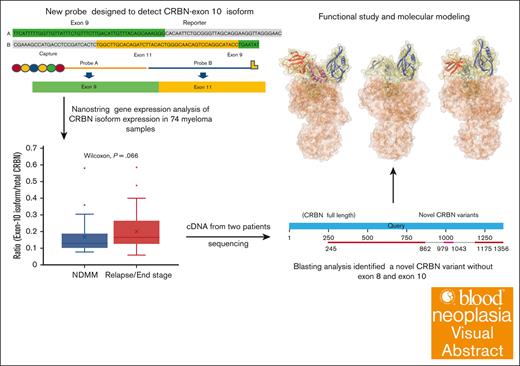
Key Points
A newly described CRBN variant with both exon 8 and 10 transcript deletions in myeloma was identified using a novel digital gene expression assay.
Functional and molecular modeling demonstrate alterations to the binding pocket and compactness of CRBN protein.
Visual Abstract
Acquisition of drug resistance, particularly to immunomodulatory drugs (IMiDs) in multiple myeloma (MM), is an important challenge in patient treatment. IMiDs are the canonical molecular glue that bind to cereblon (CRBN) and redirect its E3 ubiquitin ligase activity to neo-substrates, such as IKZF1/IFZF3. Genetic changes in the CRBN gene have been associated with IMiD resistance, including the exon 10–spliced CRBN transcripts that increase in incidence in parallel with IMiD-refractory states. Herein, we designed a new probe against CRBN splice isoform to detect exon 10 deletion and validated the variant expression using NanoString and messenger RNA (mRNA) sequencing analysis. We analyzed 74 MM samples, including 28 cases of newly diagnosed MM and 46 cases of relapsed and end-stage MM. Four cases were identified with 40% exon 10–deleted mRNA as compared with total CRBN mRNA (1 from newly diagnosed, 3 from relapsed and end-stage). Sequencing of complementary DNAs from 2 samples unexpectedly demonstrated additional deletion of exon 8. Extensive molecular-modeling and coimmunoprecipitation studies identified the mechanisms of CRBN dysfunction in protein structures, including impact on the interaction between CRBN with the E3 ligase machinery on deletion of exon 8 or dual deletion of exons 8 and 10. In summary, we developed a feasible approach to detect exon 10–deleted CRBN isoform on a gene expression platform and unexpectedly identified a variant with alterations of exon 10 and 8 transcripts in both newly diagnosed and refractory cases. This variant is present at diagnosis and could have separate, yet likely additive, effects on drug resistance.
Introduction
Immunomodulatory drugs (IMiDs) are frequently used therapies in the treatment of multiple myeloma (MM). These drugs are used in combinations at all stages of disease and are approved as a single-agent maintenance treatment after autologous stem cell transplantation. Acquisition of drug resistance, particularly to IMiDs, remains one of the most important challenges in the treatment of MM. Early in the disease, the plasma cells in the MM clone are often sensitive to these drugs. However, over time, after exposure to chemotherapy, the cells acquire increasing genomic complexity and mutations leading to drug resistance and ultimately patient death. Cereblon (CRBN), a substrate receptor for CRL4 E3 ubiquitin ligase, is the primary target for the widely used IMiDs, such as lenalidomide and pomalidomide.1,2 CRBN is essential to the newer CRBN E3 ligase modulator drugs and proteolysis targeting chimeras.3 IMiDs and other CRBN-targeting compounds function as a molecular glue between the E3 ligase substrate receptor CRBN and protein substrate.3 The mechanisms of IMiD resistance have been extensively investigated and multiple mechanisms identified, including CRBN-associated and CRBN-independent mechanisms.4-7 We recently described an algorithm based on NanoString digital gene expression profiling of purified CD138+ MM cells from patients at different clinical stages that we term the “MM-IP-7,” which correlated with clinical drug resistance, poor survival, and disease progression after combination treatment containing IMIDs and/or proteasome inhibitors.8
Using whole genome sequencing (n = 455) and RNA sequencing (n = 655), Gooding et al9 described the increasing frequency of 3 CRBN aberrations, including point mutations, copy losses/structural variations, and an exon 10–spliced variant,10 after IMiD exposure. Nearly one-third of IMiD-refractory patients harbored these CBRN alterations, which further correlated with poor patient outcomes.9
Given these data, we sought to build an integrated, single-platform test to measure both total and exon 10–deleted CRBN expression levels. We not only detected CRBN exon 10–deleted transcripts in untreated patients, but we also found a novel variant that lacked both exons 8 and 10. Subsequent dynamic molecular modeling was used to further explore protein function with implications for therapeutic approach.
Methods
MM cell lines and human MM cells
All human MM cell lines (HMCLs) used in this study were provided by the Bergsagel laboratory and were DNA fingerprinted to confirm their identity.11 The cells were cultured in RPMI 1640 medium with 5% fetal calf serum.
Primary human MM cells were recovered from bone marrow aspirates collected from all Mayo Clinic sites. Informed consent was given in writing for collection and research use under institutional review board approval (919-04, 15-009436, 18-003198, 2207-02) in accordance with the Declaration of Helsinki. After collection, CD138+ cells were isolated by immunomagnetic bead selection (RoboSep; STEMCELL Technologies).
Sample preparation and RNA extraction
Total RNA from HMCLs and CD138-selected plasma cells from the bone marrows of a patient with MM were isolated using RNeasy mini kit and the AllPrep DNA/RNA Kit (Qiagen), respectively. After spectrophotometric quantification (NanoDrop2000, Thermo Fisher Scientific), RNA was stored at −80°C until use.
Digital gene expression quantification
The newly designed CRBN probe_5 was added to our previously designed panel of 68 informative genes associated with IMiD and proteasome inhibitor response, along with 11 housekeeping genes (supplemental Data 1), to generate the exploratory CodeSet for this study.8 All probes were designed and named by the bioinformaticians at NanoString. The experiments were performed with nCounter Elements XT reagents in accordance with the manufacturer’s recommendations. An input of 100 ng total RNA was used for all cell lines and primary samples. The collected data were analyzed using nSolver 4.0 software and an advanced analysis software plugin (version 2.0, R-based statistical tool). The normalized data detected by all CRBN probes were analyzed. To define increased exon 10–deleted allele frequency, we calculated the ratio of the normalized counts detected by CRBN_5 to the normalized counts detected by total CRBN probe, CRBN_2. Cell lines were run several times as independent experiments using different panels, and results were highly reproducible as measured by Pearson correlation analysis (R2 > 0.93; supplemental Figure 1).
RT-PCR to amplify the complementary DNA (cDNA) of the CRBN exon 10 splicing variants from patient samples
Two samples (1 from a newly diagnosed patient and 1 from a relapsed patient) that revealed >50% of CRBN exon 10 splicing transcripts were selected for reverse transcription polymerase chain reaction (RT-PCR). Briefly, 2 primers specific for human CRBN were designed and synthesized (supplemental Data 2). Furthermore, 100 ng of total RNA was used in each reaction. RT-PCR was performed using SuperScript III Platinum One-Step RT-PCR kit according to the manufacturer’s instructions. The RT step involved incubation at 55°C for 30 minutes. The PCR cycling conditions included an initial denaturation at 94°C for 2 minutes followed by 40 cycles at 94°C for 15 seconds, 62°C for 30 seconds, and 68°C for 1.5 minutes. The PCR products were separated on a 1% agarose gel along with a DNA ladder. The DNA bands were harvested and purified using QIAquick gel extraction kit (Qiagen).
Site-directed mutagenesis by PCR to generate the CRBN with exon 8 deletion
There were 4 primers designed (supplemental Data 2) to generate the DNA that encodes the CRBN mutant without either exon 8 or exon 10. Furthermore, 100 ng of plasmid DNA containing wild-type (WT) CRBN was used as a template. The PCR cycling conditions included an initial denaturation at 98°C for 30 seconds, followed by 25 cycles at 98°C for 10 seconds, 68°C for 30 seconds, and 72°C for 30 seconds. The final incubation was at 72°C for 3 minutes. The PCR products were separated on 1% of agarose gel along with the DNA ladder. The DNA bands were harvested and purified using QIAquick gel extraction kit (Qiagen).
Cloning and sequencing of the cDNA amplified from patient samples and mutant DNA
The DNA fragments purified from either cDNA amplification or site-directed mutagenesis by PCR were digested with BamH1 and Xba1 and then inserted into the lentiviral vector pCDH-CMV-MCS-EF1-copGFP (System Bioscience, Mountain View, CA). The plasmids carrying inserted DNA were selected and sent for Sanger DNA sequencing (Azenta, Burlington, MA) with 2 primers (supplemental data 2). The sequence data were compared with human CRBN sequence from GenBank using BLAST tool from the National Institutes of Health.
Lentivirus preparation, infection, and expression
The plasmids validated by DNA sequencing were transfected into the 293FT cell line to generate lentivirus. Lentivirus harboring control vector and all expression constructs were used to infect the human myeloma cell line, OCIMY5. The infection efficiency was measured by Fluorescent-Activated Cell Sorter (FACScan) analysis of Green Fluorescent Protein (GFP) expression at day 3 after infection. Overexpression of CRBN was confirmed by immunoblotting assay.
Cell viability assay
HMCLs were cultured in RPMI 1640 media, supplemented with 5% of sterile fetal calf serum and antibiotics. Cell viability was measured by MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) dye absorbance according to the manufacturer’s instructions (Boehringer Mannheim). The MTT assay was set up either in the absence or presence of increasing doses of IMiDs. Each experimental condition was performed in triplicate and repeated at least twice. To evaluate whether it was the mutant cell line that was selected by treatment, R13 (GFP+) and WT (GFP−) expressing cells were mixed, treated, and analyzed by flow cytometry for GFP expression.
Co-IP and immunoblotting assay
Western blot was performed according to manufacturer’s protocol. Briefly, equal amounts of protein were subjected to sodium dodecyl sulfate polyacrylamide gel electrophoresis gels followed by transfer to Polyvinylidene fluoride (PVDF) or nitrocellulose membranes. Membranes were probed with primary antibodies overnight and then washed and incubated with either horseradish peroxidase-conjugated secondary antibodies or Alexa Fluor-conjugated secondary antibodies. Detection was performed using the enhanced chemical luminescence method or Li-COR Odyssey CLX imaging system. Coimmunoprecipitations (co-IPs) of CRBN with DDB1 were performed according to the protocol of Pierce classic magnetic IP/co-IP kit.
Molecular modeling
The CryoEM structures of CRBN open (PDB ID: 8CVP) and CRBN closed (PDB ID: 8D81) conformation were obtained from the Protein Data Bank. To construct the structures with deletion, we performed homology modeling using BIOVIA Discovery Studio (Discovery Studio 2023; Dassault Systèmes). Availability of CryoEM structures as dependable templates prompted our use of homology modeling for generating the deleted CRBN structures, instead of using AlphaFold. Indeed, studies indicate a better performance of template-based structure predictors than those from AlphaFold when credible template structures are present in the PDB.12 We used the 8CVP and 8D81 structures to model the deletions. The combined exon 8 and 10–deleted structures and exon 8–only deleted structures are based on the open model (8CVP). To find the effects of the deletions in the transition from open to closed CRBN structure, the 3 models (WT, combined exon 8 and 10–deleted, and exon 8–only deleted) were subjected to implicit solvent replica exchange molecular dynamics (REMD)13 using the Model Employing Limited Data (MELD) framework.14,15 For each model, 4 replicas were initiated between the temperatures Tmax = 330 K and Tmin = 300 K, and a total of 1000 ns of REMD with 20% to 30% replica exchange was performed. After extraction of the lowest temperature trajectory, root mean square deviation analysis was performed with respect to the initial structure. On the basis of the root mean square deviation histograms (supplemental Figure 2), 5 frames from the lowest temperature replica were extracted from each model. These frames were used as starting points for the explicit solvent simulations. It was previously found that the IMiD plays a pivotal role in the allosteric transition from open to closed conformation.3 As the combined exon 8 and 10–deleted structure lacks the thalidomide binding domain (TBD), we did not incorporate any IMiD in the simulation system for those models. Nonetheless, the WT and exon 8–only deleted structures retained their TBDs, rendering the possibility of IMiD binding. So, we docked the IMiD drug pomalidomide in the TBD of the WT and exon 8–deleted models extracted from the REMD trajectory, using docking tools available in BIOVIA Discovery Studio 2023 and generated the force field parameters for the IMiD using Chemistry at Harvard Macromolecular Mechanics - Graphical User Interface (CHARMM-GUI).16 All the extracted structures were then subjected to 200 ns molecular dynamics (MD) simulation using CHARMM36 force field17 and TIP318 explicit water model, using NAMD.19,20 Analysis of the trajectories was done using Visual Molecular Dynamics (VMD),21 MDAnalysis,22 and MDtraj.23 So, in total, we performed 4000 ns of implicit MD simulations and 5 × 200 ns of explicit MD simulations for each 1 of the 3 systems, simulating a cumulative time of 15 μs considering all the model systems.
Results
Detection of the exon 10–spliced CRBN transcripts in HMCLs and MM samples using NanoString digital expression technology
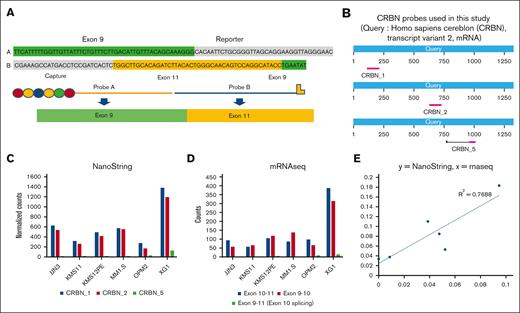
In this study, a novel CRBN probe (CRBN probe_5) was designed and added to our NanoString probe panel to detect expression of CRBN exon 10 splicing transcripts (Figure 1; supplemental Data 1). This probe was designed to have sequence homology with both exons 9 and 11 of human CRBN DNA and was expected to specifically hybridize with the CRBN isoform lacking exon 10. We also designed 2 additional CRBN probes (CRBN_1 and CRBN_2), which would detect all CRBN transcripts and measure total CRBN expression levels (Figure 1B; supplemental Data 1). To evaluate the performance of the new probe (CRBN probe_5), 6 HMCLs were evaluated and the normalized counts from hybridization were compared (Figure 1C). The expression counts measured using CRBN probe _5 was markedly low in each of the 6 tested cell lines when compared with the total CRBN expression detected by CRBN_1 and CRBN_2 probes. To further validate the results, we analyzed the messenger RNA (mRNA) sequencing data at the CRBN locus from those 6 cell lines (supplemental Data 3; supplemental Figure 3). The transcripts at 3 junctions (exons 10-11, exons 9-10, and exons 9-11) were counted. As found in Figure 1D, most counts were detected at exons 10 to 11 and exons 9 to 10. Counts detected from exons 9 to 11 were minimal. Further analysis indicated that the expression of exon 10 splice variant normalized to total CRBN as measured by mRNA sequencing correlated well with the level detected by the CRBN probe_5 in each cell line (Figure 1E). These data suggested that the newly designed CRBN probe appropriately detects CRBN exon 10 deletion.
Detection of the CRBN exon 10 splicing isoform from HMCLs with a newly designed probe on NanoString platform. (A). Newly designed CRBN probe (CRBN_5) has homology to both exon 9 and exon 11. (B) Three CRBN probes (CRBN_1, CRBN_2, and CRBN_5) were used in this study, and their locations on CRBN mRNA sequence are illustrated. (C) Expression of CRBN and the exon 10 slicing isoform in 6 HMCLs. The normalized counts from 3 CRBN probes were illustrated for each cell line. Total CRBN levels (detected by CRBN_1 and CRBN_2 probes) and the exon 10 splicing isoform (detected by CRBN_5 probe) were compared. (D) mRNA sequencing data at the CRBN locus in the same 6 cell lines were extracted and the numbers of count between exon 9 and exon 11 (represent exon 10 slicing isoform), exon 10 and exon 11, and exon 9 and exon 10 are demonstrated. (E) Expression levels of exon 10 isoform normalized to total CRBN as measured by RNA sequencing and NanoString (supplemental Data 3) were compared and plotted using Pearson correlation. mRNAseq, mRNA sequencing.
Detection of the CRBN exon 10 splicing isoform from HMCLs with a newly designed probe on NanoString platform. (A). Newly designed CRBN probe (CRBN_5) has homology to both exon 9 and exon 11. (B) Three CRBN probes (CRBN_1, CRBN_2, and CRBN_5) were used in this study, and their locations on CRBN mRNA sequence are illustrated. (C) Expression of CRBN and the exon 10 slicing isoform in 6 HMCLs. The normalized counts from 3 CRBN probes were illustrated for each cell line. Total CRBN levels (detected by CRBN_1 and CRBN_2 probes) and the exon 10 splicing isoform (detected by CRBN_5 probe) were compared. (D) mRNA sequencing data at the CRBN locus in the same 6 cell lines were extracted and the numbers of count between exon 9 and exon 11 (represent exon 10 slicing isoform), exon 10 and exon 11, and exon 9 and exon 10 are demonstrated. (E) Expression levels of exon 10 isoform normalized to total CRBN as measured by RNA sequencing and NanoString (supplemental Data 3) were compared and plotted using Pearson correlation. mRNAseq, mRNA sequencing.
Next, we evaluated 74 MM samples collected at various stages of disease and treatment, including 28 samples from newly diagnosed (N) patients (without treatment), 17 samples from relapsed (R) patients (who developed resistance from treatments with regimens containing IMiDs and other antimyeloma drugs), and 29 samples from the end-stage (E) patients (who have received multiple therapies, including IMiDs, and the samples were harvested at ∼6 months before death). Consistent with cell line data, the expression of exon 10–spliced CRBN was generally low in all samples. The ratio of this exon 10–deleted isoform to total CRBN was calculated in each sample (supplemental Data 4). The average ratio of exon 10 isoform/total CRBN in R and E patients was close to 20%, which was then selected as a cutoff value to stratify the patient samples in each group as below (low) or beyond (high) this level. As found in Figure 2A, a higher percentage of R and E patients presented >20% exon 10–deleted transcripts as compared with N patients (41.3%-44.8 vs 21.4%). Four patients had more than 40% of exon 10–deleted transcripts, including 1 N and 3 R and E patients (supplemental Data 4). Unlike previously published data,9,10 none of our samples have a ratio >0.60. Next, we analyzed the ratio of exon 10 isoform/total CRBN in samples with different IMiD sensitivity. As found in Figure 2B, R and E patients (representing IMiD-resistant group) had a higher ratio than N patients (representing IMiD-sensitive group), which trended toward statistical significance (Wilcoxon rank sum test, P < .066).
Detection of the CRBN exon 10 splicing isoform from CD138+ purified cells from MM samples. The MM samples collected at the different stages, including newly diagnosed and untreated, relapsed from treatment containing IMiDs and end-stage, were run on the NanoString panel with the newly designed CRBN_5 probe. The ratio of the normalized counts detected by CRBN_5 to the normalized counts detected by total CRBN probe, CRBN_2 was calculated. (A) The percentage of samples with low ratio (<20%) and high ratio (>20%) in the sample harvested at different stages was demonstrated. (B) The ratio of exon 10 isoform to total CRBN statistically trended higher (by Wilcoxon rank sum test) in the samples from IMiD-resistant patients (relapse and end stage) than in the samples from IMiD-sensitive patients (NDMM). NDMM = newly diagnosed multiple myeloma.
Detection of the CRBN exon 10 splicing isoform from CD138+ purified cells from MM samples. The MM samples collected at the different stages, including newly diagnosed and untreated, relapsed from treatment containing IMiDs and end-stage, were run on the NanoString panel with the newly designed CRBN_5 probe. The ratio of the normalized counts detected by CRBN_5 to the normalized counts detected by total CRBN probe, CRBN_2 was calculated. (A) The percentage of samples with low ratio (<20%) and high ratio (>20%) in the sample harvested at different stages was demonstrated. (B) The ratio of exon 10 isoform to total CRBN statistically trended higher (by Wilcoxon rank sum test) in the samples from IMiD-resistant patients (relapse and end stage) than in the samples from IMiD-sensitive patients (NDMM). NDMM = newly diagnosed multiple myeloma.
Cloning and sequencing of CRBN transcripts from 2 MM samples identified the CRBN variant with both exon 10 and 8 deletions
To further validate that patient samples contained the CRBN exon 10 isoform, we performed RT-PCR to amplify the CRBN cDNA from 2 patient samples (N17 and R13) that had >50% of exon 10–deleted CRBN transcripts in digital expression profiling and abundant remaining material. The cDNA products were inserted into a lentiviral expression construct and sent for sequencing. In addition to detecting the plasmids that carry WT CRBN cDNA, DNA sequencing also identified a novel CRBN variant with both exon 10 and 8 deletions in 2 patient samples (Figure 3A). This observation is described herein as a dual deletion but could be due to exon skipping. To know whether this CRBN variant can be translated and is functional, we generated the lentivirus to express this variant in a HMCL, OCIMY5. OCIMY5 was previously demonstrated to express low levels of WT CRBN and is known to be resistant to lenalidomide.24 Overexpression of WT CRBN into this cell line increased its sensitivity to IMiDs. Using western blots, we analyzed the OCIMY5 cell line transfected with the lentivirus carrying vector alone (Vec), WT CRBN, or the CRBN isolated from a patient sample (R13). The CRBN protein expressed from patient-derived cDNA was much shorter when compared with the WT CRBN, as found in Figure 3B.
Identifying a novel CRBN transcript with both exon 10 and 8 deletions. (A) CRBN contains several domains, including LON and CULT domains. The exon 10 encoding region is a part of CULT domain and the exon 8 encodes parts of LON-C domain. The cDNAs from 2 patients with MM (R13 and N17) were generated, cloned, and sequenced. The sequence results were compared with the WT CRBN mRNA sequence (National Center for Biotechnology Information [NCBI] reference sequence: NM_00117382.1) in GenBank, indicating exon 10 (between 1043 and 1175) and exon 8 (between 862 and 979) were both deleted from 2 clones isolated. (B) The cloned cDNA from 1 patient (R13) was introduced into OCIMY5 cell line along with WT CRBN and vector alone. The expression of CRBN in those cells was detected by western blot. MTT assay demonstrated this novel isoform cannot mediate pomalidomide-induced antimyeloma activity. (C) OCIMY5 with vector, WT CRBN, R13 were treated with pomalidomide and analyzed using MTT assay. (D) R13 (GFP+) and WT (GFP−) expressing cells were mixed, treated, and analyzed by flow cytometry. The percentage of R13 expressing cells increased after exposure to Pom. Vec = Vector, POM = pomalidomide, WT = wild type, R13= OCIMY5 cell line with cloned cDNA.
Identifying a novel CRBN transcript with both exon 10 and 8 deletions. (A) CRBN contains several domains, including LON and CULT domains. The exon 10 encoding region is a part of CULT domain and the exon 8 encodes parts of LON-C domain. The cDNAs from 2 patients with MM (R13 and N17) were generated, cloned, and sequenced. The sequence results were compared with the WT CRBN mRNA sequence (National Center for Biotechnology Information [NCBI] reference sequence: NM_00117382.1) in GenBank, indicating exon 10 (between 1043 and 1175) and exon 8 (between 862 and 979) were both deleted from 2 clones isolated. (B) The cloned cDNA from 1 patient (R13) was introduced into OCIMY5 cell line along with WT CRBN and vector alone. The expression of CRBN in those cells was detected by western blot. MTT assay demonstrated this novel isoform cannot mediate pomalidomide-induced antimyeloma activity. (C) OCIMY5 with vector, WT CRBN, R13 were treated with pomalidomide and analyzed using MTT assay. (D) R13 (GFP+) and WT (GFP−) expressing cells were mixed, treated, and analyzed by flow cytometry. The percentage of R13 expressing cells increased after exposure to Pom. Vec = Vector, POM = pomalidomide, WT = wild type, R13= OCIMY5 cell line with cloned cDNA.
Next, we evaluated whether the shortened protein affects response to IMiD. Using the MTT assay, we demonstrated that introduction of the CRBN with exon 8 and 10 deletion (R13), unlike WT CRBN, does not sensitize OCIMY5 to pomalidomide (Figure 3C). Comparable results were observed when a potent IMiD-like drug, CFT 13778, was tested in these cells (supplemental Figure 4). When we mixed R13 expressing cells with WT CRBN expressing cells (GFP−), we found that percentage of R13 (GFP+) increased after exposure to pomalidomide, which is positively associated with incubation time and drug doses, suggesting that R13 expressing cells were selected by IMiD treatment.
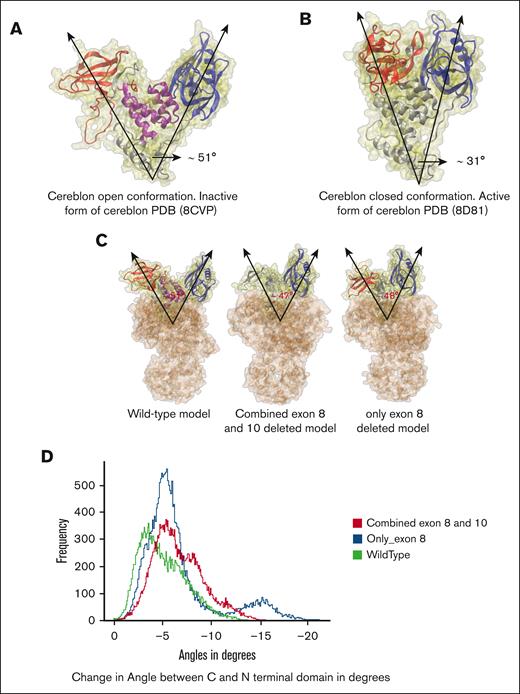
Deletions in exon 8 exhibit minimal impact on the open-to-closed transition of CRBN
The exon 8 deletion in CRBN has not been reported previously. By analysis of HMCL mRNA sequencing data, we noticed that some cell lines have lesser amounts of CRBN transcripts with exon 8 deletion, such as JJN3, which has a 5% exon 8–deleted transcript (data not revealed). So, we investigated on how this deletion could affect CRBN structure and CRBN-mediated antimyeloma effects of IMiDs. CRBN has the following 3 distinct domains: an N-terminal domain, an intermediate helical bundle, and a C-terminal domain responsible for binding thalidomide (referred to as TBD). Furthermore, CRBN displays the following 2 conformations: an open, inactive state (apo) with distant N-terminal and C-terminal TBD, and a closed, active state (holo) with closely interacting terminal domains (Figure 4A-B). According to Watson et al, the rate-limiting step for CRBN activity is the transition from open to closed state, not drug-binding kinetics, and IMiDs facilitate this transition by triggering allosteric changes that result in the active closed state, forming a binding interface for the targeted protein and thereby acting as a molecular glue.3 Following this cue, we conjectured that the deletion of the helical bundle in the middle, transcribed by exon 8 part of CRBN, could significantly impede the transition pathway, playing a pivotal role in the disruption of open-to-close transition of CRBN. To assess the transition from an open-to-close conformation, the angle between the centers of mass of the C-terminal and N-terminal domains was analyzed across all the MD simulations (Figure 4). In the homology models with combined exon 8 and 10 deletions, including those with exon 8 deletion alone, the initial angles between these domains were only 3° to 4° smaller than WT structures (Figure 4C). All the structures had similar distributions of changes in the angle between C- and N-terminal domains throughout the simulations (Figure 4D). The plot with the collective angle distribution data for all the systems (WT, combined exon 8 and 10 deletions, and only exon 8 deletions) had the most probable angle to be ∼5° lesser than that of the starting angles of the initial models (Figure 4D). This comparable trend in the distribution of changes in angles between the C- and N-terminal domains throughout the models with deletions and no deletions indicates that there might not be a significant effect of the exon 8 deletions in the open-to-close transition of CRBN. It must be noted that even though we found an average decrease of the angles between the domains in the simulations, we did not find the full transitions toward the complete closed CRBN conformation from the open conformation due to limited timescales and sparse sampling of rare events by molecular dynamics, necessitating the following experiment.
CRBN open-to-closed transition propensity in MD simulations of WT and deleted structures. (A) Molecular structure of CRBN in open conformation (WT) with C-terminal TBD (red), N-terminal domain (blue), and helical bundle in the middle (purple), PDB: 8CVP with angle between the domains ∼51°. (B) CRBN in closed conformation (also from WT), PDB: 8D81, with the same color scheme for the domains, with an angle of ∼31°. (C) All CRBN and DDB1 complex models. Structures with deletions are made through Homology Modeling in Discovery Studio using PDB 8CVP as template. The WT had an initial angle of 51°, model of combined deletion of exons 8 and 10 model had an initial angle of 47°, and only exon 8–deleted model had an initial angle of 48°. (D) Histogram of the changes in angles between the C and N-terminal domain throughout all the simulations, relative to the open conformation of WT (green), only exon 8–deleted (blue) and combined exon 8 and 10–deleted (red).
CRBN open-to-closed transition propensity in MD simulations of WT and deleted structures. (A) Molecular structure of CRBN in open conformation (WT) with C-terminal TBD (red), N-terminal domain (blue), and helical bundle in the middle (purple), PDB: 8CVP with angle between the domains ∼51°. (B) CRBN in closed conformation (also from WT), PDB: 8D81, with the same color scheme for the domains, with an angle of ∼31°. (C) All CRBN and DDB1 complex models. Structures with deletions are made through Homology Modeling in Discovery Studio using PDB 8CVP as template. The WT had an initial angle of 51°, model of combined deletion of exons 8 and 10 model had an initial angle of 47°, and only exon 8–deleted model had an initial angle of 48°. (D) Histogram of the changes in angles between the C and N-terminal domain throughout all the simulations, relative to the open conformation of WT (green), only exon 8–deleted (blue) and combined exon 8 and 10–deleted (red).
Decoupling of combined exon 10 and 8–deleted CRBN from DDB1
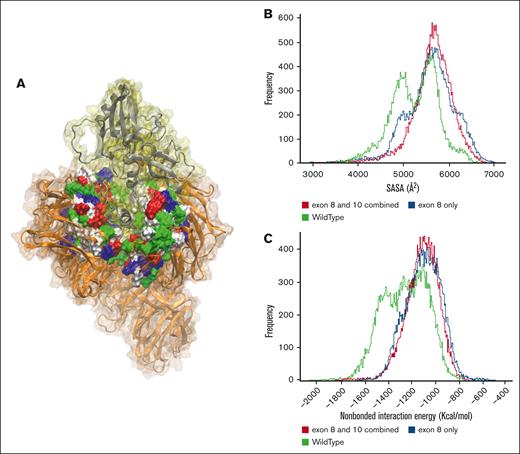
The E3 ligase consists of CRBN, DDB1, CUL4, and RBX1, all integral components in the process of ubiquitination of target proteins. Cohesion among these constituents is necessary for the seamless functioning of the E3 ligase.3,22 Deletion of the helical bundle encoded by exon 8 of CRBN might significantly diminish the crucial interactions between CRBN and DDB1. This realization prompted the second conjecture that even if IMiD binds to CRBN and induces the allosteric transition from an open to a closed state, any compromise in the connection between CRBN and DDB1 could impede the ubiquitination process of targeted proteins, potentially leading to drug failure. Thus, one probable explanation for IMiD inactivity could be attributed to the decoupling of CRBN from the rest of the E3 ligase. To measure the compactness of the connection between CRBN and DDB1, the solvent accessible surface area (SASA) of the DDB1 cleft (Figure 5A) was measured in all the simulations and then combined to get the total distribution. In the histograms of SASA values of all the simulations, the population distribution maxima in SASA space for both the combined exon 10 and 8–deleted and exon 8–deleted models extend beyond the WT model, indicating the opening of the hydrophobic cleft of the DDB1 and decoupling of CRBN (Figure 5B). The increase in SASA of the DDB1 cleft, and thereby a reduced contact area between the exon 8–deleted CRBN and DDB1, stems from the loss of major nonbonded interactions between the hydrophobic cleft of DDB1 and CRBN due to the deletion of the helical bundle encoded by exon 8. After the SASA analysis, the total nonbonded interaction energy (sum of electrostatic and Van der Waals [VdW] energy) between CRBN and DDB1 cleft was computed (Figure 5C). We found that the combined population distribution from all the simulations of the WT traversed deeper into the higher interaction energy region than that of the combined exon 8 and 10–deleted and only exon 8–deleted models. This result suggests depleting nonbonded interactions in the CRBN structures that lack the helical bundle encoded by exon 8. Our simulated data therefore bring to light the possibility of reduced IMiD activity due to exon 8 deletion-induced decoupling of CRBN and E3 ligase, prompting the co-IP experiments reported subsequently.
Comparison of SASA of DDB1 cleft in WT and deleted structures. (A) The structure of CRBN (silver) complexed with DDB1 (orange), where the DDB1 cleft is demonstrated in surface representation, where each residue type color is found; red color demonstrates negatively charged, blue for positively charged, green for polar, and white for hydrophobic residues. (B) SASA histogram of all simulations. (C) Nonbonded energy histogram of all simulations. SASA, solvent accessible surface area.
Comparison of SASA of DDB1 cleft in WT and deleted structures. (A) The structure of CRBN (silver) complexed with DDB1 (orange), where the DDB1 cleft is demonstrated in surface representation, where each residue type color is found; red color demonstrates negatively charged, blue for positively charged, green for polar, and white for hydrophobic residues. (B) SASA histogram of all simulations. (C) Nonbonded energy histogram of all simulations. SASA, solvent accessible surface area.
CRBN exon 8 deletion abolished IMiD-induced antimyeloma activity and impaired the interaction of CRBN with DDB1 in a MM cell line
To functionally confirm that exon 8 deletion alone is sufficient to abolish CRBN-mediated IMiD activity, we generated the DNA fragments encoding CRBN with either exon 8 or 10 deletion using site-directed mutagenesis. They were then inserted into the lentiviral vector and transduced into OCYMY5 cells. Western blot demonstrated that both CRBN constructs produced short CRBN proteins in OCIMY5 cells (Figure 6A). MTT assay demonstrated that neither of them could mediate IMID-induced antimyeloma activity (Figure 6B-C).
Effects of exon 8 deletion on CRBN-mediated IMiD activity and interaction with DDB1. (A) Using site-directed mutagenesis by PCR, we generated the CRBN with exon 8 and 10 deletion alone. There were expressed in OCIMY5 and detected by western blot. (B-C) MTT assay demonstrated that exon 8 deletion alone, like exon 10 deletion, cannot mediate pomalidomide- and CFT13778-induced antimyeloma activity. (D-E) co-IP experiment was performed on untreated (−) or lenalidomide treated (+) OCIMY5 cells overexpressing WT and exon 8–deleted CRBN and it demonstrated that exon 8 deletion impaired the binding of CRBN to DDB1 and Cul4A. GAPDH, glyceraldehyde-3-phosphate dehydrogenase; Vec = vector, WT = wild type, Co-IP = co-immunoprecipitation.
Effects of exon 8 deletion on CRBN-mediated IMiD activity and interaction with DDB1. (A) Using site-directed mutagenesis by PCR, we generated the CRBN with exon 8 and 10 deletion alone. There were expressed in OCIMY5 and detected by western blot. (B-C) MTT assay demonstrated that exon 8 deletion alone, like exon 10 deletion, cannot mediate pomalidomide- and CFT13778-induced antimyeloma activity. (D-E) co-IP experiment was performed on untreated (−) or lenalidomide treated (+) OCIMY5 cells overexpressing WT and exon 8–deleted CRBN and it demonstrated that exon 8 deletion impaired the binding of CRBN to DDB1 and Cul4A. GAPDH, glyceraldehyde-3-phosphate dehydrogenase; Vec = vector, WT = wild type, Co-IP = co-immunoprecipitation.
Next, we performed co-IP experiments to look at the interaction of CRBN with DDB1 and CUL4A in cells that overexpress WT or exon 8–deleted CRBN. Compared with WT CRBN expressing cells, the cells with CRBN exon 8 deletion had much less binding to DBB1 and CUL4A (Figure 6D) because OCIMY5 cells have low amounts of endogenous WT CRBN, which may cause faint DDB1 binding in the cells with exon 8 deletion. We also looked at whether CRBN with exon 8 deletion can bind IMiDs. We found that IKZF1 was pulled down by co-IP in lenalidomide-treated cell line expressing either WT CRBN or CRBN with exon 8 deletion (Figure 6E), suggesting that CRBN with exon 8 deletion binds IMiDs and recruits IKZF proteins. However, unlike WT CRBN, exon 8 deletion impaired the interaction with other components of E3 ligase and could not mediate IKZF protein degradation (Figure 6E).
Discussion
The development of MM drug resistance has long plagued patients and their treating hematologists. Recently, interest has focused on the role of CRBN alterations in IMiD-refractory patients. To provide a potential tool to identify these patients, our current findings demonstrate that inclusion of probes bridging CRBN exon 10 deletion (a known drug resistance feature) with homology to exons 9 and 11 is a feasible approach to use on a gene expression platform, which allows for combined evaluation of both full-length CRBN expression and exon 10–deleted isoform changes. We have previously used this same digital gene expression platform for developing other assays in lymphoid malignancies with robust results and implementation in a College of American Pathologists - Clinical Laboratory Improvement Amendments (CAP-CLIA)–certified hospital medical laboratory.25 With this precedent, if standardized, the current technique could become a potentially useful clinical test to assess IMiD drug responsiveness for patients with MM before spending months on therapy with these drugs. Because IMiD drugs are being used in several other hematological malignancies and tumors, including follicular lymphoma, diffuse large B-cell lymphoma, mantle cell lymphoma, and chronic lymphocytic leukemia,26-28 these results may have broader implications.
We further demonstrated that the CRBN 10–deleted isoform may already be present at diagnosis in untreated patients with MM. Consistent with the recent work describing increasing frequency of CRBN alterations in treatment-refractory patients, our data also revealed a higher percentage of exon 10 isoform transcript in the samples from IMiD-resistant patients compared with IMID-sensitive patients. However, according to Gooding et al,9 the cutoff of exon 10–spliced/full-length transcript ratio for defining an exon 10 splice variant patient is 2.6. None of our patient samples reached that ratio, even those harvested at relapse and end stages. This could be due to our small cohort size and the type of patient samples to which we had access. Differences in technology and approach to analysis may also be contributory. All the R and E patients in this study were treated with multiple drugs and other therapies in addition to IMiDs (such as bone marrow transplant). Therefore, the tumor clones with exon 10 splice isoforms could be eliminated by non-IMiD treatment affecting our ability to see more widespread IMiD resistance at later disease stages through this mechanism. Follow-up data on patient survival differences were only available for a very few patients in our study cohort, and it is a limitation of our study. A large patient cohort treated with IMiD monotherapy may be required to make a definitive clinical correlation.
Our study also identified a novel CRBN transcript with both exon 10 and 8 deletions, which has not been reported before, which could result from exon skipping. Because the cDNA samples from only 2 patient cases were sequenced, it is difficult to determine whether exon 10 and 8 skipping is a rare event or perhaps not stable (limitation of this study due to use of residual samples with limited material). Both of the sequenced patient materials still had exon 9 in their cDNA clones. Because exon 8 is not a part of the IMiD drug-binding pocket, we explored on how exon 8 deletion might confer an advantage to the malignant plasma cell in resisting IMiD therapy. Using molecular modeling, and molecular dynamics simulation of the CRBN-DDB1 machinery, with the WT, combined exon 10 and 8–deleted and only exon 8–deleted CRBN variants, we demonstrated, to our knowledge, for the first time that exon 8 deletion affects the coupling of CRBN with DDB1, rendering IMiDs ineffective. This conclusion was substantiated by laboratory testing demonstrating that exon 8 of CRBN is essential to mediate IMiD-induced antimyeloma activity.
Altogether, this work contributes to our understanding of the complexity of IMiD therapy by confirming the importance of CRBN alterations in IMiD resistance and the presence of CRBN genetic alterations in patients at time of diagnosis. It also describes a novel CRBN transcript with dual deletion of exons 10 and 8, proposes how a deletion outside of the binding pocket might alter drug binding, and details a novel assay to potentially identify patients with IMiD-resistant myeloma.
Acknowledgments
The authors thank Chang-Xin Shi for providing lentiviral vector and competent cells.
This work was supported by Paula and Rodger Riney Family Foundation and the National Cancer Institute U01CA271410 grant.
Authorship
Contribution: Y.X.Z., S.D., A.S., and L.M.R. designed the experiments; Y.X.Z. and I.L. performed the NanoString, cellular, and biochemistry experiments and data analysis; S.D., A.P., and J.C. performed molecular modeling; Y.X.Z., S.D., A.S., and L.M.R. prepared the manuscript; G.J.A. and L.B. collected and processed patient samples and performed RNA extraction; M.A. collected patient treatment information; X.C. analyzed messenger RNA sequence data and performed statistical analysis; M.C. and P.L.B. provided human myeloma cell lines, advice, and editing; and R.F. provided advice and editing.
Conflict-of-interest disclosure: R.F. reports providing consulting services for AbbVie, Adaptive Biotechnologies, Amgen, AstraZeneca, Bayer, Binding Site, Bristol Myers Squibb (Celgene), Millennium Takeda, Janssen, Juno, Kite, Merck, Pfizer, Pharmacyclics, RA Capital, Regeneron, and Sanofi; serving on the scientific advisory board of Caris Life Sciences; serving on the board of directors of Antengene (for profit); and having a patent for fluorescence in situ hybridization in multiple myeloma (∼$2000 per year). The remaining authors declare no competing financial interests.
Correspondence: Lisa M. Rimsza, Department of Pathology and Laboratory Medicine, College of Medicine, The University of Arizona, Arizona Health Sciences Center, 1501 N Campbell Ave, PO Box 245043, Room 5205, Tucson AZ 85724; email: lrimsza@arizona.edu.
References
Author notes
Deidentified participant data from the final research data set will be shared on request with those researchers from noncommercial entities who provide a methodologically sound research inquiry.
The full-text version of this article contains a data supplement.


![Identifying a novel CRBN transcript with both exon 10 and 8 deletions. (A) CRBN contains several domains, including LON and CULT domains. The exon 10 encoding region is a part of CULT domain and the exon 8 encodes parts of LON-C domain. The cDNAs from 2 patients with MM (R13 and N17) were generated, cloned, and sequenced. The sequence results were compared with the WT CRBN mRNA sequence (National Center for Biotechnology Information [NCBI] reference sequence: NM_00117382.1) in GenBank, indicating exon 10 (between 1043 and 1175) and exon 8 (between 862 and 979) were both deleted from 2 clones isolated. (B) The cloned cDNA from 1 patient (R13) was introduced into OCIMY5 cell line along with WT CRBN and vector alone. The expression of CRBN in those cells was detected by western blot. MTT assay demonstrated this novel isoform cannot mediate pomalidomide-induced antimyeloma activity. (C) OCIMY5 with vector, WT CRBN, R13 were treated with pomalidomide and analyzed using MTT assay. (D) R13 (GFP+) and WT (GFP−) expressing cells were mixed, treated, and analyzed by flow cytometry. The percentage of R13 expressing cells increased after exposure to Pom. Vec = Vector, POM = pomalidomide, WT = wild type, R13= OCIMY5 cell line with cloned cDNA.](https://ash.silverchair-cdn.com/ash/content_public/journal/bloodneoplasia/2/3/10.1016_j.bneo.2025.100099/3/m_bneo_neo-2024-000380-gr3.jpeg?Expires=1764605488&Signature=d7YwVNPkV~3VwvxTEKuyeDOZK9-AQUVoZ-OgkHcEsqij8TL~LEAu7EvmaVuMhq0p1HRH2JLmCwknShL9bfTRRos5mnMBOD80r8MxVHT~svfExekrIaM7NnDNvFYecr0py8dJYN7fG2UwdGlUit395lMVNT4rncZWF2PaURemuiaf08WOvny0PqsF-VW4y1cyLRzSG~wunlAbIVG1AK2uAq-aoVR-mIKGYN1SCD2HWIbEFvrhFpdRnCm0qODFq-0Afzc5oHKQynsMA7DxnoAoCh2rRtLu6oy-QYduWedt5nVhojP-Fzcz1Oc8Cwir1yb5gmdypWL6wN0TXJ5SMuuHuQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)