Key Points
Low platelet counts and low CD4/CD8 T-cell ratios at apheresis increase the risk of CAR T-cell manufacturing failure in multiple myeloma.
Alkylating agents within 6 months before apheresis were associated with decreased platelet counts and CD4/CD8 ratios at apheresis.
Visual Abstract
Chimeric antigen receptor (CAR) T-cell (CAR-T) therapy has significantly improved management of relapsed or refractory multiple myeloma. However, manufacturing failure due to poor cell growth necessitates revision of treatment strategies that negatively impact patients. To identify risk factors for CAR-T manufacturing failure in patients with myeloma, a nationwide cohort study was performed, analyzing patients who underwent apheresis for idecabtagene vicleucel in Japan. Of 154 patients analyzed, 13 cases (8.4%) experienced manufacturing failure. We compared clinical factors between patients with manufacturing failure (failed group) and those who met specifications (successful group). Patients in the failed group had a higher prevalence of deletion 17p at diagnosis (38.5% vs 14.9%), were more likely to have been treated with alkylating agents within 6 months before apheresis (53.8% vs 23.4%), and had undergone more chemotherapy lines before apheresis (median, 6 vs 5). Additionally, patients with manufacturing failure exhibited significantly lower hemoglobin levels (8.6 vs 10.0 g/dL), platelet counts (5.9 × 104/μL vs 13.8 × 104/μL), and CD4/CD8 ratios (0.169 vs 0.474) than patient with successful manufacturing. Multivariate analysis revealed that low platelet counts (odds ratio [OR], 0.130 for every increase of 105/μL; P = .041), or low CD4/CD8 ratios (OR, 0.100 for each doubling; P = .003) at apheresis increased the risk of manufacturing failure. Alkylating agents within 6 months before apheresis were associated with decreased platelet counts and CD4/CD8 ratios. Manufacturing failure remains an obstacle to CAR-T therapy for patients with myeloma. Avoiding risk factors, such as alkylating agents, and adopting risk-adapted strategies may optimize CAR-T therapy for patients with myeloma.
Introduction
Chimeric antigen receptor (CAR) T-cell (CAR-T) therapy targeting B-cell maturation antigen (BCMA), including idecabtagene vicleucel (ide-cel), has shown favorable outcomes in patients with relapsed or refractory multiple myeloma,1-3 leading to a dramatic increase in the number of patients awaiting this treatment in clinical practice.
Numerous barriers exist to successful CAR-T therapy. Among them, manufacturing failures result in treatment delay and loss of opportunities for this treatment, posing a significant challenge. We previously reported that in CD19-targeted CAR-T therapy for B-cell lymphoma, manufacturing failure occurs in ∼7% of clinical cases, and that those who once experienced it did not reach CAR-T infusion because of reduced remanufacturing chances and a low success rate.4
In clinical trials of BCMA-targeted CAR-T therapies, manufacturing failure was reportedly rare, with only 1 case among 140 for ide-cel, and no cases among 113 for ciltacabtagene maraleucel.1,3 However, a subsequent real-world study on ide-cel identified manufacturing failure in 12 of 196 cases (6.1%), which raises concerns that manufacturing failures may occur more frequently in clinical practice than in clinical trials.5 However, risk factors for manufacturing failure remain unknown. Identifying those factors is urgently required for BCMA-targeted CAR-T therapies.
Therefore, we conducted a nationwide cohort study in Japan in order to determine the frequency of manufacturing failures of ide-cel treatment in real-world practice, and to identify risk factors for them. Our findings provide valuable information for treatment decision-making in relapsed or refractory multiple myeloma, including treatment sequences, and optimization of CAR-T therapy.
Patients and methods
Patients and date collection
This retrospective nationwide study, conducted by the CAR-T therapy taskforce established by the Japan Society of Transfusion Medicine and Cell Therapy, enrolled multiple patients with myeloma who underwent CAR-T production for ide-cel using T cells harvested at Japanese institutions from May 2022 to December 2023. Out-of-specification cases for reasons other than cell growth issues, such as matters of mycoplasma testing or vector copy numbers, were excluded. We reviewed medical records and extracted clinical factors, including diagnosis and treatment history of underlying disease, laboratory tests at apheresis, factors related to apheresis procedures, as well as results of CAR-T production. Cell counts in the peripheral blood at apheresis were obtained within 2 days before apheresis. Our protocol complied with the Declaration of Helsinki and was approved by the institutional review boards of participating institutions or the central review board at Kyoto University Hospital.
Cell collection and ide-cel manufacturing
Autologous peripheral blood mononuclear cell concentrates were collected using Spectra Optia (Terumo BCT, Tokyo, Japan) with either the MNC or CMNC program, or COM.TEC (Fresenius Kabi, Lake Zurich, IL), as determined by the institution. Peripheral blood mononuclear cell concentrates were shipped to manufacturing facilities for ide-cel production. CD3+ cell counts in the peripheral blood and in collection bags were measured by flow cytometry after staining with fluorochrome-conjugated anti-CD3 antibody at each facility. All processes, from cell collection to shipment, were performed according to procedural guidelines provided by Bristol Myers Squibb. Ide-cel was manufactured, and production results were judged by Bristol Myers Squibb, using several parameters, including cell growth. Manufacturing failure due to poor cell growth was defined as a case in which the number of CAR-expressing cells did not reach 2.8 × 106 cells.
Definition
Disease status at apheresis was assessed using International Myeloma Working Group uniform response criteria.6 Disease status was then categorized as follows: complete response (CR), partial response (PR), stable disease, or progressive disease (PD). CR included CR and stringent CR; PR included PR, very good PR, and near CR. Definitions of stable disease and PD were based on the aforementioned criteria. Disease stage at diagnosis was assessed according to the International Staging System.7 Cytogenetic abnormalities were evaluated by G-banding and fluorescence in situ hybridization without sorting. Alkylating agents administered included cyclophosphamide, melphalan, and ifosfamide.
Statistical analyses
Categorical and continuous variables were compared between groups using the Fisher exact test and 2-tailed unpaired Student t test, respectively. In comparisons between groups, CD4/CD8 ratios were log transformed to normalize skewed distributions. We evaluated the effect of each factor on the manufacturing outcome using a logistic regression model. We first performed univariate logistic regression to examine potential predictors of manufacturing failure. In multivariate analyses, all variables with P < .1 in univariate analyses were included, and multiple regression with backward stepwise elimination (significance level = .05) was performed. P values < .05 were considered statistically significant. Statistical analyses were performed using Stata, version 17 (Stata Corp, College Station, TX).
Results
Patient characteristics
We analyzed 154 cases with multiple myeloma from 16 institutes (supplemental Figure 1). The median age at apheresis was 64 years (range, 36-76; Table 1). Cases included 86 male (55.8%) and 68 female patients (44.2%). Deletion 17p was detected at diagnosis in 26 cases (16.9%), whereas karyotyping data were unavailable for a substantial number of cases. Disease status was PD at apheresis in 101 (65.6%). Median time between diagnosis and apheresis was 74.7 months (range, 8.2-364.7). Overall, 131 patients (85.1%) had received high-dose chemotherapy with autologous stem cell transplantation, and 65 (42.2%) had received radiation therapy before apheresis. The median number of chemotherapy lines was 6 (range, 2-13). A total of 40 patients (26.1%) received chemotherapy regimens that included alkylating agents within 6 months before apheresis. Cell counts in the peripheral blood at apheresis were as follows: hemoglobin, 9.8 g/dL (range 6.4-15.0); platelets, 130.0 × 103/μL (range, 10.4 × 103 to 780.4 × 103); lymphocytes, 851/μL (range, 20-2836); CD3+ cells, 665/μL (range, 20-1946); CD4/CD8 T-cell ratios, 0.424 (range, 0.096-4.133; Table 2). A median of 3.8 × 109 (range, 1.0 × 109-13.1 × 109) CD3+ cells was harvested for CAR-T production (supplemental Table 1).
Patient characteristics
| . | Total . | Failed . | Successful . | P value . |
|---|---|---|---|---|
| N = 154 . | n = 13 . | n = 141 . | ||
| Age at apheresis, y (range) | 63 (36-76) | 58 (44-71) | 63 (36-76) | .116 |
| Sex | 1.000 | |||
| Male | 86 (55.8%) | 7 (53.8%) | 79 (56.0%) | |
| Female | 68 (44.2%) | 6 (46.2%) | 62 (44.0%) | |
| M protein subclass | .468 | |||
| IgG | 80 (51.9%) | 7 (53.8%) | 73 (51.8%) | |
| IgA | 31 (20.1%) | 1 (7.7%) | 30 (21.3%) | |
| BJP | 41 (26.6%) | 5 (38.5%) | 36 (25.5%) | |
| Nonsecreting | 1 (0.6%) | 0 (0.0%) | 1 (0.7%) | |
| ISS at diagnosis | .581 | |||
| I | 41 (26.6%) | 2 (15.4%) | 39 (27.7%) | |
| II | 52 (33.8%) | 5 (38.5%) | 47 (33.3%) | |
| III | 43 (27.9%) | 6 (46.2%) | 37 (26.2%) | |
| Deletion 17p at diagnosis∗ | 26 (16.9%) | 5 (38.5%) | 21 (14.9%) | .031† |
| t(4;14) (IGH-FGFR3) at diagnosis∗ | 27 (17.5%) | 2 (15.4%) | 25 (17.7%) | 1.000 |
| t(14;16) (IGH-MAF) at diagnosis∗ | 5 (3.2%) | 0 (0.0%) | 5 (3.5%) | 1.000 |
| Gain 1q at diagnosis∗ | 28 (18.2%) | 4 (30.8%) | 24 (17.0%) | .234 |
| Disease status at apheresis | .073 | |||
| CR | 1 (0.6%) | 1 (7.7%) | 0 (0.0%) | |
| PR | 20 (13.0%) | 1 (7.7%) | 19 (13.5%) | |
| SD | 22 (14.3%) | 1 (7.7%) | 21 (14.9%) | |
| PD | 101 (65.6%) | 8 (61.5%) | 93 (66.0%) | |
| Time from diagnosis to apheresis, mo | 74.7 (8.2-364.7) | 77.5 (23.7-139.0) | 74.3 (8.2-364.7) | .637 |
| Number of chemotherapy lines before apheresis | 6 (2-13) | 6 (4-12) | 5 (2-13) | .008† |
| Time from last chemotherapy to apheresis, d | 35 (5-770) | 45 (18-770) | 34 (5-676) | .213 |
| History of ASCT | .442 | |||
| No | 23 (14.9%) | 1 (7.7%) | 22 (15.6%) | |
| Once | 96 (62.3%) | 7 (53.8%) | 89 (63.1%) | |
| ≥2 | 35 (22.7%) | 5 (38.5%) | 30 (21.3%) | |
| Time from last ASCT to apheresis, mo | 48.7 (2.4-178.4) | 23.2 (7.2-125.1) | 53.2 (2.4-178.4) | .200 |
| IMiDs in last regimen | 85 (55.2%) | 9 (69.2%) | 76 (53.9%) | .386 |
| Time from last IMiDs to apheresis, mo | 1.2 (0.0-71.5) | 1.5 (0.5-11.5) | 1.2 (0.0-71.5) | .432 |
| Protease inhibitors in last regimen | 64 (41.6%) | 6 (46.2%) | 58 (41.1%) | .774 |
| Daratumumab in last regimen | 30 (19.5%) | 0 (0.0%) | 30 (21.3%) | .074 |
| Isatuximab in last regimen | 45 (29.2%) | 2 (15.4%) | 43 (30.5%) | .348 |
| Alkylating agents in last regimen | 29 (18.8%) | 4 (30.8%) | 25 (17.7%) | .269 |
| Alkylating agents within 6 months before apheresis | 40 (26.1%) | 7 (53.8%) | 33 (23.4%) | .041† |
| History of RT | 65 (42.2%) | 9 (69.2%) | 56 (39.7%) | .075 |
| Total dose of RT (Gy) | 40 (20-174) | 38 (30-174) | 40 (20-160) | .454 |
| Time from last RT to apheresis, mo | 3.7 (0.0-133.9) | 1.9 (0.3-75.8) | 3.7 (0.0-133.9) | .910 |
| . | Total . | Failed . | Successful . | P value . |
|---|---|---|---|---|
| N = 154 . | n = 13 . | n = 141 . | ||
| Age at apheresis, y (range) | 63 (36-76) | 58 (44-71) | 63 (36-76) | .116 |
| Sex | 1.000 | |||
| Male | 86 (55.8%) | 7 (53.8%) | 79 (56.0%) | |
| Female | 68 (44.2%) | 6 (46.2%) | 62 (44.0%) | |
| M protein subclass | .468 | |||
| IgG | 80 (51.9%) | 7 (53.8%) | 73 (51.8%) | |
| IgA | 31 (20.1%) | 1 (7.7%) | 30 (21.3%) | |
| BJP | 41 (26.6%) | 5 (38.5%) | 36 (25.5%) | |
| Nonsecreting | 1 (0.6%) | 0 (0.0%) | 1 (0.7%) | |
| ISS at diagnosis | .581 | |||
| I | 41 (26.6%) | 2 (15.4%) | 39 (27.7%) | |
| II | 52 (33.8%) | 5 (38.5%) | 47 (33.3%) | |
| III | 43 (27.9%) | 6 (46.2%) | 37 (26.2%) | |
| Deletion 17p at diagnosis∗ | 26 (16.9%) | 5 (38.5%) | 21 (14.9%) | .031† |
| t(4;14) (IGH-FGFR3) at diagnosis∗ | 27 (17.5%) | 2 (15.4%) | 25 (17.7%) | 1.000 |
| t(14;16) (IGH-MAF) at diagnosis∗ | 5 (3.2%) | 0 (0.0%) | 5 (3.5%) | 1.000 |
| Gain 1q at diagnosis∗ | 28 (18.2%) | 4 (30.8%) | 24 (17.0%) | .234 |
| Disease status at apheresis | .073 | |||
| CR | 1 (0.6%) | 1 (7.7%) | 0 (0.0%) | |
| PR | 20 (13.0%) | 1 (7.7%) | 19 (13.5%) | |
| SD | 22 (14.3%) | 1 (7.7%) | 21 (14.9%) | |
| PD | 101 (65.6%) | 8 (61.5%) | 93 (66.0%) | |
| Time from diagnosis to apheresis, mo | 74.7 (8.2-364.7) | 77.5 (23.7-139.0) | 74.3 (8.2-364.7) | .637 |
| Number of chemotherapy lines before apheresis | 6 (2-13) | 6 (4-12) | 5 (2-13) | .008† |
| Time from last chemotherapy to apheresis, d | 35 (5-770) | 45 (18-770) | 34 (5-676) | .213 |
| History of ASCT | .442 | |||
| No | 23 (14.9%) | 1 (7.7%) | 22 (15.6%) | |
| Once | 96 (62.3%) | 7 (53.8%) | 89 (63.1%) | |
| ≥2 | 35 (22.7%) | 5 (38.5%) | 30 (21.3%) | |
| Time from last ASCT to apheresis, mo | 48.7 (2.4-178.4) | 23.2 (7.2-125.1) | 53.2 (2.4-178.4) | .200 |
| IMiDs in last regimen | 85 (55.2%) | 9 (69.2%) | 76 (53.9%) | .386 |
| Time from last IMiDs to apheresis, mo | 1.2 (0.0-71.5) | 1.5 (0.5-11.5) | 1.2 (0.0-71.5) | .432 |
| Protease inhibitors in last regimen | 64 (41.6%) | 6 (46.2%) | 58 (41.1%) | .774 |
| Daratumumab in last regimen | 30 (19.5%) | 0 (0.0%) | 30 (21.3%) | .074 |
| Isatuximab in last regimen | 45 (29.2%) | 2 (15.4%) | 43 (30.5%) | .348 |
| Alkylating agents in last regimen | 29 (18.8%) | 4 (30.8%) | 25 (17.7%) | .269 |
| Alkylating agents within 6 months before apheresis | 40 (26.1%) | 7 (53.8%) | 33 (23.4%) | .041† |
| History of RT | 65 (42.2%) | 9 (69.2%) | 56 (39.7%) | .075 |
| Total dose of RT (Gy) | 40 (20-174) | 38 (30-174) | 40 (20-160) | .454 |
| Time from last RT to apheresis, mo | 3.7 (0.0-133.9) | 1.9 (0.3-75.8) | 3.7 (0.0-133.9) | .910 |
ASCT, autologous stem cell transplantation; BJP, Bence-Johns proteins; IMiDs, immunomodulatory drugs; RT, radiation therapy; SD, stable disease.
Number of cases without available data in karyotyping is as follows: 48 (failed, 5; successful 43) for 17p deletion, 46 (failed, 4; successful 42) for t(4;14), 49 (failed, 4; successful, 45) for t(14;16), and 62 (failed, 6; successful, 56) for Gain 1q.
indicates P < .05.
Laboratory data at apheresis
| . | Total . | Failed . | Successful . | P value . |
|---|---|---|---|---|
| N = 154 . | n = 13 . | n = 141 . | ||
| Hemoglobin, g/dL | 9.8 (6.4-15.0) | 8.6 (7.6-11.5) | 10.0 (6.4-15.0) | .026∗ |
| Platelet count, 103/μL | 130.0 (10.4-780.0) | 59.0 (14.4-153.0) | 138.0 (10.4-780.0) | .003∗ |
| WBC count, 103/μL | 3.4 (0.90-9.4) | 3.36 (1.62-6.18) | 3.40 (0.90-9.40) | .455 |
| Monocyte count, /μL | 366 (30-1036) | 365 (130-1030) | 368 (30-1036) | .930 |
| Lymphocyte count, /μL | 851 (20-2836) | 934 (234-2773) | 850 (20-2836) | .575 |
| CD3+ cell count, /μL | 665 (20-1946) | 613 (222-1266) | 680 (20-1946) | .893 |
| CD4+ cell count, /μL | 190 (12-798) | 110 (39-174) | 208 (12-798) | .019∗ |
| CD8+ cell count, /μL | 453 (7-1566) | 509 (148-977) | 428 (7-1566) | .489 |
| CD4/CD8 ratio | 0.424 (0.096-4.133) | 0.169 (0.096-0.519) | 0.474 (0.106-4.133) | < .001∗ |
| CD56+ cell count, /μL | 72 (3-835) | 88 (17-348) | 69 (3-835) | .925 |
| CD19+ cell count, /μL | 14 (0-636) | 10 (0-76) | 14 (0-636) | .479 |
| LDH, U/L | 201 (89-1344) | 210 (130-629) | 201 (89-1344) | .541 |
| . | Total . | Failed . | Successful . | P value . |
|---|---|---|---|---|
| N = 154 . | n = 13 . | n = 141 . | ||
| Hemoglobin, g/dL | 9.8 (6.4-15.0) | 8.6 (7.6-11.5) | 10.0 (6.4-15.0) | .026∗ |
| Platelet count, 103/μL | 130.0 (10.4-780.0) | 59.0 (14.4-153.0) | 138.0 (10.4-780.0) | .003∗ |
| WBC count, 103/μL | 3.4 (0.90-9.4) | 3.36 (1.62-6.18) | 3.40 (0.90-9.40) | .455 |
| Monocyte count, /μL | 366 (30-1036) | 365 (130-1030) | 368 (30-1036) | .930 |
| Lymphocyte count, /μL | 851 (20-2836) | 934 (234-2773) | 850 (20-2836) | .575 |
| CD3+ cell count, /μL | 665 (20-1946) | 613 (222-1266) | 680 (20-1946) | .893 |
| CD4+ cell count, /μL | 190 (12-798) | 110 (39-174) | 208 (12-798) | .019∗ |
| CD8+ cell count, /μL | 453 (7-1566) | 509 (148-977) | 428 (7-1566) | .489 |
| CD4/CD8 ratio | 0.424 (0.096-4.133) | 0.169 (0.096-0.519) | 0.474 (0.106-4.133) | < .001∗ |
| CD56+ cell count, /μL | 72 (3-835) | 88 (17-348) | 69 (3-835) | .925 |
| CD19+ cell count, /μL | 14 (0-636) | 10 (0-76) | 14 (0-636) | .479 |
| LDH, U/L | 201 (89-1344) | 210 (130-629) | 201 (89-1344) | .541 |
LDH, lactate dehydrogenase; WBC, while blood cell.
indicates P < .05.
Although ide-cel products in-specification were successfully manufactured in 141 cases (successful group), 13 cases (8.4%) experienced manufacturing failure due to poor cell growth (failed group). Of those 13 cases, infusion of in-specification ide-cel through remanufacturing was achieved in only 4 case (30.8%), and expanded use of out-of-specification products was observed in another 4 cases (30.8%). The remaining 5 cases (38.5%) did not progress to ide-cel infusion. These results suggest that patients experiencing manufacturing failure have reduced chances of undergoing subsequent CAR-T therapy.
Comparison of characteristics in the failed and successful groups
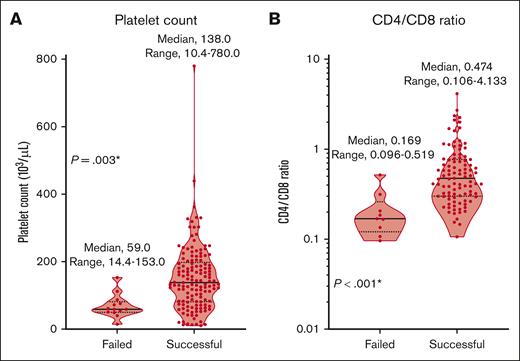
Then, we compared clinical factors between the failed group (n = 13) and the successful group (n = 141; Tables 1 and 2; supplemental Table 1). The proportion of patients with deletion 17p at diagnosis (38.5% vs 14.9%; P = .031), the number of chemotherapy lines (median, 6 vs 5; P = .008) before apheresis, and the proportion of patients previously treated with regimes with alkylating agents within 6 months before apheresis (53.8% vs 23.4%; P = .041) were significantly higher in the failed group than the successful group. Hemoglobin levels (8.6 vs 10.0 g/dL; P = .026), platelet counts (59.0 × 103 vs 138.0 × 103/μL; P = .003), and CD4/CD8 ratios (0.169 vs 0.474; P < .001) were significantly lower in the failed group (Figure 1; Table 2). There were no significant differences in failure rates related to apheresis device type or collection program. There were also no significant differences between groups in total numbers of CD3+ cells harvested (3.5 × 109 vs 3.8 × 109; P = .892) or shipped (3.5 × 109 vs 3.8 × 109; P = .895) for ide-cel production (supplemental Table 1).
Comparison of platelet counts and CD4/CD8 ratios in the peripheral blood at apheresis between the failed and successful groups. (A) Platelet counts. (B) CD4/CD8 ratios of T cells. ∗ indicates P < .05.
Comparison of platelet counts and CD4/CD8 ratios in the peripheral blood at apheresis between the failed and successful groups. (A) Platelet counts. (B) CD4/CD8 ratios of T cells. ∗ indicates P < .05.
Logistic analysis for manufacturing failure using laboratory data at apheresis
In order to evaluate risk of manufacturing failure by identifying risk factors at the time of apheresis, we performed univariate and multivariate logistic regression analyses for manufacturing failure using laboratory data at apheresis (Table 3). Multivariate analysis revealed that lower platelet counts (odds ratio, 0.130 per 100 × 103/μL; 95% confidence interval [CI], 0.018-0.921; P = .041), and lower CD4/CD8 ratios (odds ratio, 0.100 per log2; 95% CI, 0.021-0.467; P = .003) in the peripheral blood at apheresis constituted risk factors for manufacturing failure. However, absolute lymphocyte counts in the peripheral blood at apheresis were not significantly associated with manufacturing failure. On the basis of the multiple regression model, estimated probability for manufacturing failure was calculated using the following formula (see also supplemental Figure 2).
Logistic regression for manufacturing failure
| . | . | Univariate . | Multivariate . | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Odds ratio . | Lo_95% CI . | Up_95% CI . | P value . | Odds ratio . | Lo_95% CI . | Up_95% CI . | P value . | ||
| Hemoglobin | Per g/dL | 0.650 | 0.440 | 0.961 | .031∗ | ||||
| Platelet count | Per 100 × 103/μL | 0.152 | 0.045 | 0.510 | .002∗ | 0.130 | 0.018 | 0.921 | .041∗ |
| WBC count | Per 103/μL | 0.850 | 0.557 | 1.299 | .453 | ||||
| Monocyte count | Per 103/μL | 1.133 | 0.071 | 17.995 | .930 | ||||
| Lymphocyte count | Per 103/μL | 1.327 | 0.496 | 3.549 | .573 | ||||
| CD3+ cell count | Per 102/μL | 0.989 | 0.849 | 1.153 | .892 | ||||
| CD4+ cell count | Per 102/μL | 0.336 | 0.134 | 0.841 | .020 | ||||
| CD8+ cell count | Per 102/μL | 1.068 | 0.887 | 1.286 | .487 | ||||
| CD4/CD8 ratio | Per log2 | 0.109 | 0.030 | 0.397 | .001∗ | 0.100 | 0.021 | 0.467 | .003∗ |
| CD56+ cell count | Per 102/μL | 1.026 | 0.613 | 1.716 | .923 | ||||
| CD19+ cell count | Per 102/μL | 0.364 | 0.019 | 6.880 | .500 | ||||
| LDH | Per 102 IU/L | 1.101 | 0.806 | 1.503 | .545 | ||||
| . | . | Univariate . | Multivariate . | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Odds ratio . | Lo_95% CI . | Up_95% CI . | P value . | Odds ratio . | Lo_95% CI . | Up_95% CI . | P value . | ||
| Hemoglobin | Per g/dL | 0.650 | 0.440 | 0.961 | .031∗ | ||||
| Platelet count | Per 100 × 103/μL | 0.152 | 0.045 | 0.510 | .002∗ | 0.130 | 0.018 | 0.921 | .041∗ |
| WBC count | Per 103/μL | 0.850 | 0.557 | 1.299 | .453 | ||||
| Monocyte count | Per 103/μL | 1.133 | 0.071 | 17.995 | .930 | ||||
| Lymphocyte count | Per 103/μL | 1.327 | 0.496 | 3.549 | .573 | ||||
| CD3+ cell count | Per 102/μL | 0.989 | 0.849 | 1.153 | .892 | ||||
| CD4+ cell count | Per 102/μL | 0.336 | 0.134 | 0.841 | .020 | ||||
| CD8+ cell count | Per 102/μL | 1.068 | 0.887 | 1.286 | .487 | ||||
| CD4/CD8 ratio | Per log2 | 0.109 | 0.030 | 0.397 | .001∗ | 0.100 | 0.021 | 0.467 | .003∗ |
| CD56+ cell count | Per 102/μL | 1.026 | 0.613 | 1.716 | .923 | ||||
| CD19+ cell count | Per 102/μL | 0.364 | 0.019 | 6.880 | .500 | ||||
| LDH | Per 102 IU/L | 1.101 | 0.806 | 1.503 | .545 | ||||
LDH, lactate dehydrogenase; Lo, lower limit; Up, upper limit; WBC, while blood cell.
indicates P < .05.
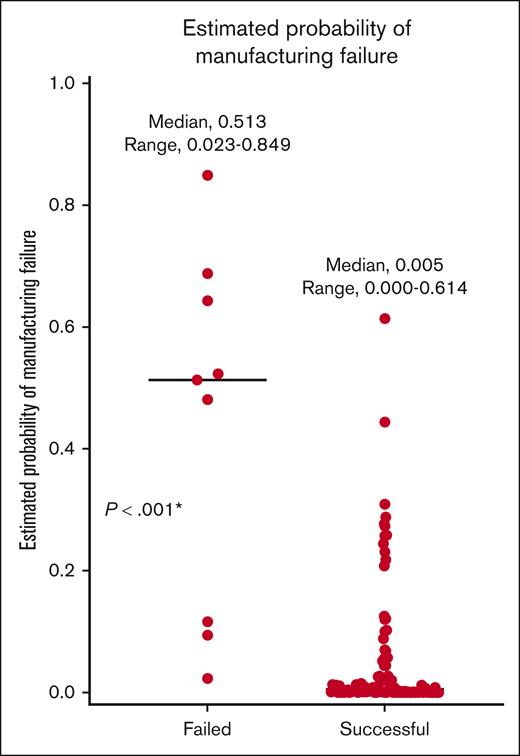
Estimated probability = odds/(1 + odds), in which odds = 0.008 × 0.980(platelet counts [10E3/μL]) × (CD4/CD8 ratios)−3.325. Using this formula, the estimated probability of manufacturing failure was significantly higher in the failed group than in the successful group (median, 0.513 vs 0.005; P < .001; Figure 2).
Estimated probability of manufacturing failure, based on multivariate logistic analysis. ∗P < .05.
Estimated probability of manufacturing failure, based on multivariate logistic analysis. ∗P < .05.
Factors associated with lower platelet counts and CD4/CD8 ratios at apheresis
Next, to identify clinical factors associated with lower platelet counts and CD4/CD8 ratios at apheresis, we analyzed correlation of preapheresis factors with platelet counts and CD4/CD8 ratios. Use of alkylating agents within 6 months before apheresis significantly reduced platelet counts at apheresis (coefficient, −42.073 × 103/μL; 95% CI, −77.144 to −.7003; P = .019; Table 4). For CD4/CD8 ratios (per log2), use of alkylating agents within 6 months (coefficient, −0.594; 95% CI, −1.101 to −0.086; P = .022), as well as advanced disease stage (International Scoring System [ISS] stage III) at diagnosis (coefficient, −0.559; 95% CI, −1.104 to −0.013; P = .045), and radiation therapy prior to apheresis (coefficient, −0.536; 95% CI, −0.965 to −0.108; P = .015) were significantly associated with low CD4/CD8 ratios at apheresis (Table 5). For instance, alkylating agents within 6 months before apheresis were expected to decrease both platelet counts and CD4/CD8 ratios at apheresis by 4.207 × 104/μL, and to 0.663 times, respectively. These results suggest that patients at an advanced disease stage at diagnosis, or those requiring disease control through radiation therapy or alkylating agents, are at increased risk of experiencing thrombocytopenia and a reduced CD4/CD8 ratio. These conditions, in turn, elevate risk of manufacturing failure of ide-cel.
Correlation between platelet counts and preapheresis factors
| . | . | Univariate . | Multivariate . | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Coefficient . | Lo_95% CI . | Up_95% CI . | P value . | Coefficient . | Lo_95% CI . | Up_95% CI . | P value . | ||
| Age at apheresis | Per year | 0.425 | −1.582 | 2.432 | .676 | ||||
| Sex | Female vs male | 17.604 | −13.563 | 48.770 | .266 | ||||
| ISS at diagnosis | II vs I | 13.569 | −27.975 | 55.112 | .519 | ||||
| III vs I | 9.170 | −34.248 | 52.588 | .677 | |||||
| deletion 17p | Yes vs no | −1.400 | −47.989 | 45.189 | .953 | ||||
| Disease status at apheresis | SD/PD vs CR/PR | 0.920 | −44.581 | 46.421 | .968 | ||||
| Time from diagnosis to apheresis | Per month | 0.032 | −0.228 | 0.291 | .810 | ||||
| Number of prior lines | Per line | −4.892 | −11.484 | 1.700 | .145 | ||||
| Time from last chemotherapy to apheresis | Per day | −0.076 | −0.226 | 0.075 | .322 | ||||
| History of ASCT | Once vs no | −18.285 | −62.695 | 26.126 | .417 | −22.324 | −66.203 | 21.554 | .316 |
| ≥2 vs no | −46.019 | −97.367 | 5.330 | .079 | −43.196 | −93.835 | 7.442 | .094 | |
| Time from last ASCT to apheresis | Per month | −0.009 | −0.425 | 0.408 | .967 | ||||
| IMiDs in last regimen | Yes vs no | −4.102 | −35.344 | 27.140 | .796 | ||||
| Time from last IMiDs to apheresis | Per month | 0.486 | −0.650 | 1.622 | .398 | ||||
| Protease inhibitors in last regimen | Yes vs no | −5.845 | −37.364 | 25.673 | .715 | ||||
| Anti-CD38 antibodies in last regimen | Yes vs no | 10.341 | −20.705 | 41.387 | .512 | ||||
| Alkylator in last regimen | Yes vs no | −35.697 | −75.031 | 3.637 | .075 | ||||
| Prior alkylating agent within 6 months | Yes vs no | −44.055 | −78.784 | −9.326 | .013∗ | −42.073 | −77.144 | −7.003 | .019∗ |
| History of RT | Yes vs no | −2.587 | −34.048 | 28.874 | .871 | ||||
| Total dose of RT | Per Gy | −0.069 | −0.807 | 0.669 | .852 | ||||
| Time from last RT to apheresis | Per month | −0.147 | −0.993 | 0.699 | .728 | ||||
| . | . | Univariate . | Multivariate . | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Coefficient . | Lo_95% CI . | Up_95% CI . | P value . | Coefficient . | Lo_95% CI . | Up_95% CI . | P value . | ||
| Age at apheresis | Per year | 0.425 | −1.582 | 2.432 | .676 | ||||
| Sex | Female vs male | 17.604 | −13.563 | 48.770 | .266 | ||||
| ISS at diagnosis | II vs I | 13.569 | −27.975 | 55.112 | .519 | ||||
| III vs I | 9.170 | −34.248 | 52.588 | .677 | |||||
| deletion 17p | Yes vs no | −1.400 | −47.989 | 45.189 | .953 | ||||
| Disease status at apheresis | SD/PD vs CR/PR | 0.920 | −44.581 | 46.421 | .968 | ||||
| Time from diagnosis to apheresis | Per month | 0.032 | −0.228 | 0.291 | .810 | ||||
| Number of prior lines | Per line | −4.892 | −11.484 | 1.700 | .145 | ||||
| Time from last chemotherapy to apheresis | Per day | −0.076 | −0.226 | 0.075 | .322 | ||||
| History of ASCT | Once vs no | −18.285 | −62.695 | 26.126 | .417 | −22.324 | −66.203 | 21.554 | .316 |
| ≥2 vs no | −46.019 | −97.367 | 5.330 | .079 | −43.196 | −93.835 | 7.442 | .094 | |
| Time from last ASCT to apheresis | Per month | −0.009 | −0.425 | 0.408 | .967 | ||||
| IMiDs in last regimen | Yes vs no | −4.102 | −35.344 | 27.140 | .796 | ||||
| Time from last IMiDs to apheresis | Per month | 0.486 | −0.650 | 1.622 | .398 | ||||
| Protease inhibitors in last regimen | Yes vs no | −5.845 | −37.364 | 25.673 | .715 | ||||
| Anti-CD38 antibodies in last regimen | Yes vs no | 10.341 | −20.705 | 41.387 | .512 | ||||
| Alkylator in last regimen | Yes vs no | −35.697 | −75.031 | 3.637 | .075 | ||||
| Prior alkylating agent within 6 months | Yes vs no | −44.055 | −78.784 | −9.326 | .013∗ | −42.073 | −77.144 | −7.003 | .019∗ |
| History of RT | Yes vs no | −2.587 | −34.048 | 28.874 | .871 | ||||
| Total dose of RT | Per Gy | −0.069 | −0.807 | 0.669 | .852 | ||||
| Time from last RT to apheresis | Per month | −0.147 | −0.993 | 0.699 | .728 | ||||
ASCT, autologous stem cell transplantation; IMiDs, immunomodulatory drugs; Lo, lower limit; RT, radiation therapy; Up, upper limit.
indicates P < .05.
Correlation between CD4/CD8 ratios and preapheresis factors
| . | . | Univariate . | Multivariate . | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Coefficient . | Lo_95% CI . | Up_95% CI . | P value . | Coefficient . | Lo_95% CI . | Up_95% CI . | P value . | ||
| Age at apheresis | Per year | 0.022 | −0.005 | 0.049 | .107 | ||||
| Sex | Female vs male | 0.037 | −0.380 | 0.454 | .860 | ||||
| ISS at diagnosis | II vs I | −0.394 | −0.941 | 0.152 | .155 | −0.387 | −0.906 | 0.131 | .141 |
| III vs I | −0.708 | −1.293 | −0.123 | .018∗ | −0.559 | −1.104 | −0.013 | .045∗ | |
| deletion 17p | Yes vs no | −0.408 | −0.976 | 0.160 | .157 | ||||
| Disease status at apheresis | SD/PD vs CR/PR | 0.081 | −0.464 | 0.626 | .768 | ||||
| Time from diagnosis to apheresis | Per month | 0.000 | −0.004 | 0.004 | .885 | ||||
| Number of prior lines | Per line | −0.093 | −0.176 | −0.010 | .028∗ | −0.056 | −0.146 | 0.034 | .223 |
| Time from last chemotherapy to apheresis | Per day | −0.001 | −0.003 | 0.001 | .596 | ||||
| History of ASCT | Once vs no | −0.126 | −0.783 | 0.530 | .704 | ||||
| ≥2 vs no | −0.724 | −1.443 | −0.005 | .048 | |||||
| Time from last ASCT to apheresis | Per month | 0.002 | −0.003 | 0.008 | .434 | ||||
| IMiDs in last regimen | Yes vs no | −0.111 | −0.532 | 0.309 | .601 | ||||
| Time from last IMiDs to apheresis | Per month | 0.008 | −0.013 | 0.029 | .443 | ||||
| Protease inhibitor in last regimen | Yes vs no | 0.292 | −0.126 | 0.709 | .169 | ||||
| Anti-CD38 antibodies in last regimen | Yes vs no | 0.033 | −0.382 | 0.448 | .874 | ||||
| Alkylator in last regimen | Yes vs no | −0.648 | −1.140 | −0.157 | .010∗ | ||||
| Prior alkylating agent within 6 months | Yes vs no | −0.823 | −1.254 | −0.393 | <.001∗ | −0.594 | −1.101 | −0.086 | .022∗ |
| History of RT | Yes vs no | −0.760 | −1.154 | −0.367 | <.001∗ | −0.536 | −0.965 | −0.108 | .015∗ |
| Total dose of RT | Per Gy | −0.004 | −0.013 | 0.005 | .332 | ||||
| Time from last RT to apheresis | Per month | −0.006 | −0.017 | 0.006 | .333 | ||||
| . | . | Univariate . | Multivariate . | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Coefficient . | Lo_95% CI . | Up_95% CI . | P value . | Coefficient . | Lo_95% CI . | Up_95% CI . | P value . | ||
| Age at apheresis | Per year | 0.022 | −0.005 | 0.049 | .107 | ||||
| Sex | Female vs male | 0.037 | −0.380 | 0.454 | .860 | ||||
| ISS at diagnosis | II vs I | −0.394 | −0.941 | 0.152 | .155 | −0.387 | −0.906 | 0.131 | .141 |
| III vs I | −0.708 | −1.293 | −0.123 | .018∗ | −0.559 | −1.104 | −0.013 | .045∗ | |
| deletion 17p | Yes vs no | −0.408 | −0.976 | 0.160 | .157 | ||||
| Disease status at apheresis | SD/PD vs CR/PR | 0.081 | −0.464 | 0.626 | .768 | ||||
| Time from diagnosis to apheresis | Per month | 0.000 | −0.004 | 0.004 | .885 | ||||
| Number of prior lines | Per line | −0.093 | −0.176 | −0.010 | .028∗ | −0.056 | −0.146 | 0.034 | .223 |
| Time from last chemotherapy to apheresis | Per day | −0.001 | −0.003 | 0.001 | .596 | ||||
| History of ASCT | Once vs no | −0.126 | −0.783 | 0.530 | .704 | ||||
| ≥2 vs no | −0.724 | −1.443 | −0.005 | .048 | |||||
| Time from last ASCT to apheresis | Per month | 0.002 | −0.003 | 0.008 | .434 | ||||
| IMiDs in last regimen | Yes vs no | −0.111 | −0.532 | 0.309 | .601 | ||||
| Time from last IMiDs to apheresis | Per month | 0.008 | −0.013 | 0.029 | .443 | ||||
| Protease inhibitor in last regimen | Yes vs no | 0.292 | −0.126 | 0.709 | .169 | ||||
| Anti-CD38 antibodies in last regimen | Yes vs no | 0.033 | −0.382 | 0.448 | .874 | ||||
| Alkylator in last regimen | Yes vs no | −0.648 | −1.140 | −0.157 | .010∗ | ||||
| Prior alkylating agent within 6 months | Yes vs no | −0.823 | −1.254 | −0.393 | <.001∗ | −0.594 | −1.101 | −0.086 | .022∗ |
| History of RT | Yes vs no | −0.760 | −1.154 | −0.367 | <.001∗ | −0.536 | −0.965 | −0.108 | .015∗ |
| Total dose of RT | Per Gy | −0.004 | −0.013 | 0.005 | .332 | ||||
| Time from last RT to apheresis | Per month | −0.006 | −0.017 | 0.006 | .333 | ||||
Abbreviations are explained in Table 4.
indicates P < .05.
Discussion
We retrospectively evaluated 154 cases of ide-cel production in patients with multiple myeloma and found the following: (1) manufacturing failure due to poor cell growth occurred in 8.4% of patients with myeloma who underwent apheresis for ide-cel; (2) lower precollection platelet counts and lower CD4/CD8 T-cell ratios in the peripheral blood significantly increased the risk of manufacturing failure; and (3) lower precollection platelet counts and CD4/CD8 ratios were associated with treatment involving alkylating agents within 6 months before apheresis, as well as advanced stage disease status at diagnosis and a history of radiation therapy.
The proportion of patients who experienced manufacturing failure in this real-world study (8.4%) is similar to that reported in real-world data of CD19 CAR-T therapy for B-cell lymphoma (7.4%), or of BCMA CAR-T therapy for myeloma (6.1%)4,5 but was much higher than that reported in clinical trials for BCMA CAR-T therapies for myeloma (<1%).1,3 Ide-cel manufacturing may be conducted for patients with more unfavorable conditions in clinical practice than those in clinical trials.8 For example, in the current cohort, compared with past clinical trial cohorts, there were more cases at advanced stages at diagnosis (27.9% ISS stage III in this study vs 14% ISS stage III in the phase 1b/2 CARTITUDE-1 trial; NCT03548207) and 16% revised-ISS stage III in the phase 2 KarMMa trial; NCT03361748). Thus, cell proliferation during manufacturing is likely influenced by patient background. Our results indicate that manufacturing failure remains an obstacle to ide-cel treatment in clinical practice, irrespective of underlying diseases.
Of 13 cases who experienced manufacturing failure, only 4 cases (30.8%) reached infusions of in-specification ide-cel products. Even after including another 4 expanded-use cases (30.8%) with out-of-specification products, the proportion of patients who proceeded to infusion was low. Further studies are required to clarify prognostic impact of manufacturing failure.
Then, we analyzed association of various laboratory variables available before apheresis, with manufacturing failure. Low platelet counts and low CD4/CD8 T-cell ratios were identified as significant risk factors for subsequent manufacturing failure. Including many cases with platelet counts lower than those in KarMMa study, which excluded cases with platelet counts of <75.0 × 103/μL,3 may have contributed to a high manufacturing failure rate observed in this study. Low platelet counts may reflect residual chemotherapy-induced T-cell toxicity. Although several groups have tried to assess T-cell fitness through detailed analyses of T-cell subsets, or cytokine production quantification,9-11 evaluating T-cell fitness in clinical practice remains challenging. Because the CD4/CD8 ratio among T cells in the peripheral blood diminishes with ablative chemotherapies, it is a convenient surrogate marker of T-cell fitness.12,13 We previously reported that both of these factors increased the risk of manufacturing failure in CD19 CAR-T therapy for B-cell lymphoma.4 In this cohort of patients with myeloma, platelet counts and CD4/CD8 ratios in the failed group were lower than those observed in our previous study cohort of patients with lymphoma (median of platelet counts, 59.0 vs 119.0 × 103/μL; median of CD4/CD8 ratios, 0.17 vs 0.30). Although the impact of these factors on the risk of manufacturing failure needs to be assessed in relation to patient background, our results suggest that these 2 factors at apheresis universally serve as predictive markers for manufacturing failure, regardless of type of underlying disease or type of CAR-T product. Furthermore, it has been reported that proliferation and therapeutic effects of CAR-Ts are improved by a manufacturing process in which collected T cells were separated into CD4+ cells and CD8+ cells, and CAR was introduced with a balanced CD4/CD8 ratio.14,15 Additionally, there is a report that presence of CD4+ cells helps to enhance proliferation of CD8+ cells after CAR introduction.16 Therefore, our results suggest that the CD4/CD8 ratios of T cells may also affect efficiency of the manufacturing process. The adverse effect of low CD4/CD8 ratios on CAR-T manufacturing may be minimized by optimizing the manufacturing process.
Next, we explored patient background factors related to low platelet counts and low CD4/CD8 ratios, and identified several preapheresis factors, including advanced disease stage at diagnosis, use of alkylating agents within 6 months before apheresis, and history of radiation therapy before apheresis. Among these factors, use of alkylating agents within 6 months before apheresis significantly decreased both platelet counts and CD4/CD8 ratios at apheresis. Use of alkylating agents before apheresis suggests difficulty in controlling progression of myeloma. Actually, alkylating agents within 6 months before apheresis were often used in intensive chemotherapy regimens (supplemental Table 2), which may lead to bone marrow suppression and reduce T-cell fitness,12 which potentially cause poor cell growth during manufacturing. Recently, it has been reported that impaired T-cell fitness caused by chemotherapy before apheresis not only leads to CAR-T manufacturing failure, but also reduces efficacy of CAR-T therapy.17 Given this context, findings of this study are important in optimizing therapeutic strategies for patients with myeloma, especially for timing of CAR-T therapy in treatment courses. Our results were consistent with those of a previous study showing that CD4/CD8 ratios were reduced in heavily treated patients with myeloma compared with patients in early stage.18 Early harvesting of T cells, before heavy treatment, may be crucial to maintain CAR-T quality. Moreover, for patients at a high risk of manufacturing failure, early preparation for alternative treatments may be beneficial, and introduction of allogeneic CAR-T therapies, which are currently under development, is eagerly anticipated.18,19 Further understanding of biological mechanisms behind the decline in T-cell function could lead to more accurate assessment of T-cell fitness for CAR-T therapy and optimal T-cell harvest.
Although this study includes detailed analyses of real-world data, some limitations exist. First, because it examined data from multiple facilities, differences in collection procedures and cell counts measurement among facilities could potentially affect risk of manufacturing failure. Second, because the ide-cel manufacturing process is proprietary to Bristol Myers Squibb, aspects of that process that may potentiate manufacturing failure cannot be evaluated. Although it is considered reasonable to assume that issues on mycoplasma testing, or on vector copy number, or aggregates in formulation, which were excluded from the analysis, are not related to poor cell growth, process of selecting cases for analysis in this study could potentially affect results. Third, although no significant correlation was observed between disease status at apheresis and platelet counts or CD4/CD8 ratios, proportion of plasma cells in the bone marrow, which was not collected in this study, may affect thrombocytopenia or CD4/CD8 ratios. Fourth, in this study, most patients underwent apheresis for ide-cel before bispecific T-cell engagers, bispecific antibody agents, and antibody–drug conjugate agents were available in Japan. Thus, an investigation into how these agents affect the manufacturing process is essential to optimize treatment sequences involving bispecific antibody agents, antibody–drug conjugate agents, and CAR-T therapies in myeloma. Fifth, this study did not analyze prognostic aspects of administering out-of-specification (OOS) products or formulations obtained from second manufacturing attempts after initial manufacturing failures. There is a report indicating that OOS products exhibit inferior therapeutic effects compared with in-specification products in CD19 CAR-T therapy for B-cell lymphoma.20 Therefore, further information on this issue for myeloma is necessary.
In conclusion, this study showed that manufacturing failure is a substantial obstacle to CAR-T therapy for patients with myeloma in real-world settings. Low platelet counts and low CD4/CD8 ratios at apheresis, which are influenced by treatment history, such as recent use of alkylating agents, increase risk of manufacturing failure. Strategies, including optimization of treatment sequences and apheresis early in treatment course, may help optimize CAR-T therapy.
Acknowledgments
The authors thank all physicians, clinical engineers, nurses, and coordinators at apheresis sites who contributed valuable data on ide-cel apheresis, and express their appreciation to the Japan Society of Transfusion Medicine and Cell Therapy.
This work was supported, in part, by the Lotte Foundation (Y.A.).
Authorship
Contribution: T.J., K.Y., S.Y., and Y.A. designed the study, reviewed and analyzed data, and wrote the paper; M.R., N.T., N.M., K. Fujii, K. Fukushima, S.-I.F., K. Kahata, Y.S., K.H., K. Kato, A.S., R.T., A.Y., and R.S. contributed to data collection; J.I., K.I., W.T., N.Y., M.Y.-F., and T.N.-I. interpreted data; and all authors critiqued the manuscript.
Conflict-of-interest disclosure: N.M. and S.-I.F. report honoraria from Bristol Myers Squibb. K. Kato reports honoraria and research funding from Bristol Myers Squibb and consulting or serving in an advisory role for Bristol Myers Squibb. The remaining authors declare no competing financial interests.
Correspondence: Yasuyuki Arai, Department of Hematology, Graduate School of Medicine, Kyoto University, and Center for Research and Application of Cellular Therapy, Kyoto University Hospital, 54 Shogoin Kawahara-cho, Sakyo-ku, Kyoto city, Kyoto 606-8507, Japan; email: ysykrai@kuhp.kyoto-u.ac.jp.
References
Author notes
T.J. and K.Y. contributed equally to this study.
The data of this study are not publicly available because of ethical restrictions that they exceed the scope of the patients’ consent for research use in the registry.
The full-text version of this article contains a data supplement.