalization | £2992.13 | ||
| Joint surgery costs | £1383.50 | ||
| Weight inputs | Males | Females | |
| Average patient weight, kg20 | |||
| 11-12 y | 46.90 | 47.06 | |
| 13-15 y | 60.99 | 58.29 | |
| 16-24 y | 77.54 | 65.80 | |
| 25-34 y | 85.65 | 72.56 | |
| 35-44 y | 86.82 | 74.47 | |
| 45-54 y | 88.84 | 75.69 | |
| 55-64 y | 87.98 | 73.86 | |
| 65-74 y | 87.45 | 71.73 | |
| 75+ y | 79.98 | 67.34 | |
| Utility inputs | PS 0 | PS 1-27 | Surgery |
| Health state utility weights21-24 | |||
| Age 0-30 y | 0.94 | 0.82 | 0.72 |
| Age 31-40 y | 0.84 | 0.74 | 0.65 |
| Age 41-50 y | 0.86 | 0.69 | 0.61 |
| Age 51-60 y | 0.83 | 0.63 | 0.56 |
| Age 61-100 y | 0.73 | 0.54 | 0.48 |
IU, international unit; PS, Pettersson score.
Model inputs
All model inputs can be found in Table 1. We describe these inputs in the following subsections.
General inputs
The model population assumed an average patient age of 43.98 years, with 51.95% male, all having VWD and being eligible for prophylaxis. An internal systematic literature review and pooled analysis of clinical studies in VWD conducted by CSL Behring estimated each of these assumptions. Patient weight was based on age.20
There is substantial heterogeneity within the VWD population, and the decision to treat with LTP is not necessarily uniform. As such, we did not model the cost-effectiveness of treatment within a particular subgroup of the VWD population (eg, type 1, type 2, or type 3). Instead, we modeled based on the baseline ABR within the population to determine the potential cost-effectiveness of LTP vs ODT among individuals who may be eligible for LTP. We assumed that the distribution of the modeled VWD population is similar to the populations eligible for prophylaxis clinical trials.7-9
Bleed risk inputs
The modeled ABR for patients on ODT and the relative risk of bleeds for LTP compared with ODT were derived from published clinical studies.7-9 Due to variability in the risk of bleeds within the VWD population and the expected impact of bleed risk on results, 3 different baseline bleed risk scenarios were considered: lower ABR (11); base-case ABR (26.5); and higher ABR (39.6). Based on a previous study, major bleeding events (nonsurgical) included any bleeding into a joint or muscle, or in the brain, or a mucosal bleeding of the gastrointestinal tract (excluding nasal or oral bleeding). All other bleeding events were classified as “minor,” unless the investigator assessment noted otherwise.8 Based on this study, we estimated that 62% of bleeds experienced by individuals on ODT were minor, and 70% of bleeds for those on LTP were minor.8
Joint surgery risk inputs
The joint surgery health state represents the treatment for arthropathy within the model. The per-cycle risk of joint surgery was assumed to be a function of joint bleed events. Due to limited data in the VWD population, we applied an approach similar to previous studies in hemophilia based on the Pettersson score. The Pettersson score is a radiological scoring system to classify the degree of joint damage to the elbows, knees, and ankles.25 In the model, we assume that with every 12.6 joint bleeds a patient’s Pettersson score increases by 1 point.13,14 The model assumed the baseline Pettersson score to be 14 and that joint surgery occurs when Pettersson score reaches 28.14,21 To estimate the number of joint bleeds incurred, we assumed that 24% of bleeds in VWD are joint bleeds, based on a published study of bleeding patterns in individuals with VWD.17 Thus, the increase in Pettersson score each 6-month cycle was calculated as follows: (ABR × 6 months/12 months) × 24%/12.6.
Efficacy inputs
Relative risks of bleeds for LTP regimens compared with ODT were estimated by dividing the ABR for LTP by the ABR for ODT using a published LTP study for Voncento.7,8 Specifically, we divided the observed ABR for LTP by the ABR for ODT to estimate the relative risk reduction for LTP. We then used this relative risk applied to the baseline ABR for the modeled population, assuming the relative risk to be constant regardless of baseline ABR.
Cost inputs
Because net prices are not publicly available, list prices per VWF international unit were obtained from a standard published source18 to illustrate the potential cost impact. Prophylaxis dose level and frequency were obtained from the summary of product characteristics.26 ODT costs were estimated using the recommended dose per international unit, the number of daily doses, and the duration of treatment, obtained from the summary of product characteristics.26 Early hemarthrosis and more extensive hemarthrosis were used as proxies for minor and major bleeds, respectively. Costs to treat minor and major bleeds were obtained from a standard costing source.19 We conservatively assumed no difference in VWF product dosage per bleed between patients on LTP and ODT.
Quality-of-life inputs
Due to the lack of data in patients with VWD, we used patients with hemophilia as a proxy for age-specific and joint surgery utilities from previous hemophilia studies.21-24 Quality-adjusted life year (QALY) decrements by major and minor bleeds were obtained from a published utility study of antiplatelet therapy.27
Mortality inputs
The model considered all-cause mortality based on age and sex.28 To be conservative with respect to the benefits of LTP, no disease-specific mortality risk adjustments associated with bleed risk were included.
Model calculations
For the cost-effectiveness analysis, the incremental cost-effectiveness ratio (ICER) was estimated as follows: ICER = (CL – Co)/(EL – Eo), in which CL is the cost accrued over the chosen time horizon for LTP; Co is the cost accrued over the chosen time horizon for ODT; EL is the effectiveness (QALY) accrued over the chosen time horizon for LTP; and Eo is the effectiveness accrued over the chosen time horizon for ODT.
Sensitivity analyses
To test the robustness of the model assumptions and specific parameters, we examined the effect on the ICER of changing 1 parameter at a time using 1-way sensitivity analyses (OWSAs). Individual parameters were varied within plausible ranges of values from the literature, standard errors, 95% confidence intervals, or ±20% change (when data on ranges are not available). Sensitivity results for each input were ranked from most to least sensitive and plotted on a tornado diagram. Drug price was excluded from the sensitivity analyses because prices are assumed to be known with certainty.
In addition to OWSAs, we also performed probabilistic sensitivity analyses (PSAs; second-order Monte Carlo simulation), in which all included parameters were varied simultaneously. Analyses were run 5000 times to evaluate the stability of the results. The results of the PSAs are presented in the form of a scatterplot. Both the OWSAs and PSAs were run using the base-case ABR population.
Finally, scenario analyses were conducted on the baseline ABR and on the resource use (hospitalizations and outpatient visits) required to treat bleed events. Specifically, for the ABR scenarios, we considered a lower ABR and a higher ABR scenario, in line with the baseline ABR from 2 other published clinical studies in VWD.7,9 For the resource use scenarios, we assumed a lower bound and upper bound of resource use. Details on each can be seen in the results.
Results
Results of the base-case analyses for each treatment regimen can be seen in Table 2. LTP was both less costly and more effective than ODT, that is, LTP was more effective and cost saving than ODT. Specifically, LTP was expected to result in a QALY gain of 6.14 compared with ODT, driven by a reduction in major bleeds (–323), minor bleeds (–516), and joint surgeries (–0.62). LTP was also expected to result in substantial cost savings (–£831 206) compared with ODT. Notably, these cost savings included 23.7% reductions (–£529 571) in product costs: prophylaxis costs (£1 636 822) were more than offset by savings in incremental bleed-related product costs (£2 166 393). LTP was also expected to reduce bleed-related other medical costs (–£301 352) and joint surgery costs (–£283).
Base case results: costs and outcomes in a medium baseline ABR scenario
| Parameter . | Voncento LTP . | Voncento ODT . |
|---|---|---|
| Costs | ||
| Total costs | £1 718 847 | £2 550 053 |
| Drug costs | £1 709 462 | £2 239 033 |
| Prophylaxis | £1 636 822 | £0 |
| Bleed-related | £72 640 | £2 239 033 |
| Other medical costs | ||
| Outpatient visit | £1010 | £28 440 |
| Hospitalization | £8375 | £282 297 |
| Joint surgeries | £0 | £283 |
| Outcomes | ||
| QALYs | 11.30 | 5.16 |
| Life years | 32.63 | 32.63 |
| Joint bleeds | 7.32 | 192.00 |
| Joint surgeries | 0.00 | 0.62 |
| Minor bleeds | 23.01 | 538.69 |
| Major bleeds | 9.86 | 332.38 |
| Incremental cost per QALY gained | ||
| Voncento LTP vs Voncento ODT | –£135 311/QALY gained (Voncento LTP dominates) |
| Parameter . | Voncento LTP . | Voncento ODT . |
|---|---|---|
| Costs | ||
| Total costs | £1 718 847 | £2 550 053 |
| Drug costs | £1 709 462 | £2 239 033 |
| Prophylaxis | £1 636 822 | £0 |
| Bleed-related | £72 640 | £2 239 033 |
| Other medical costs | ||
| Outpatient visit | £1010 | £28 440 |
| Hospitalization | £8375 | £282 297 |
| Joint surgeries | £0 | £283 |
| Outcomes | ||
| QALYs | 11.30 | 5.16 |
| Life years | 32.63 | 32.63 |
| Joint bleeds | 7.32 | 192.00 |
| Joint surgeries | 0.00 | 0.62 |
| Minor bleeds | 23.01 | 538.69 |
| Major bleeds | 9.86 | 332.38 |
| Incremental cost per QALY gained | ||
| Voncento LTP vs Voncento ODT | –£135 311/QALY gained (Voncento LTP dominates) |
Scenario analyses results can be seen in Table 3. Baseline bleed risk was a substantial determinant in the cost-effectiveness results, whereas hospital and outpatient resource use per bleed did not have a significant effect on the results. In all but the lower baseline ABR scenario, LTP remained the dominant strategy in all scenarios.
Scenario analysis results
| Scenario . | Total cost . | Total QALY . | ICER . | ||
|---|---|---|---|---|---|
| LTP . | ODT . | LTP . | ODT . | ||
| Base case | £1 718 847 | £2 550 053 | 11.30 | 5.16 | –£135 311 |
| Baseline ABR, low (11) | £1 670 870 | £1 058 395 | 11.42 | 8.86 | £239 150 |
| Baseline ABR, high (39.6) | £1 759 395 | £3 810 809 | 11.20 | 2.04 | –£223 864 |
| Lower bound bleed resources used∗ | £1 709 928 | £2 255 026 | 11.30 | 5.16 | –£88 736 |
| Upper bound bleed resources used† | £1 730 338 | £2 900 505 | 11.30 | 5.16 | –£190 490 |
| Scenario . | Total cost . | Total QALY . | ICER . | ||
|---|---|---|---|---|---|
| LTP . | ODT . | LTP . | ODT . | ||
| Base case | £1 718 847 | £2 550 053 | 11.30 | 5.16 | –£135 311 |
| Baseline ABR, low (11) | £1 670 870 | £1 058 395 | 11.42 | 8.86 | £239 150 |
| Baseline ABR, high (39.6) | £1 759 395 | £3 810 809 | 11.20 | 2.04 | –£223 864 |
| Lower bound bleed resources used∗ | £1 709 928 | £2 255 026 | 11.30 | 5.16 | –£88 736 |
| Upper bound bleed resources used† | £1 730 338 | £2 900 505 | 11.30 | 5.16 | –£190 490 |
Lower bound for minor bleeds, outpatient 0% and inpatient 0%; for major bleeds, outpatient 50% and inpatient 0%.
Upper bound for minor bleeds, outpatient 10% and inpatient 10%; for major bleeds, outpatient 0% and inpatient 100%.
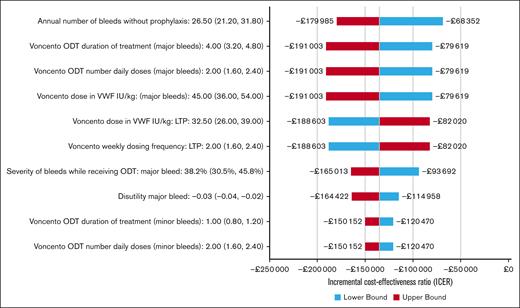
Results of the base-case OWSAs can be seen in Figure 2; because many parameters were examined in the OWSAs, we have plotted the top 10 most sensitive parameters in the tornado diagram for easy viewing. Results were most sensitive to the estimates of baseline annual number of bleeds; dose strength, duration, and number of daily doses for treatment of major bleeds; LTP dosing and dose frequency; and severity of bleeds with ODT. However, LTP remained more effective and cost saving in all individual parameter variation.
Tornado diagram for OWSAs. The OWSAs are presented as a tornado diagram, which illustrates the impact of individual parameter variation on incremental cost-effectiveness results. The 10 most sensitive parameters are presented.
Tornado diagram for OWSAs. The OWSAs are presented as a tornado diagram, which illustrates the impact of individual parameter variation on incremental cost-effectiveness results. The 10 most sensitive parameters are presented.
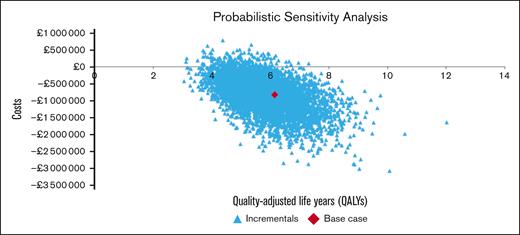
The PSAs results for the base-case analyses can be seen in Figure 3. Compared with ODT, LTP was found to be cost saving in 96.12% of simulations and cost-effective in 97.68% of simulations at willingness-to-pay thresholds of ₤20 000 per QALY.
Probabilistic cost-effectiveness scatterplot. The PSAs are presented as a scatterplot of the 5000-iteration, second-order Monte Carlo simulation.
Probabilistic cost-effectiveness scatterplot. The PSAs are presented as a scatterplot of the 5000-iteration, second-order Monte Carlo simulation.
Discussion
This analysis evaluated the potential economic impact of treatments for patients with VWD from a UK perspective. Specifically, we examined the cost-effectiveness (ie, value for money) of prophylaxis treatment with Voncento vs using only ODT. This is, to our knowledge, the first published cost-effectiveness modeling analysis of VWD prophylaxis from a UK perspective.
In the base-case analysis comparing LTP vs ODT in patients treated with Voncento in the United Kingdom, LTP was found to reduce VWF product costs (including LTP and bleed-related product costs) by 23.7%, in addition to reducing costs for other medical resource use (hospitalizations, outpatient visits, and joint surgeries). Patients on LTP were also expected to incur fewer bleeds and joint surgeries while gaining more QALYs than those on ODT.
The scenario analyses conducted suggest that, despite substantial heterogeneity in the population and parameter uncertainty, LTP with Voncento is likely to be cost-effective. In OWSAs, no parameters resulted in LTP not being cost saving. In scenario analyses comparing LTP with ODT, all scenarios showed LTP to be cost saving and more effective except when considering a baseline ABR of 11. In PSAs, Voncento LTP was dominant over ODT in >95% of simulations.
There are a few limitations of this analysis. First, studies used to obtain efficacy measurements had small sample sizes, which may be problematic in a VWD population because bleed risk is highly variable. Second, limited data on the long-term risk of arthropathy in a VWD population made it challenging to assess the risk and impact of developing arthropathy in these patients. However, any underestimation of the risk of joint surgery in a VWD population would only underestimate the value of LTP, and thus, our findings are likely conservative. Furthermore, the use of data from a hemophilia population for arthropathy risk introduces uncertainty. However, as seen in the OWSAs, the risk of arthropathy was not found to be a major driver of cost-effectiveness in this study. Additionally, the price of VWF product is based on published list prices and, as such, may not reflect locally agreed prices. Thus, although these results are approximations and illustrative, they importantly demonstrate relative cost savings irrespective of product price. This study shows that there are important cost savings to the health care system with prophylaxis; the exact amount is dependent on the confidential price agreements VWD products have in place. Lastly, substantial heterogeneity exists among the baseline bleed risks observed in VWD.8 Thus, we explored scenarios with different baseline bleed risks. Despite these limitations, the results were fairly robust to parameter variation.
Although this study was conducted assuming a UK VWD population, the results themselves may be illustrative for other countries. Because the majority of the VWD care costs modeled were found to be VWF product related, the applicability of these results to other country settings would depend on VWF product costs and the baseline ABR within that country. For countries where patients tend to have a higher ABR, LTP will likely be more cost-effective. Conversely, in countries where patients typically have a lower ABR, LTP will be less cost-effective. The price of VWF and the acceptable cost-effectiveness ratio will also have an impact on the cost-effectiveness of LTP within a given country.
Because there is substantial heterogeneity within the VWD population, we would not interpret the cost-effectiveness of LTP to be applicable to all patients with VWD; for those with a low ABR, we would not expect LTP to be cost-effective. However, the results of this analysis suggest that LTP is cost-effective compared with ODT in medium to high ABR populations. Despite the product costs associated with LTP, the reduction in bleed events with LTP compared with ODT and their associated direct medical costs (product costs, hospitalization, and outpatient visits) more than offset the incremental product costs of LTP. LTP can also improve the patient's quality of life by reducing the risk of bleeds and the associated morbidity from experiencing these bleeds. As such, LTP should be the recommended strategy for patients with medium to high ABRs.
Acknowledgments
The authors acknowledge the following individuals for their helpful input during the development of this model: Andrew Davies (University of Glasgow, Glasgow, United Kingdom); Jaime Garcia-Chavez (La Raza National Medical Center, Mexico City, Mexico); Paula James (Queens University, Kingston, Ontario, Canada); Jose Luis Lamas (Sotero del Rio Hospital, Puente Alto, Chile); Sandra Santos (formerly at CSL Behring, Hattersheim am Main, Germany); and Pavel Zarkhov (Dmitry Rogachev National Medical Research Centre of Pediatric Hematology, Oncology and Immunology, Moscow, Russia).
This study was conducted by RTI Health Solutions under the direction of CSL Behring and was funded by CSL Behring. Editorial support was provided by Meridian HealthComms (part of the Bioscript Group), funded by CSL Behring.
Authorship
Contribution: M.W. contributed to the model conceptualization and development, data collection, running of analyses, and manuscript development; C. McDade contributed to the data collection, model programming, running of analyses, and drafting of the manuscript; S.Y. contributed to the study concept, model conceptualization and development, data collection, and drafting of the manuscript; W.M., G.E., and G.C. contributed to the model conceptualization and critical revision of the manuscript; W.T., C. Millar, and R.T. contributed to critical review of the project scope with respect to the UK perspective and critical revision of the manuscript; and all authors agreed on manuscript content, provided critical review and revisions to the manuscript during its development, and read and approved the final manuscript.
Conflict-of-interest disclosure: M.W. and C. McDade are employees of RTI Health Solutions, which received funding from CSL Behring for the development of the model and manuscript. G.C. has served as a speaker/participant on advisory committees for Baxalta, Bayer, CSL Behring, Pfizer, SOBI, Novo Nordisk, uniQure, Roche, and Kedrion; and received research support from Pfizer, SOBI, and CSL Behring. W.T. has received speaker fees from AstraZeneca, Alexion, Bayer, CSL Behring, Novo Nordisk, Portola, Pfizer, SOBI, Sanofi, and Takeda; and participated in advisory boards for Sanofi, Takeda, Pfizer, LFB Biopharmaceuticals, Grifols, and Ablynx. C. Millar has received research support from Baxter/Takeda, CSL Behring, and Grifols; and honoraria or consultation fees from CSL Behring, LFB, Octapharma, and Takeda. G.E. has received honoraria/consultant fees from Bayer, Bristol Myers Squibb, Boehringer Ingelheim, CSL Behring, Novo Nordisk, and Pfizer. W.M. has received consultant and personal fees from Bayer, Biotest, CSL Behring, Pfizer, Octapharma, LFB, SOBI, Biogen, and BPL. R.T. and S.Y. are employees of CSL Behring.
Correspondence: Songkai Yan, CSL Behring, 1020 First Ave, King of Prussia, PA 19406; email: Songkai.Yan@cslbehring.com.
References
Author notes
Data are available on request from the corresponding author, Songkai Yan (Songkai.Yan@cslbehring.com).