Overview of purine metabolism and adenosine signaling in humans with potential relevance to the pathophysiology of sickle cell disease.
Overview of Purine Metabolism and Adenosine Signaling in Humans With Potential Relevance to the Pathophysiology of Sickle Cell Disease
Overview of Purine Metabolism and Adenosine Signaling in Humans With Potential Relevance to the Pathophysiology of Sickle Cell Disease
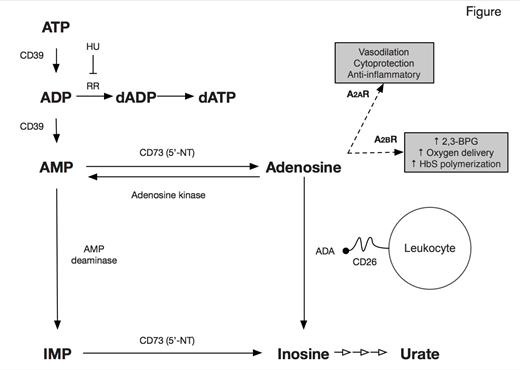
Adenosine is a purine nucleoside derived primarily from the catabolism of adenine nucleotides, ATP and ADP (Figure). The abundance of adenosine is determined by the action of a number of enzymes (Figure). In the extracellular compartment, the ectonucleotidases, CD39 (ectonucleoside triphosphate diphosphohydrolase 1) and CD73 (ecto-5’-nucleotidase), promote the formation of adenosine. Adenosine is degraded by adenosine deaminase (ADA), which can be present on cell surfaces in a complex with CD26. Adenosine signals through four G-protein coupled receptors, which mediate different actions. Recently, potential roles for adenosine in the pathophysiology of sickle cell disease (SCD) have been identified. In the May/June 2013 issue of The HematologistMay/June 2013 issue of May/June 2013 issue of The HematologistThe Hematologist, I reviewed a study that examined the therapeutic potential of agonism of the adenosine 2A receptor (A2AR) by regadenoson to decrease the activation of invariant natural killer T (iNKT) cells and thereby ameliorate the ischemia-reperfusion injury of SCD.1 Another function of adenosine, mediated in this case by signaling through A2BR, is stimulation of erythrocyte 2,3-bisphosphoglycerate (2,3-BPG) production, a process that enhances delivery of oxygen to ischemic tissues by decreasing the oxygen affinity of hemoglobin (Hb). While normally a beneficial compensation in the case of ischemia, decreasing Hb oxygen affinity in SCD favors the formation of deoxyhemoglobin S polymers and consequently promotes vaso-occlusion.2
Hydroxyurea (hydroxycarbamide, HU), an inhibitor of ribonucleotide reductase (Figure), has many salutary effects in SCD, most of which are mediated by the induction of fetal Hb (HbF) synthesis. However, clinical benefits can occur before a significant increase in HbF concentration is observed, so additional mechanisms of action have been posited, including alterations in cell-surface expression of adhesion molecules3 and greater bioavailability of nitric oxide.4 In the current paper by Dr. Ana C. Silva-Pinto and colleagues from the University of São Paulo, Brazil, reduction of adenosine activity was investigated as a novel mechanism of action of hydroxyurea. Dr. Silva-Pinto and coworkers studied 15 patients with SCD (HbSS) treated with HU, 17 patients with SCD who did not receive HU, and 10 healthy controls. The main findings were that monocytes in HU-treated patients expressed CD26, which forms a complex with ADA on cell surfaces (Figure), while monocytes from both untreated patients and controls did not. ADA transcripts were higher in monocytes of HU-treated patients than in untreated and control individuals, and HU-treated patients also had higher ADA activity. Together, these effects would serve to promote degradation of adenosine by ADA. Further, a lower percentage of T-lymphocytes expressing CD39 was observed in HU-treated patients compared with untreated patients and controls, and there was a suggestion that CD73 expression might be lower in iNK cells in HU-treated patients. Together, the effects of HU on CD39 and CD73 would be expected to reduce production of adenosine. In the HU-treated patients, several of the above findings were more pronounced in those with laboratory evidence of a good response to HU.
In Brief
Dr. Silva-Pinto and colleagues have reported intriguing evidence for an alteration in adenosine metabolism in HUtreated patients, specifically a decreased potential for synthesis of adenosine (via reduction in expression of CD39 on T-lymphocytes and CD73 in iNK cells) and an increased potential for its catabolism (via increased expression of CD26 and co-localization with ADA on monocytes). It is unclear how HU might lead to those changes, although altered cell-surface expression of adhesion molecules on erythrocytes by HU was first reported nearly 20 years ago.3 Both CD39 and CD73 are hypoxia-responsive, so it is possible that HU could reduce their expression indirectly because it ameliorates anemia, consequently decreasing tissue hypoxia. Regardless of the pathways involved, lower levels of adenosine may decrease the detrimental (in SCD) signaling though A2BR, thus accentuating the benefits of HU, although the cytoprotective and anti-inflammatory effects of signaling through A2BR (Figure) would also be decreased. One issue not considered by the investigators is that HU, as a ribonucleotide reductase inhibitor (Figure), may increase substrate (ADP) availability for the generation of adenosine, at least in the intracellular compartment. The greater abundance of adenosine in SCD, however, is proposed to occur in the extracellular compartment due to lysis of ATP- and ADP-containing erythrocytes. Much remains to be learned both about the role of adenosine in pathophysiology of SCD and about how adenosine metabolism is modulated by HU. If confirmed, the studies of Dr. Silva-Pinto and colleagues would add to the growing list of mechanisms of action for the multiple therapeutic benefits of HU beyond induction of HbF expression.
References
Competing Interests
Dr. Quinn indicated no relevant conflicts of interest.