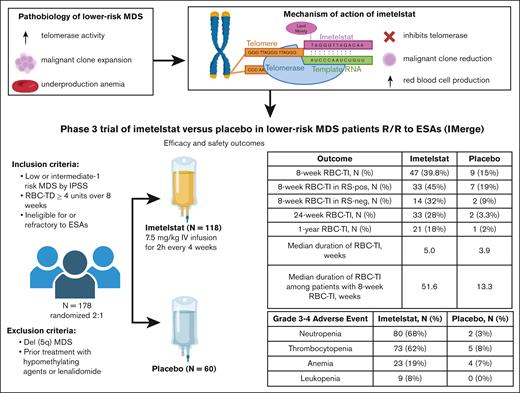
Imetelstat for lower-risk MDS. ESA, erythropoietin-stimulating agent; IPSS, International Prognostic Scoring System; neg, negative; pos, positive; R/R, relapsed/refractory; RBC-TD, red blood cell transfusion-dependent; RS, ring sideroblast.
Imetelstat for lower-risk MDS. ESA, erythropoietin-stimulating agent; IPSS, International Prognostic Scoring System; neg, negative; pos, positive; R/R, relapsed/refractory; RBC-TD, red blood cell transfusion-dependent; RS, ring sideroblast.
Concept
Telomerase maintains telomere length at the terminal strands of DNA to protect adjacent coding regions during cell replication.1 Imetelstat is a 13-mer oligonucleotide and first-in-class telomerase inhibitor (see figure), initially studied in early-phase trials, that achieved red blood cell transfusion independence (RBC-TI) end points in lower-risk myelodysplastic syndrome (MDS; LR-MDS).2
Evolution
The IMerge study (see figure) met the primary end point of 8-week RBC-TI, resulting in the US Food and Drug Administration approval of imetelstat for the treatment of LR-MDS with transfusion-dependent anemia.3,4 Rates of grade 3 to 4 bleeding and febrile neutropenia were similar between both groups.
Application
Imetelstat represents a new treatment option for patients with transfusion-dependent LR-MDS who are ineligible for, or refractory to, erythropoietin-stimulating agents or luspatercept.5 Of note, the US Food and Drug Administration approval of imetelstat after erythropoietin-stimulating agent failure or ineligibility is not limited to patients with ring sideroblast morphology. The likelihood of RBC-TI will have to be weighed against the agent’s significant myelosuppressive effects, as outlined in the figure. Additional data are needed regarding relative efficacy of imetelstat in patients previously treated with luspatercept and the optimal sequencing of these therapies.
As reported in the IMerge data, imetelstat resulted in numerically higher cytogenetic responses, decrease in ring sideroblast morphology, and a modest decrease in variant allele frequency in pathogenic mutations including SF3B1 in LR-MDS. Responses were observed across a broad spectrum of mutations, including high-risk molecular alterations.3
Future steps
Whether the molecular, cytogenetic, and morphologic responses reported represent a significant disease-modifying treatment effect is worthy of further study. Imetelstat is also being investigated as a single agent and in combination regimens in high-risk MDS and acute myeloid leukemia.
Conflict-of-interest disclosure: O.O. discloses research funding (paid to institution) from AbbVie, AstraZeneca, Celgene, Curis, Incyte, Shattuck Labs, and K-Group Alpha; scientific advisory board participation for AbbVie, Celgene/Bristol Myers Squibb, Novartis, Incyte, Kymera Therapeutics, Servier, and Rigel; and service on data safety board for Treadwell Therapeutics. The remaining authors declare no competing financial interests.
Correspondence: Olatoyosi Odenike, Section of Hematology/Oncology, Department of Medicine, The University of Chicago, 5841 S Maryland Ave, MC 2115, Chicago, IL 60637; email: todenike@medicine.bsd.uchicago.edu.
References
Author notes
J.C. and R.M.-M. contributed equally to this study.
Authorship Contribution: J.C. and R.-M.M. drafted, reviewed, and edited the manuscript; and O.O. conceptualized the project, reviewed, and edited the manuscript.