Abstract
We used a pretargeting technique to treat a nonobese diabetic/severe combined immunodeficient murine model of human adult T-cell leukemia with an anti-Tac antibody-streptavidin (HAT-SA) conjugate, which recognizes CD25, followed by bismuth 213 (213Bi)-1,4,7,10-tetraazacyclododecane-N,N′,N′′,N′′′-tetraacetic acid (DOTA)- biotin. In the 3-step pretargeting radioimmunotherapy protocol, HAT-SA (140 or 400 μg) was administered intravenously (i.v.) to bind to the interleukin 2 receptor α (IL-2Rα; CD25)–expressing tumor cells. After 24 hours, 100 μg of a synthetic clearing agent was administered i.v. to remove unbound circulating HAT-SA conjugate from the circulation. Four hours later,213Bi–DOTA-biotin was administered i.v. for therapy. Tumor growth was significantly inhibited in 3 trials by using 250 μCi (9.25 MBq) of 213Bi–DOTA-biotin with a pretargeting technique as monitored by serum levels of soluble IL-2Rα and/or human β-2-microglobulin (P < .05, t test) and by survival of tumor-bearing mice in the treatment groups (P < .02, log rank test) as compared with the control groups. No prolongation of survival was observed with a nonspecific antibody-SA conjugate or in the absence of the radionuclide. Additionally, no prolongation of survival resulted from administration of 213Bi directly linked to intact HAT. Furthermore, there was no prolongation of survival when the β-emitting radionuclide yttrium 90 instead of the α-emitting radionuclide213Bi was used. The pretargeting approach with213Bi inhibited tumor growth more effectively than did immunotherapy with unmodified HAT. The best results were obtained with combination therapy that involved 213Bi–DOTA-biotin with a pretargeting technique supplemented by 4 weekly doses of HAT. The findings of this study support the use of this combination approach in a clinical trial in patients with IL-2Rα–expressing leukemias.
Introduction
Adult T-cell leukemia (ATL) develops in a small proportion of individuals infected with human T-cell lymphotrophic virus-I (HTLV-I).1 The leukemia consists of an overabundance of malignant activated T cells, which are characterized by the expression of CD25 (interleukin 2 receptor α [IL-2Rα]) on their cell surfaces.2-4 Presently, there is no accepted curative therapy for ATL.1
The observation that IL-2Rα is not expressed by normal resting cells, but is expressed by ATL cells, provided the rationale for the use of monoclonal antibodies directed toward IL-2Rα to deliver therapeutic agents. Some partial and rare complete remissions were obtained in patients with ATL treated in clinical trials with intact murine anti-Tac, humanized anti-Tac (HAT), as well as these intact antibodies armed with yttrium 90 (90Y) used in an effort to develop yet more effective IL-2Rα–directed agents.5 A preclinical in vivo murine model of ATL was developed to facilitate further improvements.6 In initial studies, antibodies to IL-2Rα, including HAT, murine anti-Tac, and 7G7/B6, inhibited the progression of the leukemia and prolonged the survival of the mice. However, in general, cures were not achieved.6
Leukemia generally provides better access than solid tumor to target antigens. This quality, along with the inherent radiosensitivity of hematologic malignancies, has made clinical radioimmunotherapy (RIT) of lymphomas one of the few applications with established clinical efficacy.5,7-9 However, the long serum half-lives of antibodies prolong radiation exposure to normal organs and to radiosensitive bone marrow, which limits the radiation dose that can be safely administered.10 11 Furthermore, the large size of antibodies yields only slow access to malignant cells in large tumors, precluding the use of short-lived radionuclides, including most available α-emitting radionuclides.
To overcome some of the obstacles encountered by conventional RIT, a technique involving a pretargeting system was introduced for tumor targeting to take advantage of the extremely high affinity of avidin-biotin binding and rapid pharmacokinetics of the small molecule biotin.12-22 In this system, antibody and radionuclides are administered separately, and radioactivity is rapidly and selectively accumulated in tumors with a parallel reduction of radioactivity in normal tissues.
A promising approach, reported by the NeoRx Corporation (Seattle, WA),19 consists of 3 steps. In step 1, the antibody-streptavidin (SA) conjugate is administered intravenously (i.v.) and allowed to target and accumulate in the tumor. In step 2, the unbound antibody-SA is cleared from the circulation by in vivo complexation with a synthetic biotinylated poly(GalNAc)–clearing agent (sCA) to prevent it from binding the biotin-radionuclide used in the following step. The resultant complexes are rapidly cleared into the liver by the asialogalactose receptor and metabolized.23The clearing step is essential to maintain low absolute blood concentration of antibody-SA that would bind to the radiolabeled reagent administered in the next step. In step 3, radiation is delivered to the antibody-SA on the tumor by the administration of radiolabeled biotinidase-resistant 1,4,7,10-tetraazacyclododecane-N,N′,N′′,N′′′-tetraacetic acid (DOTA)-biotin. This low molecular weight cytotoxic molecule readily reaches the tumor where it is captured by the prelocalized antibody-SA. Unbound radiolabeled DOTA-biotin is rapidly eliminated from the body by the urine. Rapid uptake of the therapeutic nuclide at the tumor and efficient elimination of excess radioactivity represent the fundamental advantages of the pretargeting approach as compared with conventional RIT, in which the radionuclide is directly conjugated to the antibody. Animal studies have characterized the pharmacokinetics and optimized the 3 reagents used in this approach in solid tumor models.19 The results have shown favorable specific and rapid targeting of radionuclide that has resulted in good tumor responses. Clinical trials have also been performed, and results showed the feasibility of the technique.20-22
For select situations, especially with isolated malignant cells as in leukemia, α-emitting radionuclides appear to have several advantages when compared with β-emitting radionuclides.24-26 The high linear energy transfer of α-particles makes them highly cytotoxic with a relative biologic effectiveness of 5 to 20 times that of β-particles. Another advantage of α-particles compared with β-particles is that they exhibit a low dependence on dose rate and oxygen enhancement effects.26,27 In addition, α-particles have relatively short effective path lengths in tissue, decreasing the radiation delivered to normal tissues. However, α-emitters, when conjugated to intact antibody, may be of only limited value in tumor therapy because of the short physical half-life and the time required to achieve a useful tumor-to-normal tissue ratio of the radionuclide following administration of the radiolabeled antibody.28 29 Two main candidates for α therapy are astatine 211 (211At; T1/2, 7 hours) and bismuth 213 (213Bi; T1/2, 46 minutes).
In this study, we investigated the use of the pretargeting technique with the α-emitting radionuclide, 213Bi, to treat ATL, taking advantage of the rapid delivery of the radioactivity to the tumor and the rapid elimination of excess radioactivity from healthy tissues. We compared the pretargeting technique with that using a directly labeled monoclonal antibody. Furthermore, we compared the213Bi radionuclide with the β-emitting radionuclide,90Y. We observed that in this model of leukemia213Bi– but not 90Y–DOTA-biotin used with the pretargeting technique provided effective therapy for ATL.
Materials and methods
Tumor cell lines
The ATL cell line, MET-1, was established from the peripheral blood of a patient with ATL. The cells were maintained by serial transfer in nonobese diabetic/severe combined immunodeficient (SCID/NOD) mice (Jackson Lab, Bar Harbor, ME). MET-1 cells have a distinct phenotype elucidated by flow-activated cell sorter analysis: CD3dim, CD7−, and CD25+. Kit225-IG3, HUT102, and MT1 are leukemic T-cell lines, which also express CD25 on their cell surfaces. SP2/Tac and ATAC4 are nonlymphoid cells transfected with the IL-2Rα (Tac) gene and, therefore, express CD25.
Monoclonal antibody
Preparation of antibody-SA conjugate
HAT or B3 was conjugated to SA by use of succinimidyl 4-(N-maleimido-methyl) cyclohexane-1-carboxylate described previously.19
Synthetic clearing agent
The sCA, provided by NeoRx, consists of a bifunctional moiety with multiple N-acetyl-galactosamine residues linked to biotin (molecular weight = 8651).23 The sCA binds rapidly to the circulating antibody-SA conjugate and clears rapidly from the circulation into the liver by the asialogalactose receptor present on hepatocytes. In this process, it carries with it any SA-bound antibody that had attached to it.23
Radiolabeling
Bismuth 213 was eluted from an actinium 225 (225Ac) generator.33 The 225Ac was supplied by Oak Ridge National Laboratory (Oak Ridge, TN), and 90Y was obtained from NEN (Boston, MA) as 90YCl3. For pretargeting therapy, biotinidase-resistant DOTA-biotin (molecular weight = ∼900) (NeoRx) was labeled with 213Bi or90Y at specific activities of 1 mCi/μg (37 MBq/μg), as previously described,19 and these labeled DOTA-biotin reagents consistently bound more than 95% to avidin gel.
For directly labeled antibody therapy, HAT-CHX-A" was labeled with213Bi at a specific activity of 16.2 μCi/μg (0.6 MBq/μg) as previously described.34 Unmodified HAT and HAT-SA conjugate were labeled with iodine 125 (125I) for immunoreactivity assay and internalization studies at a specific activity of 3 μCi/μg (111 kBq/μg) by using the chloramine-T method.
Immunoreactivity assay and internalization study
Tumor model and tumor burden evaluation
The leukemia model was established by intraperitoneal injection of 1.5 to 2.0 × 107 MET-1 cells into SCID/NOD mice. MET-1 cells were separated from enlarged spleens or from subcutaneous tumors of MET-1 tumor-bearing mice with a high (> 400 000 pg/mL) serum soluble IL-2Rα (sIL-2Rα) level.6 The therapy experiment was performed on these mice when their sIL-2Rα levels were more than 1000 pg/mL serum, which occurs approximately 10 to 14 days after tumor inoculation.
Pretargeting technique
Tumor-bearing mice were injected i.v. with 140 or 400 μg (0.67 or 1.91 nmol) of HAT-SA conjugate for pretargeting. After 24 hours were allowed for distribution and tumor localization, 100 μg (11.56 nmol) sCA was injected i.v. to clear circulating HAT-SA conjugate from the blood. Four hours after injection of the sCA, 0.3 or 1 μg (0.33 or 1.11 nmol) 213Bi–DOTA-biotin was injected i.v.
Seven days before pretargeting, the mice were fed with a biotin-free diet to reduce the endogenous biotin level (Biotin Deficient Rodent Diet 5836C-I; Purina Mills, Richmond, IN). One day after the administration of 213Bi, the normal diet was restored.
Therapy study
The therapeutic protocol is shown in Table1. In the dose-escalating therapeutic trial, different doses (0, 50, 150, 250, or 350 μCi [0, 1.85, 5.55, 9.25, or 12.95 MBq]) of 213Bi–DOTA-biotin were used. Tumor-bearing mice with sIL-2Rα from 1000 to 10 000 pg/mL were selected and divided into 6 groups of 5 mice. The mice were injected intravenously with 140 μg HAT-SA conjugate for pretargeting for 24 hours. Then, 100 μg sCA was injected intravenously 4 hours later.213Bi–DOTA-biotin (0.3 μg) was administered intravenously. One group without treatment served as a control.
In the small-tumor–burden therapeutic trial, there were 4 groups, 10 mice each, with the same sIL-2Rα range (1000-10 000 pg/mL). Group 1, pretargeting radioimmunotherapy (PRIT), was treated with 250 μCi (9.25 MBq) 213Bi–DOTA-biotin (0.3 μg) following the same HAT-SA conjugate pretargeting approach. Group 2, nonspecific PRIT, received 250 μCi (9.25 MBq) 213Bi–DOTA-biotin, following the administration of the B3-SA conjugate and the sCA. Group 3, immunotherapy (HAT), was injected with 100 μg HAT at 0, 7, 14, and 21 days, respectively. Group 4 did not receive treatment.
In the large-tumor–burden therapeutic trial, mice with serum sIL-2Rα values ranging from 20 000 to 70 000 pg/mL were treated. A larger pretargeting dose (400 μg HAT-SA conjugate) and a correspondingly larger DOTA-biotin dose (1 μg) were used. There were 7 groups in this experiment. The first 4 groups were the same as those in the small-tumor–burden therapeutic trial with the exception that the larger doses of HAT-SA and DOTA-biotin were used. Group 5, combination therapy (PRIT + HAT), received combined therapy of PRIT and immunotherapy (group 1 plus group 3). Group 6, no radionuclide PRIT, received the same HAT-SA conjugate pretargeting, sCA, but received the DOTA-biotin without any radioactivity. Group 7, RIT, received 50 μCi (1.85 MBq) 213Bi directly labeled HAT for comparison.
To compare the therapeutic effect of a β-emitter with that of an α-emitter, escalating doses (0, 100, 175, and 250 μCi [0, 3.7, 6.475, and 9.25 MBq]) of 90Y–DOTA-biotin were studied with the same pretargeting technique and same tumor-bearing mice.
Monitoring of tumor growth
Measurements of the serum concentrations of the sIL-2Rα and/or soluble β-2-microglobulin (β2μ) were performed by using enzyme-linked immunosorbent assay at 2-week intervals after therapy to monitor the growth of the leukemia. The assay kits were purchased from R&D System (soluble Tac-Cat. No. DR2A00; soluble β2μ Cat. No. DBM200; Minneapolis, MN).
Toxicity study
Six groups of 10 healthy SCID/NOD mice each were used to define the toxicity of 213Bi. For groups 1 to 3, increasing doses of 213Bi–DOTA-biotin (100, 250, and 500 μCi [3.7, 9.25, and 18.5 MBq]) were administered following the pretargeting approach with larger doses of HAT-SA and DOTA-biotin as used in the large-tumor–burden therapeutic trial. Group 4 also received 250 μCi (9.25 MBq) 213Bi–DOTA-biotin but without pretargeting and sCA. Group 5 received pretargeting, sCA, and 250 μCi (9.25 MBq)213Bi–DOTA-biotin followed by 100 μg unmodified HAT at days 1, 7, 14, and 21, respectively. Group 6 did not receive any treatment.
The body weight and the complete blood count were measured before and after treatment (initially at weekly and subsequently at monthly intervals). The serum levels of creatinine, blood urea nitrogen (BUN), alanine aminotransferase, aspartate aminotransferase, creatine kinase, and γ-glutamyl transpeptidase were also measured at 2 and 5 weeks and at 2 and 4 months after treatment. Two to 3 animals in each group were killed at 5 weeks and at 2 and 4 months after treatment, and the tissues (liver, kidneys, lung, spleen, intestine, and femur) were evaluated histopathologically (Pathology Laboratory, National Cancer Institute, Frederick Cancer Research and Development Center, Frederick, MD).
Definition of the maximum-tolerated dose
Prior to initiation of PRIT, the maximum-tolerated dose of213Bi–DOTA-biotin was determined in both healthy SCID/NOD and MET-1 tumor-bearing SCID/NOD mice. Doses of 0, 50, 150, 250, and 350 μCi (0, 1.85, 5.55, 9.25, and 12.95 MBq)213Bi–DOTA-biotin for tumor-bearing mice and doses of 100, 250, and 500 μCi (3.7, 9.25, and 18.5 MBq)213Bi–DOTA-biotin for healthy mice were evaluated. Renal functional abnormality developed in the mice receiving 500 μCi (18.5 MBq). Therefore, doses of 250 μCi (9.25 MBq)213Bi–DOTA-biotin were used in the PRIT studies.
Statistical analysis
The serum levels of sIL-2Rα, β2M, and BUN, as well as body weight, at different time points for the different treatment groups and the data of internalization studies were analyzed statistically using the t test for unpaired data. In terms of the mouse survival plots, StatView was used to generate Kaplan-Meier cumulative survival plots.
Results
Immunoreactivity
The 125I labeled HAT-SA conjugate and HAT antibody bound to Kit225-IG3 cells comparably. They showed similar maximal bindings of 83% to 87% and 86% to 89%, respectively, suggesting that SA conjugation did not affect the bindability of the antibody.
Internalization of HAT-SA conjugate
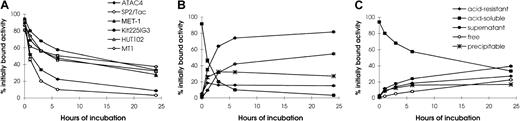
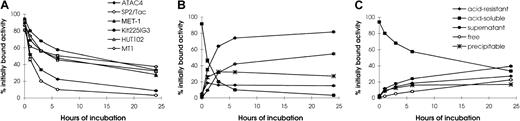
It is important for the pretargeting technique that antibody-SA conjugate stay on the tumor cell surface until the radiolabeled biotin is administered. Therefore, the rate of internalization of HAT-SA by leukemic cell lines (MET-1, Kit225-IG3, HUT102, and MT1), as well as nonlymphoid cell lines (SP2/Tac and ATAC4), was investigated. All 6 cell lines had more than 80% of the cell-associated radioactivity on the cell surface initially (Figure 1). Acid soluble activity decreased rapidly in nonlymphoid SP2/Tac and ATAC4 cells. For the SP2/Tac cell line, only 10% and 3% were left on the cell surface after incubation for 6 and 24 hours, respectively. In contrast, approximately one half and one third were present on the cell surface after 6 and 24 hours' incubation, respectively, with the 4 leukemic cell lines, values significantly higher than those with SP2/Tac and ATAC4 cell lines (Figure 1A, P < .001).
Internalization of 125I–HAT-SA.
Acid-soluble radioactivity (cell surface bound HAT-SA conjugate) at different times of incubation is shown for the 6 cell lines studied (A). Kinetics of internalization and metabolism of125I–HAT-SA of (B) SP2/Tac or (C) Kit225IG3 cells are shown. Cells were incubated with 125I–HAT-SA for 1 hour at 4°C for surface labeling, then incubated at 37°C for 0, 1, 3, 6, or 24 hours. The relative percentages of radioactivity on the cell surface (acid soluble), intracellularly (acid resistant), and in the supernatant, which is further differentiated by methanol precipitation as free and precipitable, are depicted as a function of time (B,C). Each point represents the mean ± SD of triplicate measurements.
Internalization of 125I–HAT-SA.
Acid-soluble radioactivity (cell surface bound HAT-SA conjugate) at different times of incubation is shown for the 6 cell lines studied (A). Kinetics of internalization and metabolism of125I–HAT-SA of (B) SP2/Tac or (C) Kit225IG3 cells are shown. Cells were incubated with 125I–HAT-SA for 1 hour at 4°C for surface labeling, then incubated at 37°C for 0, 1, 3, 6, or 24 hours. The relative percentages of radioactivity on the cell surface (acid soluble), intracellularly (acid resistant), and in the supernatant, which is further differentiated by methanol precipitation as free and precipitable, are depicted as a function of time (B,C). Each point represents the mean ± SD of triplicate measurements.
As the radioactivity on the cell surface decreased, that of the supernatant increased accordingly. In particular, the amount in the supernatant with SP2/Tac cells (Figures 1B) was significantly higher than that with Kit225-IG3 cells (Figures 1C). At 6 hours after incubation, more than 70% was found in the supernatant with SP2/Tac cells, whereas less than 30% was present with Kit225-IG3 cells. The radioactivity in the supernatant of SP2/Tac cells was mainly free iodine (more than 40% at 6 hours), which reflects the release of iodine from the cell after internalization and processing of the labeled conjugate. In contrast, the amount of free iodine in the supernatant with Kit225-IG3 cells was less than 10% at 6 hours of incubation, significantly lower than that of SP2/Tac cells (P < .001). The pattern of catabolism with the ATAC4 cells was similar to that of the SP2/Tac cells, and the patterns with MET-1, HUT102, and MT1 cell lines were similar to that of the Kit225-IG3 cells. The internalization of HAT-SA by the 4 leukemic cell lines studied was relatively slow, which provides binding sites for the subsequent radiolabeled biotin for tumor targeting.
Therapeutic study
PRIT with the α-emitting radionuclide 213Bi.
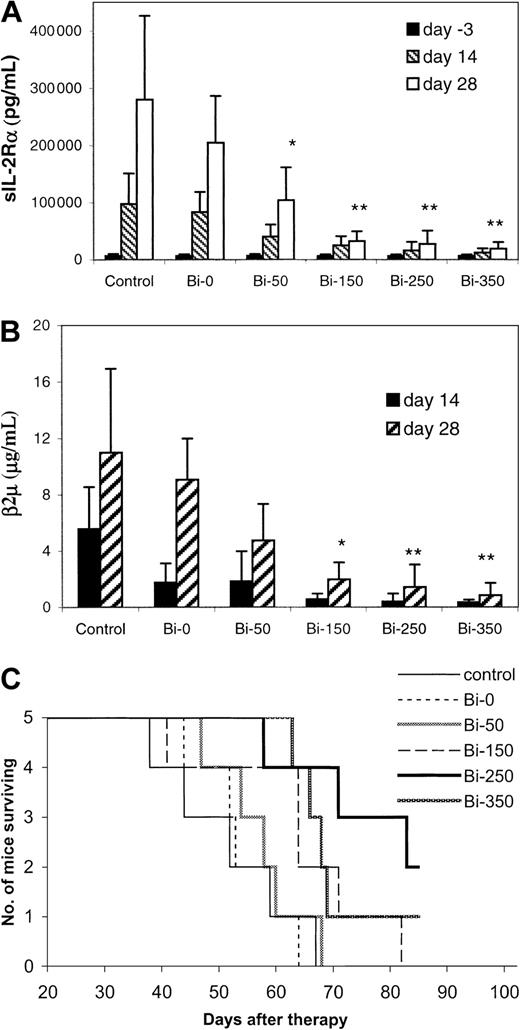
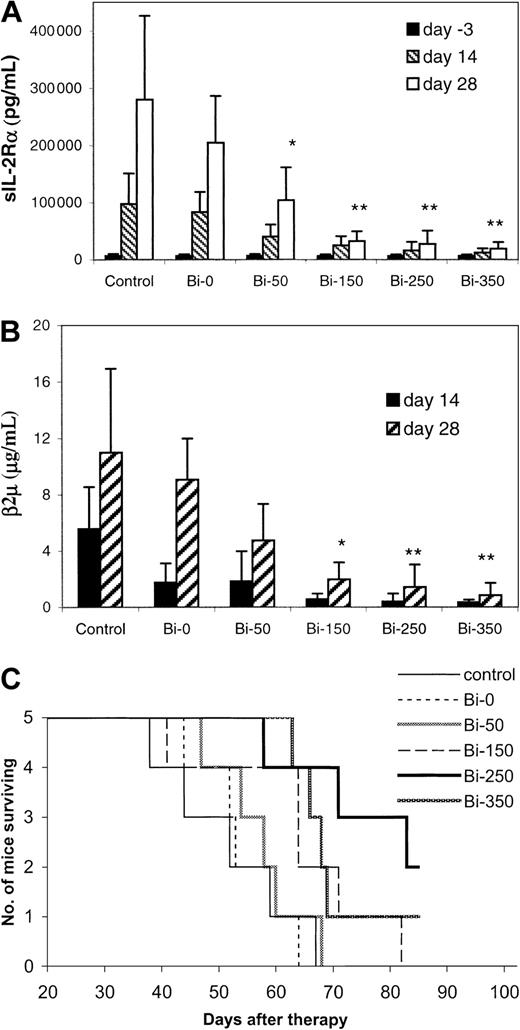
Escalating doses of 213Bi–DOTA-biotin, from 50 to 350 μCi (1.85-12.95 MBq), were used to treat the MET-1 tumor-bearing mice with sIL-2Rα levels of 1000 to 10 000 pg/mL with the pretargeting approach. When assessed at 28 days after therapy, the tumor growth was inhibited in a dose-related manner (Figure2A,B). The serum levels of sIL-2Rα were 19 150, 27 340, 32 560, and 104 280 pg/mL in groups receiving 350, 250, 150, and 50 μCi (12.95, 9.25, 5.55, and 1.85 MBq)213Bi–DOTA-biotin, respectively, significantly lower than the 280 000 pg/mL in the control group (Figure 2A,P < .05). The survival of the mice in the 250 and 350 μCi (9.25 and 12.95 MBq) groups was significantly prolonged as compared with that of the control group (Figure 2C,P < .02). The median survival duration of the control group was 52 days and was more than 75.6 days in the 250 μCi (9.25 MBq) group. On the basis of this observation, 250 μCi (9.25 MBq)213Bi was used in the therapeutic studies thereafter.
Dose escalation therapeutic study of213Bi–DOTA-biotin with the pretargeting technique in MET-1 tumor-bearing SCID/NOD mice.
At the time of the experiment, the mice had sIL-2Rα levels of 1000-10 000 pg/mL. The mice in the control group did not receive any treatment. Mice in other groups received 140 μg HAT-SA pretargeting for 24 hours, 100 μg sCA for 4 hours, and 0.3 μg213Bi–DOTA-biotin at different doses of 213Bi: 0 μCi (0 MBq; Bi-0), 50 μCi (1.85 MBq; Bi-50), 150 μCi (5.55 MBq; Bi-150), 250 μCi (9.25 MBq; Bi-250), or 350 μCi (12.95 MBq; Bi-350). Serum collections from the mice were taken at 3 days before and at 14 and 28 days after therapy. The serum concentrations of (A) sIL-2Rα, (B) β2M, and (C) Kaplan-Meier survival plot were shown. *P < .05, **P < .01 as compared with the control group.
Dose escalation therapeutic study of213Bi–DOTA-biotin with the pretargeting technique in MET-1 tumor-bearing SCID/NOD mice.
At the time of the experiment, the mice had sIL-2Rα levels of 1000-10 000 pg/mL. The mice in the control group did not receive any treatment. Mice in other groups received 140 μg HAT-SA pretargeting for 24 hours, 100 μg sCA for 4 hours, and 0.3 μg213Bi–DOTA-biotin at different doses of 213Bi: 0 μCi (0 MBq; Bi-0), 50 μCi (1.85 MBq; Bi-50), 150 μCi (5.55 MBq; Bi-150), 250 μCi (9.25 MBq; Bi-250), or 350 μCi (12.95 MBq; Bi-350). Serum collections from the mice were taken at 3 days before and at 14 and 28 days after therapy. The serum concentrations of (A) sIL-2Rα, (B) β2M, and (C) Kaplan-Meier survival plot were shown. *P < .05, **P < .01 as compared with the control group.
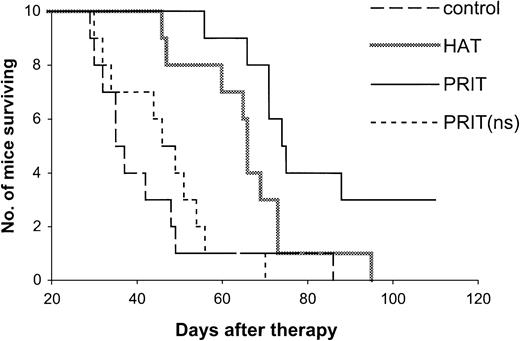
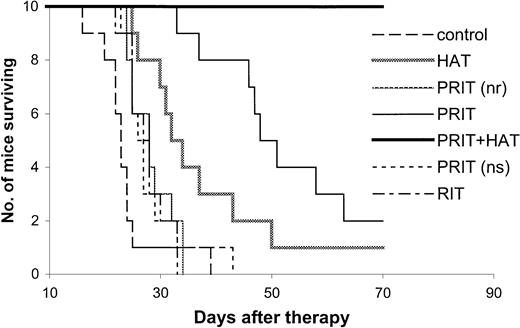
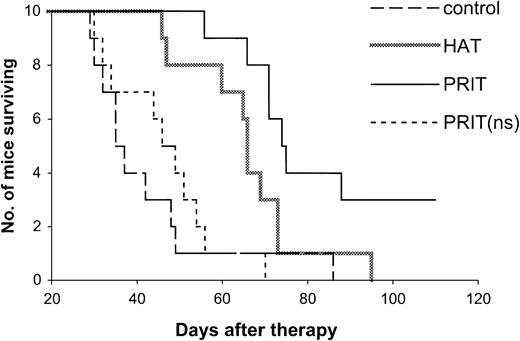
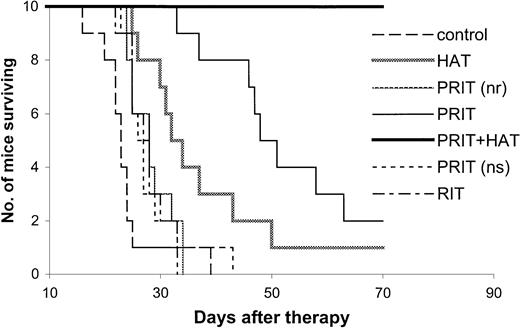
The effective results with PRIT were repeated in mice with both small- and large-tumor burdens (Tables2,3 and Figures3,4). The tumor growth was inhibited with PRIT in both therapeutic studies. On day 28 after therapy in the small-tumor–burden therapeutic trial, the serum concentration of β2M was 0.44 μg/mL in the PRIT group as compared with 7.74 μg/mL in the control group (Table 2,P < .0001). At 14 days after therapy in the large-tumor–burden therapeutic trial, β2M was 0.38 μg/mL in the PRIT group as compared with 7.17 μg/mL in the control group (Table 3,P < .0001). Furthermore, the survival of the mice in the PRIT groups was significantly prolonged as compared with the control groups (Figures 3,4; P < .0005). The median survival durations of the control groups were 42.3 and 23.8 days in the small- and large-tumor–burden therapeutic trials, respectively, whereas they were prolonged to 89.8 and 53.7 days, respectively, in the PRIT groups.
Kaplan-Meier survival plot of MET-1 tumor-bearing SCID/NOD mice in the small-tumor–burden therapeutic study.
At the time of the experiment, the mice had sIL-2Rα levels of 1000 to 10 000 pg/mL. Mice in the control group did not receive any treatment. Mice in the HAT group received 100 μg HAT once per week for 4 weeks. Mice in the PRIT group received 140 μg HAT-SA pretargeting for 24 hours, 100 μg sCA for 4 hours, and 0.3 μg213Bi–DOTA-biotin at a dose of 250 μCi (9.25 MBq). Mice in the PRIT (nonspecific, ns) group received 140 μg B3-SA pretargeting for 24 hours, 100 μg sCA for 4 hours, and 0.3 μg213Bi–DOTA-biotin at a dose of 250 μCi (9.25 MBq).
Kaplan-Meier survival plot of MET-1 tumor-bearing SCID/NOD mice in the small-tumor–burden therapeutic study.
At the time of the experiment, the mice had sIL-2Rα levels of 1000 to 10 000 pg/mL. Mice in the control group did not receive any treatment. Mice in the HAT group received 100 μg HAT once per week for 4 weeks. Mice in the PRIT group received 140 μg HAT-SA pretargeting for 24 hours, 100 μg sCA for 4 hours, and 0.3 μg213Bi–DOTA-biotin at a dose of 250 μCi (9.25 MBq). Mice in the PRIT (nonspecific, ns) group received 140 μg B3-SA pretargeting for 24 hours, 100 μg sCA for 4 hours, and 0.3 μg213Bi–DOTA-biotin at a dose of 250 μCi (9.25 MBq).
Kaplan-Meier survival plot of MET-1 tumor-bearing SCID/NOD mice in the large-tumor–burden therapeutic study.
At the time of the experiment, the mice had sIL-2Rα levels of 20 000 to 70 000 pg/mL. Mice in the control group did not receive any treatment. Mice in the HAT group received 100 μg HAT once per week for 4 weeks. Mice in the PRIT group or PRIT (no radionuclide, nr) group received 400 μg HAT-SA pretargeting for 24 hours, 100 μg sCA for 4 hours, and 1 μg DOTA-biotin labeled with 250 or 0 μCi (9.25 or 0 MBq) 213Bi. Mice in the PRIT + HAT group received the same pretargeting approach as those in the PRIT group, followed by 100 μg HAT once per week for 4 weeks. Mice in the PRIT (nonspecific, ns) group received 400 μg B3-SA pretargeting for 24 hours, 100 μg sCA for 4 hours, and 1 μg 213Bi–DOTA-biotin at a dose of 250 μCi (9.25 MBq). Mice in the RIT group received 50 μCi (1.85 MBq)213Bi-HAT.
Kaplan-Meier survival plot of MET-1 tumor-bearing SCID/NOD mice in the large-tumor–burden therapeutic study.
At the time of the experiment, the mice had sIL-2Rα levels of 20 000 to 70 000 pg/mL. Mice in the control group did not receive any treatment. Mice in the HAT group received 100 μg HAT once per week for 4 weeks. Mice in the PRIT group or PRIT (no radionuclide, nr) group received 400 μg HAT-SA pretargeting for 24 hours, 100 μg sCA for 4 hours, and 1 μg DOTA-biotin labeled with 250 or 0 μCi (9.25 or 0 MBq) 213Bi. Mice in the PRIT + HAT group received the same pretargeting approach as those in the PRIT group, followed by 100 μg HAT once per week for 4 weeks. Mice in the PRIT (nonspecific, ns) group received 400 μg B3-SA pretargeting for 24 hours, 100 μg sCA for 4 hours, and 1 μg 213Bi–DOTA-biotin at a dose of 250 μCi (9.25 MBq). Mice in the RIT group received 50 μCi (1.85 MBq)213Bi-HAT.
Compared with HAT immunotherapy, PRIT showed more therapeutic efficacy (Tables 2,3 and Figures 3,4). The survival of the mice in the PRIT groups was prolonged significantly as compared with HAT therapy groups (Figures 3,4; P < .05). In the therapy with large-tumor burden, β2M was also significantly lower with PRIT than with HAT treatment on day 14 after therapy (Table 3, P < .0001).
The specificity of therapeutic effect with PRIT was confirmed by comparing specific HAT-SA conjugate PRIT with nonspecific PRIT using an irrelevant control antibody-SA conjugate, B3-SA. The levels of β2M were significantly lower with the specific PRIT than with the nonspecific PRIT (Tables 2,3; P < .001), and there were significant prolongations of survival of the mice in the specific PRIT groups compared with the nonspecific PRIT groups (Figures 3,4;P < .0001).
Immunotherapy (HAT).
Treatment of MET-1 tumor-bearing mice with the unmodified HAT antibody showed effective therapeutic results with partial remissions of the leukemia and a prolongation of the life of the leukemia-bearing mice, similar to those we previously reported.6 In the therapeutic study with small-tumor burden, initial sIL-2Rα 1000 to 10 000 pg/mL, tumor growth was inhibited as seen by the effect on the serum levels of the surrogate tumor markers of human sIL-2Rα and human β2M (Table 2). On day 28 after therapy, the serum concentrations of sIL-2Rα and β2M were 4800 pg/mL and 0.63 μg/mL, respectively, in mice receiving 4 weekly doses of 100 μg HAT, significantly lower than 526 300 pg/mL and 7.74 μg/mL for sIL-2Rα and β2M, respectively, that were observed in the control group (Table2; P < .002). Furthermore, there was a significant prolongation of survival of the mice in the HAT-treated group (Figure3; P < .02). The median survival duration of the control group was 42 days, whereas it increased to 66 days in the HAT-treated group. In the therapeutic study involving a large-tumor burden, sIL-2Rα levels 20 000 to 70 000 pg/mL, an effective therapeutic response with HAT therapy was also observed. The β2M was 2.92 μg/mL in the treated group compared with 7.17 μg/mL in the control group at 14 days after therapy (Table 3; P < .0001). The survival of the mice in the HAT group was also prolonged significantly when compared with the control group (Figure 4; P < .002).
As a control for PRIT study, all of the nonradioactive reagents involved in the pretargeting technique, 140 μg or 400 μg HAT-SA, 100 μg sCA, and 0.3 or 1 μg DOTA-biotin (no radionuclide), were given. No significant effect was shown on the survival of the tumor-bearing mice as compared with the untreated control group (Figures 2,4; P > .05).
RIT with 213Bi-labeled intact HAT.
To compare the pretargeting approach with RIT with directly labeled intact antibody, 213Bi–CHX-A"–HAT was administered to mice bearing large-tumor burdens. The tolerated dose of 50 μCi (1.85 MBq) 213Bi-HAT was used. The β2M at 14 days after therapy was 4.06 μg/mL in the 213Bi-HAT group as compared with 0.38 μg/mL with the PRIT group (Table 3; P < .0001). Furthermore, the survival of the mice in the PRIT group was significantly prolonged when compared with the group receiving intact HAT armed with 213Bi (Figure 4; P < .0001). The median survival duration of the 213Bi-HAT group was 27.6 days, whereas it was 53.7 days in the PRIT group.
PRIT with the β-emitting radionuclide 90Y.
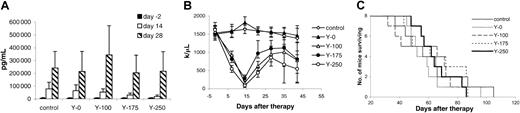
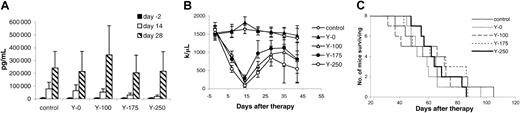
The therapeutic emission from 213Bi is an α particle with a short distance of action appropriate for isolated leukemic cells. We compared the efficacy of this radionuclide with 90Y that has a β emission wherein effective action involves crossfire, most appropriate for large-tumor masses. Escalating doses of 0, 100, 175, and 250 μCi (0, 3.7, 6.475, and 9.25 MBq)90Y–DOTA-biotin with the pretargeting protocol were evaluated in small-tumor–bearing SCID/NOD mice (sIL-2Rα, 1000-10 000 pg/mL) and compared with an untreated control group. In terms of sIL-2Rα levels at 14 days after therapy, significant differences were found with 175 and 250 μCi (6.475 and 9.25 MBq)90Y–DOTA-biotin when compared with that of the control group (Figure 5A; P < .05). This response was not maintained thereafter. Meanwhile, the platelet count showed a dose-related decrease (Figure 5B). There was no significant difference in the survival of the different groups of mice in the study (Figure 5C). Meaningful therapeutic efficacy was not obtained with the β-emitting 90Y in contrast to the significant effects observed when the α-emitting radionuclide213Bi was used in the leukemia model.
Therapeutic study of 90Y–DOTA-biotin with the pretargeting technique.
At the time of the experiment, the mice had sIL-2Rα levels of 1000 to 10 000 pg/mL. Mice in the control group did not receive any treatment, and mice in other groups received 140 μg HAT-SA pretargeting for 24 hours, 100 μg sCA for 4 hours, followed by different doses of90Y–DOTA-biotin: 0 μCi (0 MBq; Y-0), 100 μCi (3.7 MBq; Y-100), 175 μCi (6.475 MBq; Y-175), or 250 μCi (9.25 MBq; Y-250). (A) Growth of MET-1 tumor in SCID/NOD mice as shown by the serum concentrations of sIL-2Rα. Serum collections from the mice were taken at 2 days before and at 14 and 28 days after therapy, and the concentrations of sIL-2Rα were measured. (B) Platelet count as measured weekly in MET-1 tumor-bearing SCID/NOD mice. (C) Kaplan-Meier survival plot of the MET-1 tumor-bearing SCID/NOD mice.
Therapeutic study of 90Y–DOTA-biotin with the pretargeting technique.
At the time of the experiment, the mice had sIL-2Rα levels of 1000 to 10 000 pg/mL. Mice in the control group did not receive any treatment, and mice in other groups received 140 μg HAT-SA pretargeting for 24 hours, 100 μg sCA for 4 hours, followed by different doses of90Y–DOTA-biotin: 0 μCi (0 MBq; Y-0), 100 μCi (3.7 MBq; Y-100), 175 μCi (6.475 MBq; Y-175), or 250 μCi (9.25 MBq; Y-250). (A) Growth of MET-1 tumor in SCID/NOD mice as shown by the serum concentrations of sIL-2Rα. Serum collections from the mice were taken at 2 days before and at 14 and 28 days after therapy, and the concentrations of sIL-2Rα were measured. (B) Platelet count as measured weekly in MET-1 tumor-bearing SCID/NOD mice. (C) Kaplan-Meier survival plot of the MET-1 tumor-bearing SCID/NOD mice.
Combination therapy involving PRIT with 213Bi and immunotherapy with unmodified HAT.
Combination therapy, involving pretargeting 250 μCi (9.25 MBq)213Bi in the protocol used above followed by weekly doses of HAT at 100 μg on day 1, 7, 14, and 21 showed improved therapeutic results when compared with either PRIT or HAT alone. In the therapeutic study involving mice with large-tumor burdens (sIL-2Rα, 20 000-70 000), the serum level of β2M was significantly lower in the combination therapy group when compared with that in the PRIT or HAT-treated group at 28 days after therapy (Table 3;P < .05). On day 42 after therapy, β2M could be detected in only 1 of 10 mice in the combination group but was detectable in 9 of 10 animals in the PRIT group and in all of the 10 mice in the HAT-treated group. There was also a significant prolongation of survival of the mice in the combination group as compared with either the PRIT or HAT-treated groups (Figure 4;P < .0005). Up to 70 days after therapy, all mice in the combination group were alive, whereas only 2 mice in the PRIT group and one mouse in the HAT-treated group remained alive.
Toxicity
In the 3-step pretargeting regimen in SCID/NOD mice, the animal body weight did not show significant changes with 250 μCi (9.25 MBq) or less 213Bi–DOTA-biotin with or without HAT-SA pretargeting, whereas mice that received 500 μCi (18.5 MBq)213Bi–DOTA-biotin showed weakness and significant loss of body weight (P < .01).
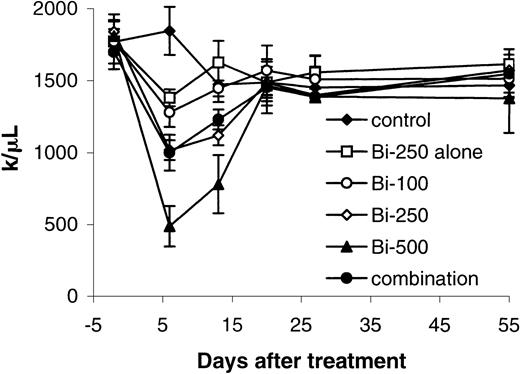
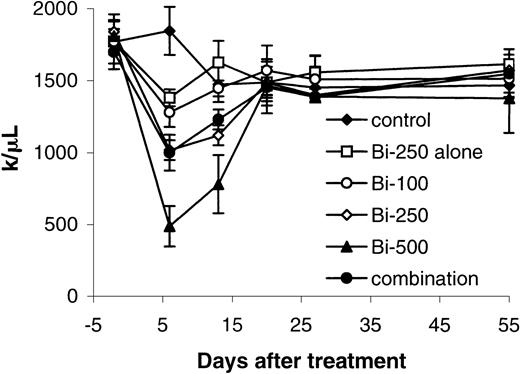
The platelet count was reduced with PRIT in a dose-related manner (Figure 6). The nadir occurred 1 week after radiation therapy and recovered 2 to 3 weeks later. Minor changes of the platelet count were shown with 250 μCi (9.25 MBq)213Bi–DOTA-biotin without pretargeting. The serum values of BUN in the mice receiving 500 μCi (18.5 MBq)213Bi–DOTA-biotin were significantly higher than those of the mice in the control group at 2 and 5 weeks and at 2 months after treatment (P < .05). However, significant elevations in BUN were not observed in the groups receiving 250 μCi (9.25 MBq)213Bi–DOTA-biotin. Histopathologic examination showed that the mice treated with 250 μCi (9.25 MBq)213Bi–DOTA-biotin without pretargeting or 500 μCi (18.5 MBq) 213Bi–DOTA-biotin manifested hydronephrosis 4 months after treatment. In contrast, the mice receiving 100 to 250 μCi (3.7-9.25 MBq) 213Bi–DOTA-biotin with the pretargeting did not show the pathologic changes.
Platelet counts were measured in healthy SCID/NOD mice that received different treatments for the toxicity of213Bi (initially at weekly and subsequently at monthly intervals).
Mice in the control group did not receive any treatment (control). Mice in another group received 250 μCi (9.25 MBq)213Bi–DOTA-biotin without pretargeting and sCA (Bi-250 alone), and mice in remaining groups received 400 μg HAT-SA pretargeting for 24 hours, 100 μg sCA for 4 hours, followed by 100 μCi (3.7 MBq; Bi-100) or 250 μCi (9.25 MBq; Bi-250) or 500 μCi (18.5 MBq; Bi-500) 213Bi–DOTA-biotin. Mice in the combination group received the same pretargeting, clearing steps and 250 μCi (9.25 MBq) 213Bi–DOTA-biotin followed by 100 μg HAT once per week for 4 weeks.
Platelet counts were measured in healthy SCID/NOD mice that received different treatments for the toxicity of213Bi (initially at weekly and subsequently at monthly intervals).
Mice in the control group did not receive any treatment (control). Mice in another group received 250 μCi (9.25 MBq)213Bi–DOTA-biotin without pretargeting and sCA (Bi-250 alone), and mice in remaining groups received 400 μg HAT-SA pretargeting for 24 hours, 100 μg sCA for 4 hours, followed by 100 μCi (3.7 MBq; Bi-100) or 250 μCi (9.25 MBq; Bi-250) or 500 μCi (18.5 MBq; Bi-500) 213Bi–DOTA-biotin. Mice in the combination group received the same pretargeting, clearing steps and 250 μCi (9.25 MBq) 213Bi–DOTA-biotin followed by 100 μg HAT once per week for 4 weeks.
Discussion
ATL is a malignancy of T lymphocytes with a median survival duration of 9 months in the acute form of the disease.1Various combination chemotherapies have not significantly increased the survival of patients with ATL.1 In light of the disappointing results using conventional combination chemotherapy, IL-2R–directed therapy was developed that included the use of unmodified murine and humanized antibodies (eg, anti-Tac) directed toward IL-2R. Although such therapy yielded partial or complete remissions in one third to one half of patients, most suffered a disease relapse.5 37 The use of monoclonal antibodies armed with toxins or radionuclides to specifically target these cytotoxic agents to the leukemic cells provides a valuable augmentation of therapy. There are a number of components that must be considered in designing an optimal RIT agent, including (1) the selection of the monoclonal antibody and thus the antigenic target, (2) the choice of the delivery system used to target the radionuclide to the tumor cell, and (3) the choice of the radionuclide.
As just noted, a pivotal issue to be addressed is the selection of the monoclonal antibody that targets the tumor and thereby the type of malignancy chosen as the target for RIT. In the present study we have chosen the human IL-2Rα subunit identified by the anti-Tac monoclonal antibody as our target for immunotherapy. The scientific basis for this choice using the α subunit of the IL-2R is that resting cells do not express this receptor, whereas this receptor is expressed by a high proportion of the abnormal cells in certain forms of lymphoid neoplasia, in select autoimmune diseases, and in individuals rejecting allografts. In terms of neoplasia, certain T-cell, B-cell, monocytic, and granulocytic leukemias express IL-2Rα identified by anti-Tac.38 In our clinical trials, we exploit the differential expression of IL-2Rα between normal resting cells and malignant T cells. A series of modifications in the anti-Tac monoclonal antibody have been made to increase its effector function, to reduce its immunogenicity, and to improve its pharmacokinetics. To increase its effector function, anti-Tac has been armed with the β-emitting radionuclide 90Y. A proportion of the 19 patients with HTLV-I–associated Tac-expressing ATL treated with anti-Tac armed with90Y developed a partial (7 patients) or complete (2 patients) remission.5 Although intact anti-Tac armed with this radionuclide provided meaningful therapy for this form of leukemia that was previously universally fatal, only 2 of the 16 patients manifested a complete remission.
A second issue in designing an optimal RIT reagent is the choice of the method used to deliver the radionuclide to the tumor cell. In our clinical trials, we have used intact monoclonal antibodies to deliver the radionuclide 90Y. There are a number of limitations in this approach. First, there are physiologic and structural barriers that limit rapid delivery of high molecular weight molecules such as intact antibodies to the tumor cells. Second, meaningful tumor uptake of antibody may not occur until 24 to 48 hours after injection. Unfortunately, the long serum half-lives of the monoclonal antibody prolong radiation exposure to normal organs, including radiosensitive bone marrow which limits the radiation dose that can be safely administered.10 11 Finally, because of the slow equilibration of intact monoclonal antibodies with the tumor cell, it is limited to relatively long-lived β-emitting radionuclides rather than short-lived α-emitting radionuclides to deliver low-dose irradiation that may be insufficient in a particular tumor cell to overcome the continual proliferation and repair of such tumor cells.
To obviate some of the obstacles encountered by conventional RIT with intact radiolabeled monoclonal antibodies, a series of multistep strategies has been described to de-couple the pharmacokinetics of the radionuclide delivery from that of the antibody.12-17,19-22,39 40 The approach used in the present study involves a pretargeting approach that includes 3 steps. This approach delivered large quantities of radioactivity to the tumor with the remaining radionuclide rapidly cleared by the kidney. A pivotal requirement for the success of this technique is that the conjugated antibody stays on the tumor cell surface until the radiolabeled biotin is administered. In this study, the internalization of HAT-SA conjugate into the leukemic cells was investigated with 6 cell lines. Although a similar percentage of the cell-associated radioactivity was present on the cell surface at the beginning, HAT-SA was internalized rapidly in the nonlymphoid SP2/Tac and ATAC4 cells with 60% internalized by 6 hours. In contrast, approximately 50% was present on the cell surface with the 4 leukemic cell lines examined. Thus, internalization of HAT-SA by the 4 leukemic cell lines studied was relatively slow, which made the pretargeting technique promising for the therapy of ATL leukemia with radiolabeled biotin.
The third component of an optimal RIT regiment to consider is the nature of the radionuclide used. There are various α- and β-emitting radionuclides that have a relatively short distance of action. Large tumors may include antigen-negative cells and may contain necrotic regions. For such tumors, a high-energy β-emitting radionuclide such as 90Y may be preferable. Because of greater penetration, high-energy β emissions, with their longer path length, may sterilize nontargeted tumor cells through a crossfire effect from neighboring antigen-bearing cells that have been targeted by the radiolabeled monoclonal antibody.
We have observed such an advantage of the β-emitting radionuclide90Y with its crossfire effect over the α-emitting213Bi with a very short distance of action in a solid tumor A431 (xenograft) model in a pretargeted B3-SA system.41Nevertheless, the use of β-emitting radionuclides has limitations. As the tumor mass decreases, the benefit of the crossfire effect also decreases. With various small tumors, including micrometastases and individual tumor cells including leukemic cells, therapeutic efficacy may be limited because high-energy β-emitting radionuclides yield a high dose of irradiation delivered outside the target volume because of the long path of β irradiation. Additionally, the required number of β emission traversals provided by crossfire that are required may not be possible in smaller tumors, micrometastases, and single cells. For such forms of malignancy, the future development of isotopic monoclonal antibody–mediated approaches may focus on α-emitting radionuclides that may be the most effective agents at killing tumor cells without damaging adjacent normal tissues. Thus, for agents that target the surface of leukemic cells, one would require only the binding of a relatively small number of α-emitting radiolabeled molecules per cell to provide the limited number of nuclear transversals required for tumor-killing activity. Macklis et al27 reported the212Bi–anti-Thy 1.2 immunoconjugates were capable of extraordinary cytotoxicity in vitro, requiring approximately 3212Bi-labeled conjugates per target cell to suppress [3H]thymidine incorporation to background levels.27
One potential radionuclide that is studied in the present report,213Bi, decays with a physical half-life of 46 minutes and with an α emission energy of 8 MeV that is deposited over a very short distance (40-100 μm in tissue), yielding a dense track of ionizing radiation of high energy. We demonstrated in the present study that when 213Bi linked to biotin was given as the third step in the pretargeting protocol, there was a reduction in the concentrations of the surrogate tumor markers human β2M and sIL-2Rα as well as a significant increase in the survivals of the tumor-bearing mice. This efficacy in the therapy in the MET-1 model with the pretargeting approach contrasts sharply with the limited efficacy observed with 213Bi linked to an intact monoclonal antibody. Thus, the rapid delivery of a high proportion of the administered 213Bi–DOTA-biotin coupled with the rapid renal clearance of the radionuclide unbound to the tumor provided an increase in the therapeutic efficacy. Furthermore, in contrast to the efficacy observed with the α-emitting radionuclide 213Bi, there was no efficacy when the β-emitting radionuclide90Y linked to biotin was administered in the pretargeting approach. This finding supports the view that in contrast with the situation with large solid tumors, for leukemia that has isolated malignant cells, α-emitting radionuclides are more effective agents in killing tumor target cells than β-emitting radionuclides.
The results of the pretargeting trial in the MET-1 model of ATL were encouraging; however, this aggressive T-cell leukemia was not completely eliminated by a single course of therapy with213Bi. A paradigm is emerging which suggests that, for cancer therapy, the addition of 2 therapeutic agents that interrupt the cell cycle at 2 distinct points may be more than additive in their cytotoxic action leading to malignant cell death. This paradigm has been shown for monoclonal antibodies added at therapeutic doses with chemotherapeutic agents as has been reported for the combination of Herceptin and Paclitaxel.42 In our combination trial, we wanted to obtain the complementary actions of receptor-saturating doses of the anti-Tac monoclonal antibody to yield antibody-dependent cellular cytotoxicity (ADCC) and cytokine deprivation–mediated leukemic cell death with the tumor cytoreduction provided by irradiation mediated by the radionuclide 213Bi delivered to the leukemic cell surfaces. Indeed, whereas neither HAT alone nor213Bi used in a pretargeting regime yielded complete long-lasting remissions, such remissions were observed in most of the mice receiving both agents in conjunction (Figure 4). In conclusion, our emerging understanding of the IL-2/IL-2R system in normal and leukemic cells opens the possibility for novel IL-2R–directed therapeutic approaches. In particular, pretargeting of ATL cells in the MET-1 tumor model with HAT-SA followed by a clearing step and then by213Bi–DOTA-biotin has shown favorable, specific, and fast targeting that has resulted in good tumor responses. Furthermore, the conjunction of this approach with the use of saturating concentrations of HAT given in serial doses provides the desired efficacy with acceptable toxicity. These findings support the use of this combination approach in a clinical trail in patients with IL-2Rα–expressing leukemias.
We thank Karen J. Wong and Dr Chang H. Paik for iodination of proteins.
Prepublished online as Blood First Edition Paper, April 17, 2002; DOI 10.1182/blood-2002- 01-0107.
Supported in part by funding from NeoRx (D.B.A., R.W.M., and L.J.T.).
D.B.A., R.W.M., and L.J.T. are employed by NeoRx Corporation, whose product was studied in the present work.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Thomas A Waldmann, Metabolism Branch, CCR, NCI, NIH, Building 10, Room 4N115, 10 Center Dr, Bethesda, MD 20892-1374; e-mail: tawald@helix.nih.gov.