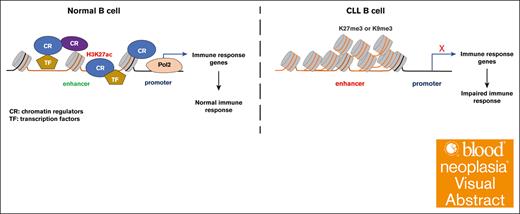
Key Points
Heterochromatin enrichment at the lost enhancers in CLL B cells is associated with the silence of immune response genes.
The aberrant heterochromatin signature also exists in the CLL precursor MBL stage and is largely resistant to ibrutinib treatment.
Visual Abstract
Epigenetic alterations have been found in chronic lymphocytic leukemia (CLL), but the functional importance of these changes remains underexplored. Here, we characterized the genome-wide histone modification landscapes of CLL using patient-derived samples across the disease course. Compared with normal B cells, we found that the enhancers specifically lost in CLL B cells are associated with the downregulation of genes involved in immune response. Importantly, these lost enhancers exhibit an increased level of heterochromatin marks, including H3K9me3 and H3K27me3. Using the EBF1 gene locus as an example, we demonstrated that acquired H3K9me3 contributes to enhancer silencing and associated gene suppression. We further found that this aberrant chromatin signature also exists in the CLL precursor stage monoclonal B-cell lymphocytosis (MBL) cells, implicating the importance of epigenetic silencing in CLL evolution. Finally, when treated with the Bruton tyrosine kinase inhibitor ibrutinib, these silenced enhancers are relatively stable during therapy compared with the CLL-gained enhancers. In summary, we described an epigenetic silencing mechanism mediated by the heterochromatin that persists throughout CLL disease development and treatment and which may increase the risk of severe infections in MBL and CLL.
Introduction
Although cancer is a genetic disease, DNA mutations alone are not always sufficient to trigger tumorigenesis, implying the involvement of alternative mechanisms. One example is epigenetic machinery, which is frequently used by oncogenic signaling pathways to regulate gene expression and facilitate the survival and progression of cancer cells.1,2 Among the components of epigenetic machinery, chromatin plays a critical role as the medium through which environmental stimulations, active signaling pathways, and transcription factors (TFs) regulate gene expression and cellular activity.3 Chromatin plasticity has essential roles at all stages of cancer development, including initiation, progression, and drug resistance.3-8 However, most of the chromatin studies in cancer have concentrated on the active chromatin signatures (such as the enhancers and promoters) associated with oncogene expression. Conversely, there is less emphasis on the loss of active chromatin of tumor suppressor genes during tumorigenesis.9 Importantly, there is little understanding regarding the role of heterochromatin-mediated epigenetic silencing in cancer cells compared with their normal cell counterpart.
Leukemic cells derived from chronic lymphocytic leukemia (CLL) offer 1 of the best models to study cancer epigenetics.10 CLL, the most common leukemia in the United States, with ∼21 000 new cases diagnosed yearly, is defined by the expansion of monoclonal, mature CD5+CD23+ B cells in the peripheral blood, secondary lymphoid tissues, and bone marrow.11 All CLL cases progress from a precursor state known as monoclonal B-cell lymphocytosis (MBL).11 A well-established prognostic factor for CLL is the mutational status of the immunoglobulin heavy chain variable (IGHV) region genes, with CLL cases with mutated IGHV exhibiting a more favorable prognosis than unmutated IGHV.11 There is genome-wide hypomethylation with localized hypermethylation in CLL.12,13 Based on this, it has been proposed that naive B cells most likely serve as the putative cell-of-origin for CLLs with unmutated IGHV (U-CLL), whereas memory B cells (MBC) are likely the cellular origin for CLLs with mutated IGHV (M-CLL).12 Recent studies have revealed that CLL B cells exhibit altered histone modification landscapes, with active enhancers or open chromatin regions associated with pro-oncogenes.14,15 Our study also indicated that B-cell receptor (BCR) signaling putatively drives genome instability by modulating the enhancer activity in CLL.16 Besides, there was a globally increased histone deacetylase activity in CLL, suggesting a potential loss of enhancer activity in CLL B cells.15 However, the functional importance and mechanism of the enhancer loss in CLL is unknown: (1) what is the function of these lost enhancers; (2) how these lost enhancers are maintained in CLL; (3) whether these lost enhancers can be detected at the MBL stage; and finally (4) whether this aberrant chromatin signature is affected by CLL treatment.
To address these questions, we mapped the genome-wide histone modification profile (including H3K4me1, H3K4me3, H3K9me3, H3K27ac, H327me3, and H3K36me3) in CLL B cells from 22 patients with CLL, 6 individuals with MBL, and B cells from 12 healthy donors by cleavage under targets and tagmentation (CUT&Tag).17 We also included CLL B cells derived from 8 patients with CLL before and after 1 year of continuous ibrutinib treatment. Our data indicate that heterochromatin plays a significant role in the enhancer loss in CLL, which may contribute to the suppression of immune response genes of B cells during CLL disease evolution.
Methods
Antibodies, methods for cell culture, western blot, Reverse Transcription-Polymerase Chain Reaction (RT-PCR), and CRISPR–directed epigenetic editing are described in the supplemental Methods.
Purification of leukemic and NBCs
All patients provide research blood samples by signing and submitting written informed consent according to the Declaration of Helsinki to the Mayo Clinic institutional review board, which approved these studies. Informed consent was also obtained from healthy donors to obtain their B cells. The relevant patient information is shown in supplemental Table 1. Primary CLL B cells and normal B cells (NBCs) were purified from blood samples using the RosetteSep B-cell enrichment kit (STEMCELL Technologies). The typical purification range of CD5+/CD19+ CLL B cells was >95%, as determined by flow cytometric analysis. NBCs were purified from blood samples using the RosetteSep B-cell enrichment kit (STEMCELL Technologies) with CD19+ CLL B cells at >95%.
CUT&Tag
CUT&Tag was used to map genome-wide histone modification profiles and performed as previously described.18 A total of 1 × 105 CLL B cells were used for each histone mark per sample. CUT&Tag libraries were sequenced to 150 base pairs on an Illumina Novaseq using pair-end mode by Novogene (Sacramento, CA).
CUT&Tag data analysis
CUT&Tag data were analyzed using the CUT&Tag data processing and analysis tutorial.19 Reads were aligned to the human genome (hg38) or Escherichia coli genome using Bowtie2.20 The fragments mapped to E coli were used for the spike-in calibration. Uniquely mapped reads were used for further analysis. Peaks were identified using SEACR, with 0.05 as the cutoff false discovery rate value.21 Mapped reads were transformed into BigWig files by deepTools for visualization.22 Read density at genomic regions was calculated by deepTools multiBigwigSummary. Gene ontology analysis was performed by Genomic Regions Enrichment of Annotation Tool (GREAT) version 3.0.0.23 The differential analysis was performed by DESeq2.24
Statistical analysis
Significance was defined as P ≤ .05 unless otherwise noted in the text (∗P < .05; ∗∗P < .01; ∗∗∗P < .001). Paired or unpaired 2-tailed Student t tests and 2-way analysis of variance statistics were calculated using GraphPad Prism 9. Two-tailed Student t tests were used for pairwise comparisons.
Results
The downregulated enhancers in CLL are associated with immune response genes
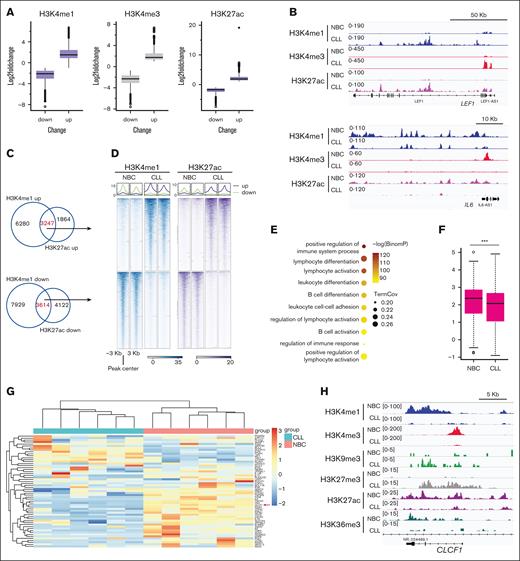
We analyzed the gene expression profile of CLL B cells from 6 patients and B cells from 6 healthy donors (NBCs) by RNA sequencing (RNA-seq). Compared with NBCs, CLL B cells had dramatic gene expression alterations (total changed genes = 2574, up = 1390, down = 1184; P < .01, fold change of >2; supplemental Figure 1A-B). We focused our analysis on the genes that are downregulated in CLL and observed that they were predominantly associated with immune response pathways (supplemental Figure 1C-D), consistent with the reported immune response dysfunction in CLL.25 To further understand the underlying epigenetic mechanisms, we systematically mapped the genome-wide distribution of major histone modifications in leukemic cells derived from 10 patients with CLL and purified blood NBCs from 6 healthy donors by CUT&Tag. We obtained high-quality epigenetic data in these samples (supplemental Table 1). Unsupervised principal component analysis of the samples based on these histone marks showed that the separation between the CLL cells and NBCs was much larger than the intra-CLL differences (supplemental Figure 2A). We noted that when NBCs are included in this analysis, the intra-CLL difference of the heterochromatin states is not based on IGHV states but on sex (data not shown). We then checked the histone marks associated with active gene transcription, including H3K4me1, H3K4me3, and H3K27ac.26 Consistent with previous reports,14,15 we observed both the gain and loss of these histone marks in CLL compared with NBCs (Figure 1A-B; supplemental Figure 2B-D). To gain insights into the gene expression changes associated with these alterations, we analyzed the changed enhancers and promoters, respectively. Using overlapped H3K4me1 and H3K27ac peaks to define the changed enhancers,27 we found 3247 increased (gained) and 3614 decreased (lost) enhancers in CLL (Figure 1C-D). Next, we checked the genes putatively regulated by the lost enhancers using GREAT23 and observed that these genes were enriched in immune response pathways (Figure 1E), and ∼70% of these genes were downregulated in CLL (Figure 1F-G; supplemental Figure 2E; supplemental Table 2). For example, cardiotrophin-like cytokine factor 1, a gene required for B-cell activation,28 showed decreased expression and loss of enhancer marks in CLL B cells (Figure 1G-H). We also observed a loss of the enhancer near the interleukin-1A (IL-1A) and IL-1B gene locus, with a reduction of expression of both genes (supplemental Figure 2F). IL-1 is an important extracellular mediator of T-cell immunity,29-31 so the decreased expression of IL-1A and IL-1B in CLL B cells may contribute to T-cell dysfunction in CLL. Collectively, these results highlight the crucial role of enhancer regulation in the immune response dysfunction in CLL.
Loss of active enhancers in CLL B cells. (A) Box plots showing the fold change of the indicated histone mark at the altered peaks in CLL B cells. The altered peaks were cut off at P < .01 and fold change of >2 between CLL and NBCs. (B) Representative snapshots showing the alteration of active histone marks in CLL B cells. (C) Venn diagrams showing the overlap of the downregulated or upregulated H3K4me1 and H3K27ac peaks in CLL B cells. (D) Representative metagene heat maps showing H3K4me1 and H3K27ac intensity of the indicated peaks in panel C. (E) Pathways associated with the genes putatively regulated by the downregulated enhancers (by GREAT) in CLL. (F) The expression of the downregulated enhance-associated genes (determined by GREAT). Gene expression was determined by RNA-seq. (G) The gene expression of leukocyte differentiation pathways genes. Gene expression was determined by RNA-seq. The gene cardiotrophin-like cytokine factor 1 (CLCF1) is marked by the red arrow. (H) A snapshot showing histone modification enrichment at gene locus CLCF1.
Loss of active enhancers in CLL B cells. (A) Box plots showing the fold change of the indicated histone mark at the altered peaks in CLL B cells. The altered peaks were cut off at P < .01 and fold change of >2 between CLL and NBCs. (B) Representative snapshots showing the alteration of active histone marks in CLL B cells. (C) Venn diagrams showing the overlap of the downregulated or upregulated H3K4me1 and H3K27ac peaks in CLL B cells. (D) Representative metagene heat maps showing H3K4me1 and H3K27ac intensity of the indicated peaks in panel C. (E) Pathways associated with the genes putatively regulated by the downregulated enhancers (by GREAT) in CLL. (F) The expression of the downregulated enhance-associated genes (determined by GREAT). Gene expression was determined by RNA-seq. (G) The gene expression of leukocyte differentiation pathways genes. Gene expression was determined by RNA-seq. The gene cardiotrophin-like cytokine factor 1 (CLCF1) is marked by the red arrow. (H) A snapshot showing histone modification enrichment at gene locus CLCF1.
Next, we evaluated the changes of promoters using overlapped H3K4me3 and H3K27ac peaks and found fewer altered promoters than enhancers in CLL (supplemental Figure 3A-B). The genes associated with lost promoters in CLL were enriched in pathways related to the cellular response to extracellular stimulus (supplemental Figure 3C), and they were consistently downregulated in CLL (supplemental Figure 3D). These findings suggest that the lost enhancers but not promoters are more related to impaired immune response genes expression status in CLL B cells.
The lost enhancers in CLL are marked with facultative heterochromatin mark H3K27me3
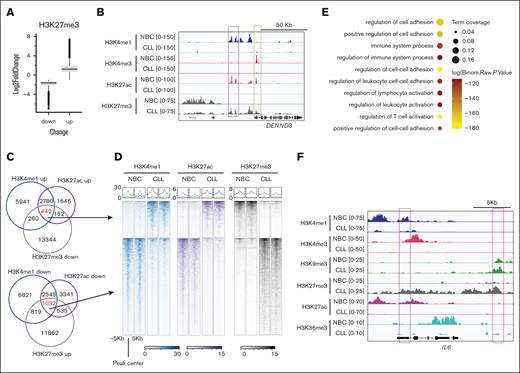
Next, we focused on these lost enhancers in CLL and assessed other epigenetic signatures within these regions. H3K27ac is associated with gene activation, whereas facultative heterochromatin mark H3K27me3 is associated with gene repression.32 Because they act on the same lysine residue, these marks are mutually exclusive. Previous studies in CLL have revealed the incomplete silencing of gene expression mediated by H3K27me3,33 but its role in enhancers has yet to be clearly defined. To this end, we assessed the H3K27me3 changes at the lost enhancers in CLL. Globally, we found increased and decreased H3K27me3 peaks in CLL (Figure 2A-B). At the gained or lost enhancer regions in CLL, we found 442 H3K4me1up/H3K27acup/H3K27me3down and 1032 H3K4me1down/H3K27acdown/H3K27me3up peaks, respectively (Figure 2C-D). Of note, among all the CLL samples studied, the changes of H3K4me1, H3K27ac, and H3K27me3 enrichment at these peaks were relatively consistent (Figure 2D; supplemental Figure 4A). We then analyzed the genes associated with H3K27me3 up lost enhancers (H3K4me1down/H3K27acdown/H3K27me3up regions) and found that these genes mostly exhibited decreased levels of expression in CLL and were enriched in pathways related to immune response (supplemental Figure 4B; Figure 2E; supplemental Table 3). For example, we observed a decrease in the enhancer mark H3K4me1 and H3K27ac, along with an increase in H3K27me3 at gene locus IL-6 (Figure 2F). To validate these epigenetic changes, we conducted additional profiling in a new cohort consisting of 6 CLL B cells and 6 healthy control NBCs (supplemental Table 1). We observed similar results (supplemental Figure 4C-D) with this additional cohort. We also analyzed the published chromatin immunoprecipitation sequencing data14 and obtained consistent results with ours in this patient cohort (supplemental Figure 4E).
Enrichment of heterochromatin H3K27me3 at the lost enhancers in CLL B cells. (A) Box plots showing the fold change of the H3K27me3 intensity at the H3K27me3 increased or decreased peaks in CLL B cells. The altered peaks were cut off at P <.01 and fold change of >2 between CLL and NBCs. (B) A snapshot showing the indicated histone marks at the DENND3 gene locus in CLL B cells. (C) Venn diagrams showing the overlap of the indicated peaks. (D) Representative metagene heat maps showing H3K4me1, H3K27ac, and H3K27me3 intensity of the indicated peaks in panel C. (E) Pathways associated with the genes putatively regulated by the H3K4me1down/H3K27acdown/H3K27me3up peaks in CLL. (F) A snapshot showing histone modification enrichment at gene locus IL-6.
Enrichment of heterochromatin H3K27me3 at the lost enhancers in CLL B cells. (A) Box plots showing the fold change of the H3K27me3 intensity at the H3K27me3 increased or decreased peaks in CLL B cells. The altered peaks were cut off at P <.01 and fold change of >2 between CLL and NBCs. (B) A snapshot showing the indicated histone marks at the DENND3 gene locus in CLL B cells. (C) Venn diagrams showing the overlap of the indicated peaks. (D) Representative metagene heat maps showing H3K4me1, H3K27ac, and H3K27me3 intensity of the indicated peaks in panel C. (E) Pathways associated with the genes putatively regulated by the H3K4me1down/H3K27acdown/H3K27me3up peaks in CLL. (F) A snapshot showing histone modification enrichment at gene locus IL-6.
Given the above results, we evaluated how these findings varied when studying U-CLL vs M-CLL cohorts as well as in comparison with NBC subsets. We checked the H3K27me3 signature at CLL lost enhancers between IGHV-mutated and -unmutated CLLs. M-CLL and U-CLL showed similar reductions of H3K4me1 and H3K27ac (supplemental Figure 5A-B). Although all the CLL samples showed increased H3K27me3 compared with NBCs, we noticed that some M-CLL showed a relatively higher H3K27me3 (supplemental Figure 5C). In addition, when analyzing the published RNA-seq database,14 we did not see differences in the expression of H3K27me up enhancer-regulated genes between U-CLL and M-CLL (supplemental Figure 5D). These results suggest that the differential increases of H3K27me3 between the M-CLL and U-CLL is likely not sufficient for significant gene expression changes.
Next, we analyzed the published data14 to compare the epigenetic signature at H3K27me3 up lost enhancers of CLL B cells vs normal mature B-cell subpopulations to cover the different stages of B-cell differentiation. We found a reduction of H3K4me1 and H3K27ac at most of these regions in CLL B cells compared with these NBC subpopulations (supplemental Figure 6A-B). We further found an increased H3K27me3 enrichment at these regions compared with naive B cells and MBC but to a lesser extent when compared with germinal center B cells and plasma B cells (supplemental Figure 6C), suggesting these regions may have been partially silenced by H3K27me3 in these 2 subpopulations.
CLL cells exhibit a MBC-like phenotype including surface immunophenotype and gene expression.34 When comparing the regions with lost enhancers across NBC subsets, it appears that a significant proportion of identified lost enhancers are also present in MBCs (supplemental Figure 6A). To further compared these enhancers between CLL and MBC, we analyzed the public assay for transposase-accessible chromatin using sequencing signals14 at H3K4me1down/H3K27acdown/H3K27me3up regions and found consistent higher chromatin accessibility in MBCs compared with CLL (supplemental Figure 7A), although MBCs have a relatively lower accessibility compared with other B-cells subgroups. We then compared the enhancer and H3K27me3 changes directly between MBCs and CLL B cells. We used the master H3K4me1 and H3K27ac peaks from our CUT&Tag data as enhancers and extracted the reads at these peaks from the published chromatin immunoprecipitation sequencing data.14 We could still see the loss of enhancers in CLL B cells compared with MBCs, and these lost enhancers are associated with increased H3K27me3 (supplemental Figure 7B). Importantly, these enhancers are also associated with immune response genes (supplemental Figure 7C), and most of their associated genes were downregulated in CLL compared with in MBCs (supplemental Figure 7D).
Together, these data indicated that H3K27me3 plays a significant role in maintaining the epigenetic signatures at the lost enhancer regions in CLL.
The downregulated enhancers in CLL are marked with heterochromatin mark H3K9me3
H3K9 methylation marked heterochromatin plays important roles in cell lineage specification during normal development35 and tumor progression.36 However, the role of H3K9me3 in CLL B cells is unknown. Recent reports indicate that H3K9 methylation also regulates enhancer activity,37,38 so we assessed the H3K9me3 enrichment at the altered enhancer regions in CLL. Globally, we identified increased and decreased H3K9me3 peaks in CLL compared with NBCs (Figure 3A-B). At altered enhancer regions, we found 189 peaks with H3K4me1up/H3K27acup/H3K9me3down and 736 peaks with H3K4me1down/H3K27acdown/H3K9me3up, respectively (Figure 3C-D). These findings suggest that H3K9me3 is more involved in the lost enhancers in CLL than the gained enhancers. The CLL samples showed a consistent change of H3K4me1, H3K27ac, and H3K9me3 enrichment at these peaks (Figure 3D; supplemental Figure 8A). Like H3K27me3, the genes associated with H3K9me3 up lost enhancers (H3K4me1down/H3K27acdown/H3K9me3up) exhibited decreased expression in CLL B cells (supplemental Figure 8B) and were enriched in pathways related to immune response (Figure 3E; supplemental Table 4). For example, the IL-6 enhancer exhibited a decreased level of H3K4me1 and H3K27ac along with an increased level of H3K9me3 in CLL (Figure 2F), suggesting H3K9me3 and H3K27me3 may coordinate to regulate the enhancer activity in CLL. Moreover, we obtained consistent results with the new validation cohort (supplemental Table 1; supplemental Figure 8C-D) as well as from published data14 (supplemental Figure 8E).
Enrichment of heterochromatin H3K9me3 at the lost enhancers in CLL B cells. (A) Box plots showing the fold change of the H3K9me3 intensity at the H3K9me3 increased or decreased peaks in CLL B cells. The altered peaks were cut off at P < .01 and fold change of >2 between CLL and NBCs. (B) A snapshot showing the indicated histone marks at the MAP4K4 gene locus in CLL B cells. (C) Venn diagrams showing the overlap of the indicated peaks. (D) Representative metagene heatmaps showing H3K4me1, H3K27ac, and H3K9me3 intensity of the indicated peaks in panel C. (E) Pathways associated with the genes putatively regulated by the H3K4me1down/H3K27acdown/H3K9me3up peaks in CLL.
Enrichment of heterochromatin H3K9me3 at the lost enhancers in CLL B cells. (A) Box plots showing the fold change of the H3K9me3 intensity at the H3K9me3 increased or decreased peaks in CLL B cells. The altered peaks were cut off at P < .01 and fold change of >2 between CLL and NBCs. (B) A snapshot showing the indicated histone marks at the MAP4K4 gene locus in CLL B cells. (C) Venn diagrams showing the overlap of the indicated peaks. (D) Representative metagene heatmaps showing H3K4me1, H3K27ac, and H3K9me3 intensity of the indicated peaks in panel C. (E) Pathways associated with the genes putatively regulated by the H3K4me1down/H3K27acdown/H3K9me3up peaks in CLL.
Nex, we checked the H3K9me3 signature at CLL lost enhancers between M- and U-CLLs. Although M-CLL and U-CLL showed similar reductions of H3K4me1 and H3K27ac (supplemental Figure 9A-B), we noticed that some M-CLL (n = 5) showed a relatively higher increase of H3K9me3 than other CLLs (n = 11; supplemental Figure 9C). When analyzing the published RNA-seq database,14 we did not see differences in the expression of genes regulated by H3K9me up lost enhancers between U-CLL and M-CLL (supplemental Figure 9D), suggesting the modest increase of H3K9me3 may be sufficient to silence the expression of these genes.
Next, we checked the published data14 to compare the epigenetic signature at H3K9me3 up lost enhancers of CLL B cells with normal mature B-cell subpopulations, covering different stages of the differentiation program. We found a reduction of H3K4me1 and H3K27ac at the majority of these regions in CLL B cells compared with the NBC subpopulations (supplemental Figure 10A-B). We further found increased H3K9me3 enrichment at these regions compared with all normal sub–B-cell populations (supplemental Figure 10C). Interestingly, although the CLL lost enhancers were partially enriched with H3K27me3 in germinal center and plasma B cells (supplemental Figure 6C), they were partially enriched with H3K9me3 in other B-cell populations (naive B cells and MBC), suggesting a different function of H3K9me3 and H3K27me3 silencing in CLL pathobiology.
We then analyzed the assay for transposase-accessible chromatin using sequencing (ATAC-seq) signals at H3K4me1down/H3K27acdown/H3K9me3up regions and found that MBCs had a relatively lower accessibility compared with other B-cell subgroups but were still higher than CLL B cells (supplemental Figure 11A). We then compared the enhancer and H3K9me3 changes between MBC and CLL B cells and observed a loss of enhancers in CLL B cells compared with MBCs, and these lost enhancers are associated with increased H3K9me3 (supplemental Figure 11B). Importantly, these enhancers are also associated with immune response genes (supplemental Figure 11C), and most of these genes were downregulated in CLL compared with MBCs (supplemental Figure 11D). With the H3K27me3 enrichment (supplemental Figure 7), these results indicate that, even comparing a more appropriate normal counterpart MBC, CLL still shows increased heterochromatin at the lost enhancers associated with immune response genes.
The aberrant enhancer/heterochromatin signature is detectable in MBL
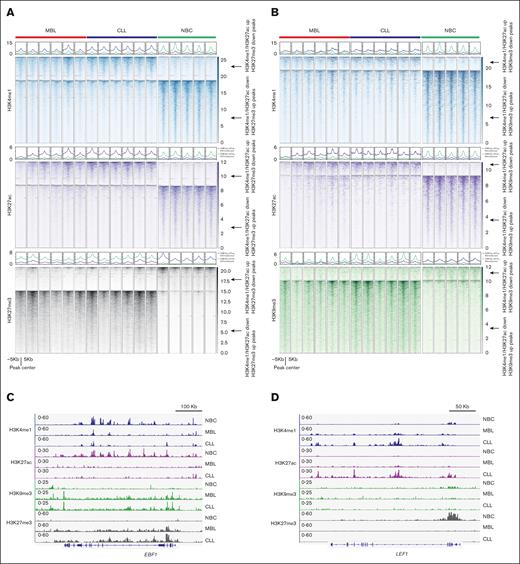
Next, we assessed the epigenetic status of MBL B cells from high-count MBL, which is commonly considered the precursor state of CLL.11 Studies using whole-exome (WES) and whole-genome sequencing (WGS) indicate that the mutational load in MBL is very similar to CLL,39 and the DNA methylation profile is also conserved between MBL and CLL B cells.40 However, little is known regarding the histone modification profiles in leukemic cells from high-count MBL compared with CLL B cells. Therefore, we further profiled the histone modifications in 6 MBL samples and 6 CLL B cells using CUT&Tag (supplemental Table 1). First, we validated our findings in these 6 CLL samples (Figure 4A-C). Moreover, like the CLL B cells, the MBL samples also exhibited an aberrant enhancer/heterochromatin signature, with increased H3K27me3 and H3K9me3 enrichment at the lost enhancers when compared with NBCs (Figure 4A-C). These findings suggested that the transition to cancer cell–specific epigenetic silencing occurs in the precursor MBL stage. Interestingly, we found that in 5 of 6 MBL samples, when checking the H3K4me1up/H3K27acup/H3K27me3down regions, although the H3K27me3 was lost, the enrichment of the H3K4me1 and H3K27ac was lower than that of the CLL samples (Figure 4A,D). This finding indicated that the loss of H3K27me3 is evident at the MBL stage before the full activation of the cancer enhancers in CLL. Together, these data suggest that the aberrant heterochromatin enrichment at enhancers has a pathophysiologic role shared by MBL and CLL.
The enrichment of heterochromatin at CLL lost enhancers is already detectable at the MBL stage. (A) Metagene heatmaps showing H3K4me1, H3K27ac, and H3K27me3 intensity at the indicated peaks in indicated samples. (B) Metagene heatmaps showing H3K4me1, H3K27ac, and H3K9me3 intensity at the indicated peaks in indicated samples. (C) A snapshot showing histone modification enrichment at gene locus EBF1. (D) A snapshot showing histone modification enrichment at gene locus LEF1.
The enrichment of heterochromatin at CLL lost enhancers is already detectable at the MBL stage. (A) Metagene heatmaps showing H3K4me1, H3K27ac, and H3K27me3 intensity at the indicated peaks in indicated samples. (B) Metagene heatmaps showing H3K4me1, H3K27ac, and H3K9me3 intensity at the indicated peaks in indicated samples. (C) A snapshot showing histone modification enrichment at gene locus EBF1. (D) A snapshot showing histone modification enrichment at gene locus LEF1.
The gain of H3K9me3 downregulates the gene expression at the EBF1 locus
Next, we investigated whether the gain of heterochromatin inhibiting enhancer activity was associated with gene expression. By assessing the genes that are regulated by the H3K4me1down/H3K27acdown/H3K9me3up enhancers, we identified early B-cell factor 1 (EBF1) as a top candidate (Figure 5A). EBF1 is an important TF that defines the NBC lineage41 and its expression is lost in CLL B cells.42,43 We also validated the downregulation of EBF1 in CLL B cells using RNA-seq, RT-PCR, and western blot (Figure 5B-D). However, we did not observe any significant expression changes for another important B-cell lineage TF, PAX5 (Figure 5B). Although there was increased H3K9me3 across the enhancer, we noted there was a sharp peak at the 3′ end of EBF1 in CLL (Figure 5A), and this peak was close to but did not cross the H3K4me1 and H3K27ac peaks. We then used CRISPR-mediated epigenetic editing to test the function of this H3K9me3 enrichment (Figure 5E). To do this we chose a CLL-derived MEC1 cell line,44 which does not have this CLL-specific H3K9me3 peak (Figure 5F), making it an appropriate model to study the gain of function of H3K9me3 peak. Of the 3 guide RNAs we tested, 2 (1 and 3) were able to generate the H3K9me3 peak at the indicated region (Figure 5F), which induced a modest reduction of H3K4me1 and H3K27ac at the enhancer (Figure 5F). We also observed reduced expression of EBF1 by this epigenetic editing (Figure 5G-H). Interestingly, the gain of this H3K9me3 peak was not sufficient to abolish all the H3K4me1 and H3K27ac as seen in the primary CLL B cells (Figure 5F), indicating both H3K9me3 and H3K27me3 or the interaction of chromatin with other signaling events are required for silencing this enhancer in CLL.
Increased H3K9me3 suppresses EBF1 expression. (A) A snapshot showing histone modification enrichment at gene locus EBF1. The gained H3K9me3 peak in CLL is highlighted. (B) Downregulated EBF1 but not PAX5 expression in CLL B cells. Gene expression is determined by RNA-seq. (C) Validation of the expression of EBF1 in the indicated samples by Reverse Transcritpion-Polymerase Chain Reaction (RT-PCR). (D) Validation of the expression of EBF1 in the indicated samples by western blot. (E) Scheme of CRISPR dCas9-Krab–mediated (Dead Cas9-Kruppel-associated box) epigenetic silencing (H3K9me3 deposition). (F) Histone modification profiles at the EBF1 locus at indicated samples. The cells with infection of indicated single-guide RNAs (sgRNAs) were incubated with 0.25 mg/mL doxycycline for 7 days to induce dCas9-Krab expression before the cells were harvested. (G) RT-qPCR analysis of EBF1 expression after the introduction of H3K9me3 at the indicated region by CRISPR. (H) Western blot analysis of EBF1 expression after the introduction of H3K9me3 at the indicated region by CRISPR. GAPDH, Glyceraldehyde-3-phosphate dehydrogenase; sgRNA, small guide RNA; ns, not significant; sgGFP, small guide RNA targeting Green fluoresent protein (GFP).
Increased H3K9me3 suppresses EBF1 expression. (A) A snapshot showing histone modification enrichment at gene locus EBF1. The gained H3K9me3 peak in CLL is highlighted. (B) Downregulated EBF1 but not PAX5 expression in CLL B cells. Gene expression is determined by RNA-seq. (C) Validation of the expression of EBF1 in the indicated samples by Reverse Transcritpion-Polymerase Chain Reaction (RT-PCR). (D) Validation of the expression of EBF1 in the indicated samples by western blot. (E) Scheme of CRISPR dCas9-Krab–mediated (Dead Cas9-Kruppel-associated box) epigenetic silencing (H3K9me3 deposition). (F) Histone modification profiles at the EBF1 locus at indicated samples. The cells with infection of indicated single-guide RNAs (sgRNAs) were incubated with 0.25 mg/mL doxycycline for 7 days to induce dCas9-Krab expression before the cells were harvested. (G) RT-qPCR analysis of EBF1 expression after the introduction of H3K9me3 at the indicated region by CRISPR. (H) Western blot analysis of EBF1 expression after the introduction of H3K9me3 at the indicated region by CRISPR. GAPDH, Glyceraldehyde-3-phosphate dehydrogenase; sgRNA, small guide RNA; ns, not significant; sgGFP, small guide RNA targeting Green fluoresent protein (GFP).
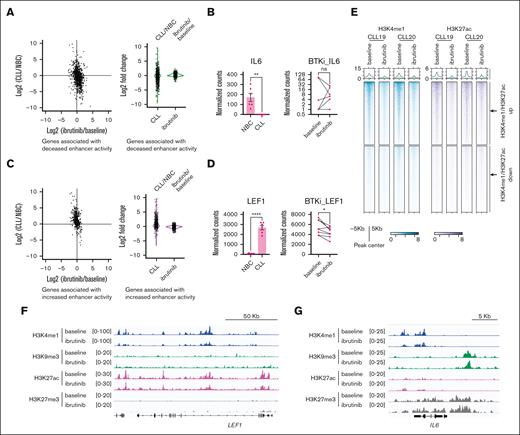
The lost enhancers marked by heterochromatin remain stable after Bruton tyrosine kinase inhibitor (BTKi) treatment
We named the regions with increased or decreased enhancer signatures (H3K4me1 and H3K27ac enrichment) compared with NBCs as “gained enhancers” or “lost enhancers,” respectively. Next, we examined the response of the gain/lost enhancers to the BCR signaling inhibitor ibrutinib in patients with CLL. We profiled the transcription and histone modification status of the primary CLL B cells derived from patients with CLL, at baseline before ibrutinib therapy, and then when treated the with ibrutinib continuously for 1 year (supplemental Table 1, n = 8 patients). These patients were all in partial or complete responses at the time of the blood collection at 12 months. We noticed that the percentage of clonal B cells was largely unchanged at this time point of ibrutinib treatment (supplemental Figure 12A), which can occur in single agent use of ibrutinib even when patients are responding.45 We compared our results with those of previous studies46,47 and found that our data showed similar results, that ibrutinib treatment decreased chromatin accessibility and H3K27ac at the closed chromatin defined by Rendeiro et al46 (supplemental Figure 12B).
Next, we focused our analysis on the aforementioned CLL-gained/lost enhancers. These enhancers are largely not overlapped with the closed enhancers modified by ibrutinib treatment (defined by Rendeiro et al46; supplemental Figure 13A). We checked the expression of these lost enhancer–associated genes (most of these genes were downregulated in CLL compared with NBCs) in CLL and found that ibrutinib treatment over 12 months slightly increased their expression, but the fold changes were much smaller than that between CLL B cells and NBCs (Figure 6A-B). Similarly, the expression of CLL-gained enhancer-regulated genes in CLL were slightly decreased by 1 year of ibrutinib treatment but to a much less extent compared with the upregulation in untreated CLL compared with NBCs (Figure 6C-D). These results suggest that the expression of the altered enhancer-regulated genes in CLL is relatively stable even during clinically effective ibrutinib treatment.
Ibrutinib treatment partially restores chromatin signatures at gained enhancers in CLL. (A) Comparison of the log2 fold changes of the genes regulated by decreased enhancers in CLL between CLL vs NBCs and ibrutinib treatment vs baseline CLL B cells. (B) Expression of IL-6 in the indicated samples. (C) Comparison of the log2 fold changes of the genes regulated by increased enhancers in CLL between CLL vs NBCs and ibrutinib treatment vs baseline CLL B cells. (D) Expression of LEF1 in the indicated samples. (E) Representative metagene heat map showing the CLL-gained and -lost enhancers in the CLL B cells derived from patients with CLL at baseline and during ibrutinib treatment. (F-G) Representative snapshots showing the indicated histone marks at the LEF1 and IL-6 gene loci. ns, not significant.
Ibrutinib treatment partially restores chromatin signatures at gained enhancers in CLL. (A) Comparison of the log2 fold changes of the genes regulated by decreased enhancers in CLL between CLL vs NBCs and ibrutinib treatment vs baseline CLL B cells. (B) Expression of IL-6 in the indicated samples. (C) Comparison of the log2 fold changes of the genes regulated by increased enhancers in CLL between CLL vs NBCs and ibrutinib treatment vs baseline CLL B cells. (D) Expression of LEF1 in the indicated samples. (E) Representative metagene heat map showing the CLL-gained and -lost enhancers in the CLL B cells derived from patients with CLL at baseline and during ibrutinib treatment. (F-G) Representative snapshots showing the indicated histone marks at the LEF1 and IL-6 gene loci. ns, not significant.
We further assessed the enrichment of histone modifications at these CLL-gained/lost enhancers to ibrutinib treatment. We observed a decrease in H3K4me1 and H3K27ac enrichment at the CLL-gained enhancers after ibrutinib treatment (Figure 6E-F; supplemental Figure 13B). However, the enrichment of H3K4me1 and H3K27ac at the CLL lost enhancers remained largely unchanged upon treatment (Figure 6E,G; supplemental Figure 13B). We further found that H3K27me3 and H3K9me3 remained stable in most of the gained and lost enhancers (supplemental Figure 13B-C; Figure 6F-G). Collectively, we found that the histone modification signatures at CLL lost enhancers are largely not responsive to BCR signaling inhibition.
Discussion
The exact etiology of an abnormal and deficient immune response in CLL has yet to be determined but is multifactorial. Here, we provide a novel explanation for immunodeficiency in CLL through epigenetic reprogramming. Epigenetic reprogramming has been considered a major hallmark of cancer.48 Aberrant epigenetic modifications cause gene expression that facilitates and promotes cancer development. One of the most well-studied mechanisms related is the activation of enhancers that promote oncogene expression.49 In CLL, cancer-specific enhancers have also been identified and implicated in important roles of leukemogenesis and drug resistance.14-16,46,50 However, the functional importance and the mechanisms of the regulation of the CLL lost enhancers are not well understood. More importantly, there is a lack of systematic analysis of suppressive histone modification in CLL. In this study, we showed that the lost enhancers in MBL and CLL are associated with immune response genes and are potentially regulated by the suppressive histone marks H3K9me3 and H3K27me3. Previous genomic analysis in CLL has shown an impaired immune response,51 and our study provides further evidence for an epigenetic basis of the deregulated immune response.
The impaired humoral and cell-mediated immune responses in MBL and CLL lead to increased infections (including COVID-19) requiring hospitalization, which is 1 of the major causes of morbidity and mortality in CLL.52-58 Therefore, with our discovery of epigenetic reprogramming in CLL, it is important to understand how this epigenetic event affects the immune response genes in CLL. We identified the genes regulated by the decreased enhancers in CLL and MBL clonal B cells and found that they are mainly associated with immune response (Figures 2E and 3E; supplemental Tables 2-4). Of the genes suppressed by heterochromatin in CLL, EBF1 is a well-defined TF that defines the NBC lineage,41 and its expression is lost in CLL B cells,42,43 and genetic disruption of EBF1 contributes to leukemogenesis.59,60 EBF1 has been shown to support the expression of the immunoglobulin gene recombination machinery,61 the immunoglobulin beta-chain gene,62 and the endogenous immunoglobulin surrogate light chain genes.63 Thus, the silenced expression of EBF1 by heterochromatin in CLL may contribute to hypogammaglobulinemia frequently seen in CLL.64 Another example marked with increased heterochromatin at its enhancer is IL-6, which is associated with B-cell antibody production,65 suggesting IL-6 silencing also contributes to impaired immunoglobulin production in CLL. It is well established that CLL B cells can suppress T-cell activity.66,67 We also observed genes important for T-cell function are suppressed by heterochromatin in CLL B cells. For example, we found that IL-1A and IL-1B are marked with heterochromatin and their expression is down in CLL. IL-1 is an important mediator of T-cell immunity,29-31 so the suppression of IL-1A and IL-1B gene expression by heterochromatin in CLL may contribute to T-cell dysfunction in CLL. Our data and prior publications indicate that B cells and other cell types are both involved in the immune response defects in CLL. Together, silencing the crucial B-cell immune response genes by epigenetic mechanism(s) may contribute to the deficient immune responses and, thus, the increased risk of serious infections in CLL and MBL. This may also apply to the known reduced efficacy of vaccinations for influenza68 and documented more recently to COVID-19 vaccines.69
It is possible that the loss of immune gene expression coordinates with the activated oncogenic genes and thus favors the selection of the clonal B cells during disease evolution.
H3K27ac is the mark for active enhancers and has important functions in cell development and tumorigenesis.70,71 H3K27ac has been exploited for cancer therapy by specifically targeting either its writer CBP/p300 or reader bromodomain and extraterminal domain (BET) proteins.72,73 However, recent studies indicate that H3K27me3 plays a determining role in the enhancer activity, which challenges the sole function of H3K27ac in enhancer transcription activation.74,75 Previous findings suggested the incomplete silencing of gene expression is mediated by H3K27me333; our study showed that a proportion of CLL lost enhancers are also marked with H3K27me3 (Figure 2C) and associated with gene downregulation in CLL (supplemental Figure 43B), suggesting an important role of gained H3K27me3 in suppressing the activity of these enhancers and gene expression.
H3K9 methylation marked heterochromatin plays important roles in lineage specification during normal tissue development35 and tumor progression.36 We also observed the enrichment of H3K9me3 at the CLL lost enhancers. Although CLL is regarded as an indolent cancer, CLL B cells still undergo cell division during evolution.76,77 Both H3K27me3 and H3K9me3 are stable epigenetic marks that can be faithfully inherited during DNA replication,78,79 and both are barriers to transcription-induced cell programming.79,80 Our findings here indicate that these repressive histone modifications safeguard the lost enhancers in CLL, which may have important roles yet to be discovered in maintaining leukemic B-cell identity. We also observed that some M-CLL showed a relatively higher increase of H3K27me3 and H3K9me3 at the lost enhancers (supplemental Figures 5C and 9C). However, the expression of these enhancer-regulated genes between U-CLL and M-CLL is very similar (supplemental Figures 5D and 9D). These genes may show different responses to cellular stimulation or other signaling pathways when compared between M-CLL and U-CLL and need further investigation.
Although we found a correlation between heterochromatin and enhancer reprogramming, the causal effect of heterochromatin on enhancer changes is not defined. Using the EBF1 locus as an example, we showed that acquired heterochromatin H3K9me3 led to moderate enhancer inhibition and EBF1 downregulation, indicating that H3K9me3 and H3K27me3 coordinate to inhibit the enhancer activity. Previous studies showed that the low expression of EBF1 in CLL is associated with hypermethylation at its promoter,42 suggesting that epigenetic mechanisms regulate EBF1 expression at multiple levels. We also profiled the histone modification landscapes of CLL-derived cell lines (HG3, MEC1, PGA, CI, and CII) and found none of these lines exhibit a similar CLL-specific heterochromatin/enhancer signature (data not shown). This latter finding further emphasizes the importance of using primary patient-derived cells to study CLL B-cell epigenetics.
During BTKi ibrutinib treatment, the CLL-gained enhancers could be partially reversed, indicating that they are dependent to some extent on the BCR signaling pathways. However, compared with gained enhancers, we found that the lost enhancers are less responsive to ibrutinib treatment in responding patients, suggesting the heterochromatin domains at the lost enhancers are stable. It is also possible that the modest gene expression change by ibrutinib treatment is independent of the epigenetic states of the enhancers. We will continue to investigate the function of these heterochromatin-marked enhancers in CLL treatment with more primary CLL samples. Notably, our study here only included ibrutinib-treated samples; we recognize that other US Food and Drug Administration–approved BTKis with less off-target activities may have the same or differential effects on this epigenetic signature and are currently under our study. Importantly, our finding indicates that ibrutinib treatment does not apparently restore immune functions based on an epigenetic basis, suggesting future development of novel agents in treating CLL should also focus on more significant reversal of the epigenetic events that dampen their immune response.
Altogether, we comprehensively mapped the histone modification profiles of NBC subsets, the precursor MBL stage, and sequentially in ibrutinib-treated CLL B-cell samples while patients were in a clinical response. Our study shed new insights into a more mechanistic understanding of the CLL epigenome. First, a proportion of the lost enhancers in CLL that are marked with heterochromatin mark H3K9me3 or H3K27me3 remain even on therapy, suggesting the stable state of these enhancers despite the use of novel agents. Second, the transition to the CLL-specific heterochromatin/enhancer signature we show also occurs at the CLL precursor stage of MBL, further underlining the malignant nature of the B cell in MBL and suggesting this event may play a role in disease progression.
Acknowledgments
The authors thank all of the Mayo Clinic Chronic Lymphocytic Leukemia (CLL) Research group members for the helpful discussions on the work. The authors thank the Henry J. Predolin Foundation for supporting the Mayo Clinic CLL Tissue Bank.
The work was supported by the Hollis Brownstein research grants program from the Leukemia Research Foundation (Z.W.), the Mayo Clinic Cancer Center-Karolinska Institutet (MCCC-KI)Cancer Research Award from the Mayo Clinic Breast Cancer Specialized Programs of Research Excellence (SPORE) (P50CA116201) (N.E.K), and R01CA251801 from the Insti National Institutes of Health (N.E.K). This work was also funded by Michael W. Michelson and Georgia Taylor Michelson.
Authorship
Contributions: Z.W. conceived and designed the project; Z.W., O.M.D., H.C.D, M.S.H., and Q.G. performed the experiments; Z.Y. helped with bioinformatic analysis tools; S.S. and W.H. provided key reagents and samples; Z.W. and O.M.D. analyzed the data; K.G.R, Y.G, C.Y, E.B, S.L.S., and S.A.P. provided key resources and supervision; and Z.W. and N.E.K. supervised the project.
Conflict-of-interest disclosure: N.E.K. reports advisory board role for AbbVie, AstraZeneca, BeiGene, Behring, Boehringer Ingelheim Pharmaceuticals, Inc, Dava Oncology, Janssen, Juno Therapeutics, and Pharmacyclics; served on the data safety monitoring committee for Agios Pharm, AstraZeneca, Bristol Myers Squibb (BMS)/Celgene, and Dren Bio Janssen; and reports research funding from AbbVie, Acerta Pharma, BMS, Celgene, Genentech, Pharmacyclics, Sunesis, and Vincerx. S.A.P. reports that research funding has been provided to the institution from Janssen, AstraZeneca, Merck, and Genentech for clinical studies in which S.A.P. is a principal investigator; reports that honoraria has been provided to the institution from Pharmacyclics, Merck, AstraZeneca, Janssen, BeiGene, Genentech, Amgen, MingSight Pharmaceuticals, TG Therapeutics, Novalgen Limited, Kite Pharma, and AbbVie for participation in consulting activities/advisory board meetings. The remaining authors declare no competing financial interests.
Correspondence: Zhiquan Wang, Mayo Clinic, 200 1st St SW, Rochester, MN 55905; email: wang.zhiquan@mayo.edu; and Correspondence: Neil E. Kay, Mayo Clinic, 200 1st St SW, Rochester, MN 55905; email: kay.neil@mayo.edu.
References
Author notes
The data sets generated during and/or analyzed during this study are available upon reasonable request from the corresponding author, Zhiquan Wang (wang.zhiquan@mayo.edu).
The full-text version of this article contains a data supplement.