Abstract
The prognostic impact of trisomy 8, alone or with other clonal aberrations, was evaluated in 849 patients with previously untreated acute myeloid leukemia (AML) who were registered to 5 Southwest Oncology Group trials. At presentation, 108 (12.7%) patients had +8 in their karyotypes, including 43 (5.1%) patients with +8 as the sole aberration; 307 (36.2%) were normal, and 434 (51.1%) had other cytogenetic abnormalities. Patients with +8 were slightly older (P = .033), had lower WBC (P = .011), and had lower percentages of peripheral blasts (P = .0004) than the patients without +8. Median survival time for all patients with +8 was 9.9 months (95% CI, 6.5-12.5), similar to that of “unfavorable” cytogenetics risk groups (8.3 months; 95% CI, 6.8-9.5.) Patients with +8 had significantly lower peripheral blasts (P = .0002), WBC (P < .0001) counts, and decreased overall survival (OS) than patients with normal cytogenetics (9.9 months vs 15.4 months; P = .006). However, survival of patients with +8 as the sole aberration did not differ significantly from those with normal cytogenetics (P = .36). Thus, the trisomy 8 group as a whole had poor survival, which was largely attributable to worsened outcomes among patients whose trisomy 8 was associated with other unfavorable cytogenetic abnormalities.
Introduction
Trisomy 8 is the most frequent numerical aberration in acute myeloid leukemia (AML), occurring at a frequency of 10% to 15%.1 Recent reports have suggested that AML patients with trisomy 8 have poor outcomes and are not responsive to cytarabine-based therapy.2,3 Although some studies have reported that trisomy 8 confers an independent prognostic risk in AML,4 a German AML cooperative group study reported that prognosis in the presence of trisomy 8 appeared to be dependent on the other associated clonal cytogenetic changes.5 In acute promyelocytic leukemia (APL), trisomy 8 appears to have little or no impact on prognosis.5 6 The clinical impact of additional copies of chromosome 8 on leukemic progression and response to therapy remains controversial.
In the present study, we examined the clinical and laboratory findings in a large, prospectively defined series of patients with leukemia (n = 849) enrolled on 5 different Southwest Oncology Group (SWOG) treatment trials to determine the impact of trisomy 8 on clinical presentation, treatment response, and survival in AML. Correlations were evaluated between chromosomal status and laboratory variables, including percentage peripheral blasts, hemoglobin, percentage marrow blasts, and platelet and WBC counts, in addition to descriptors such as age, FAB classification, and SWOG performance status (PS). The indicators of outcome for analysis were initial complete response (CR) rate (confirmed and unconfirmed), disease-free survival (DFS), and overall survival (OS).
Patients, materials, and methods
SWOG protocols and patient population
All patients included in this analysis were enrolled in SWOG 8750/9007, the prospective cytogenetic companion studies to SWOG adult leukemia treatment protocols. Patients were registered to 1 of 5 SWOG treatment trials for patients with previously untreated AML (Table1). The 4 AML trials were S9031, a phase 3, double-blind, placebo-controlled trial of daunomycin (DNR) and cytarabine (Ara-C) with or without rhG-CSF7; S9034, a phase 3 study comparing 3 intensive postremission therapies, autologous bone marrow transplantation, intensive chemotherapy, and allogeneic bone marrow transplantation8; S9333, a phase 3 randomized, controlled trial of mitoxantrone and etoposide versus DNR and Ara-C as induction therapies; and S9500, a phase 2 study of the addition of 3 days of high-dose cytarabine to standard daunomycin plus cytarabine (3 + 7 + 3), with sequential high-dose Ara-C consolidation. Cumulatively, 774 of 965 patients who were entered in these trials had successful cytogenetic evaluations at study entry. In addition, patients registered to S9129, a phase 3 randomized study of all-trans retinoic acid versus Ara-C and DNR as APL induction therapy (n = 75 patients) were also evaluated. The proportion of patients with successful cytogenetic studies ranged from 77% to 85% in all trials.
Clinical descriptors, hematologic variables, and endpoints for analysis of each set of patients are listed in Table2. PS was graded according to SWOG criteria as follows: 0 = fully active, able to carry on all predisease activities without restriction; 1 = restricted in physically strenuous activity but ambulatory and able to carry out work of a light or sedentary nature (eg, light housework, office work); 2 = ambulatory and capable of all self-care but unable to carry out any work activities; up and about for more than 50% of waking hours; 3 = capable of only limited self-care, confined to bed or chair more than 50% of waking hours; 4 = completely disabled, unable to perform any self-care, totally confined to bed or chair.
For ECOG-coordinated studies (S9034 and S9129), PS was defined by the Zubrod scale. FAB classification was determined from reports from the originating institution.
The 4 AML studies were directed at specific age groups (Table 1). Prior conditions and treatments also varied as follows: S9031 permitted patients with either de novo AML or AML secondary to MDS or prior therapy for disease other than acute leukemia. Although S9031 and S9333 had secondary AML as a stratification factor, unfortunately information was not collected on whether the AML was secondary to MDS or secondary to prior therapy. In contrast, S9034 patients who had undergone radiotherapy or cytotoxic chemotherapy earlier were ineligible. The S9129 APL study focused on induction therapy for patients with previously untreated leukemia and was not age restricted, though no SWOG patients were younger than 16 years. All patients gave informed consent to participate in both the SWOG treatment study and the cytogenetics companion study in accordance with local institutional guidelines and federal regulations.
Cytogenetic analysis, review, and definitions
The SWOG Cytogenetics Committee reviewed all pretreatment cytogenetic studies included in this analysis. Patients were accepted if they met the following criteria: all had to be processed by at least 2 different methods and at least 20 metaphase cells had to be analyzed, unless an abnormal clone was diagnosable with fewer cells. Chromosomal banding levels that would permit definition of t(15;17) or inv (16) were required. Either G- or Q-banded staining methods were acceptable. Definitions of cytogenetic clonality and karyotypic descriptions were from the International System for Human Cytogenetic Nomenclature.9 Patients in this study were evaluated for time of appearance and coexistence (simultaneous vs secondary) of +8 and other aberrations and for mosaicism with normal cells. This study included 108 patients with +8 in their presentation karyotype. In 43 patients, trisomy 8 was observed as the sole karyotypic aberration. To the best of our knowledge, none of our patients were characterized by constitutional mosaicism for +8, a rare but reported finding.10-12 This analysis pertains only to pretreatment specimens. Our data set includes patients who are listed as having clonal +8, patients with cytogenetic aberrations other than +8, and patients with normal cytogenetics.
Three patients were identified with only a single cell with +8 (nonclonal), and they were coded as normal for purposes of this analysis. One patient's sample was triploid and not otherwise evaluable. Four patients with tetrasomy 8 were classified as trisomy 8 for purposes of this analysis.
Historically, analyses have divided patients into favorable, intermediate, and unfavorable cytogenetic groups.13 14 In previous SWOG studies the favorable category included inv(16), t(15;17), and t(8;21); the intermediate group included those with normal cytogenetics, +8, and a few other single-chromosome trisomies or deletions or sex chromosome losses, and the unfavorable category included patients with complex (3 or more) aberrations, aberrations or losses of 5 and 7, inv(3q), t(6;9), t(9;22), and others. The same classification scheme was used for the present analyses.
Statistical analysis, response criteria, and follow-up
Collection and quality control of patient pretreatment and outcome data were performed according to standard SWOG procedures. Comparisons of continuous variables were based on the Kruskal-Wallis test, and comparisons of dichotomous variables were based on the χ2 approximation of the Fisher exact test. OS was measured from randomization until death from any cause, with observations censored for patients last known alive. DFS was measured from the date the complete response was established until the relapse of leukemia or death from any cause, with observations censored for patients last known to be alive without report of relapse. Distributions of OS and DFS were estimated by the method of Kaplan and Meier.15 For OS and DFS, comparisons between groups were based on the log-rank test, and analyses of prognostic factors were based on proportional hazards (PH) regression models.16All P values are 2-tailed. Analyses were based on data available as of July 1998 for S9034, September 1997 for S9129, and November 2000 for the other 3 studies.
Results
Of the 849 patients with evaluable pretreatment cytogenetic specimens, 108 (12.7%) had +8 with or without additional findings, 307 (36.2%) had normal cytogenetics, and the remaining 434 (51.1%) had other cytogenetic abnormalities excluding +8 (Table 1). Trisomy 8 was observed as the sole aberration in 43 (5.1%) patients. Of these patients, the marrow cytogenetics showed mosaicism for normal cells in 31 of 43, and 12 patients had trisomy 8 as the sole cytogenetic population. Among the 65 of 108 (60%) patients with +8 and additional aberrations, 22 had favorable aberrations—4 patients with inv(16), 15 patients with t(15;17), 3 patients with t(8;21)—and 43 patients had other aberrations. Of these, 8 of 43 patients were classified as intermediate and the remaining 35 were classified as having unfavorable cytogenetics, with 17 of 35 having complex aberrations. It should be noted that we grouped 4 patients with complex aberrations in the favorable category—1 with t(8;21), 1 with t(15;17), and 2 with inv(16).
Comparison of all patients with and without +8 in karyotype
Comparison of patients with +8 (n = 108) and without +8 (n = 741) showed that patients with +8 were slightly older (marginally significant difference, P = .033), had lower WBC (P = .011) (Table 3), and had considerably lower percentages of peripheral blasts (P = .0004). No differences were observed in DFS for the patient group as a whole (log rank P = .62) or when the comparison was stratified by study (P = .32). However, a significant benefit in OS was found for patients without +8 (median survival, 12.3 months; 95% CI, 11.2-14.0) compared with patients with +8 (median survival, 9.9 months; 95% CI, 6.5-12.5; log rank,P = .03), and this remained true when patients were stratified by study (P = .02). The estimated hazard ratio from the stratified analysis was 1.33 (95% CI, 1.06-1.67); that is, the mortality hazard rate was 33% greater with than without the +8 alteration.
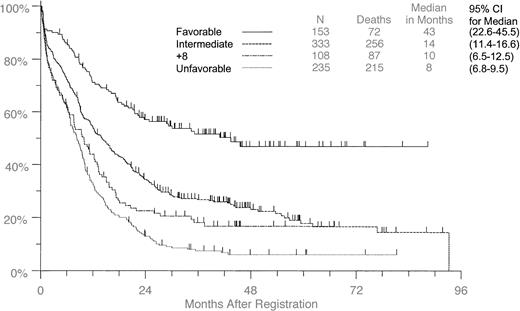
When the entire patient group was examined and those with +8 were compared with the remaining patients with leukemia classified according to favorable, intermediate, or unfavorable cytogenetic status, the OS survival curve for the +8 patients fell between those of the intermediate and the unfavorable groups, and the median survival for +8 patients was similar to that of the unfavorable group (Figure1). When we applied the same analyses to the individual trials, the numbers were too small to be meaningful statistically. However, the +8 patients had consistently similar or worse OS and DFS than patients without +8 in all studies separately. In general, patients on S9031, S9034, and S9333 had worse outcomes than the patients on S9500 (who were younger) and S9129 (who had APL). It should be noted that 20 of 849 patients were not classified as to cytogenetic risk status because their cytogenetic aberrations occur with low frequency in AML, and their clinical ramifications are not well understood.
Overall survival in all patients with +8 compared with all others classified by cytogenetic group.
Tick marks indicate censored observations.
Overall survival in all patients with +8 compared with all others classified by cytogenetic group.
Tick marks indicate censored observations.
We also compared patients with and without +8 within the favorable, intermediate, and unfavorable subgroups (Tables4, 5). In the favorable category the only difference, marginally significant, was that patients with +8 had lower peripheral blast counts (P = .05). More than 80% of the patients in the intermediate category had normal cytogenetics, and the data for these comparisons are presented in the next section. The only subgroup within which the presence of +8 altered survival was the unfavorable category, which showed a marginally worse CR rate of 23% in the presence of +8 compared with 40% for patients lacking the trisomy (P = .05). Patients with +8 were slightly older, but for them OS was significantly worse even after the adjustment for age (P = .01). Median survival was 3.4 months, whereas it was 8.3 months for those without +8. The difference remained significant when patients were stratified by study (P = .02). The hazard ratio was 1.62 (95% CI, 1.11-2.35).
The question of leukemia cutis was examined with respect to trisomy 8. Six of 104 (6%) patients with trisomy had skin involvement; data were missing for 4 patients in this group. The frequency of skin involvement in 44 of 714 (6%) patients (relevant data missing for 27 patients) was similar to that for patients lacking the trisomy (Table 2).
Comparison between patients with +8 and with normal cytogenetics
If all patients with cytogenetic aberrations other than +8 were excluded, 415 patients from the 5 trials could be separated into those with normal cytogenetics (n = 307) and those with +8 (n = 108), with or without additional aberrations. Variables of race, age, CR rate, PS, hemoglobin, marrow blast percentage, and platelets did not differ significantly between the 2 groups (Table 2). However, the sex ratio was marginally significantly different (χ2,P = .05). Although the number of females with normal cytogenetics exceeded the number of males, fewer females than males had the +8 aberration in this analysis. Peripheral blasts and WBC counts were significantly lower (P = .0002 andP < .0001, respectively) in the +8 patients than in the patients with normal cytogenetics (Table 3). Log-rank analysis for DFS, when stratified by study, was significant (P = .03); patients with +8 trisomy had shorter DFS. There was a 1.54 (95% CI, 1.04-2.25) greater risk for disease relapse or death at any specific time if the patient had the +8 abnormality than if the patient had normal cytogenetics.
A statistically significant increase in OS was shown for patients with normal cytogenetics compared to +8 patients (15.4 months [95% CI, 12.0-19.2] vs 9.9 months [95% CI, 6.5-12.5]) for all studies combined (P = .006). This survival difference was maintained when stratified by study (P = .0002). The hazard ratio was 1.65 (95% CI, [1.28-2.13]). There was a 1.65 greater risk for death if the patient had the +8 abnormality than if the patient had normal cytogenetics.
Comparison between patients with +8 as the sole abnormality and patients with normal cytogenetics
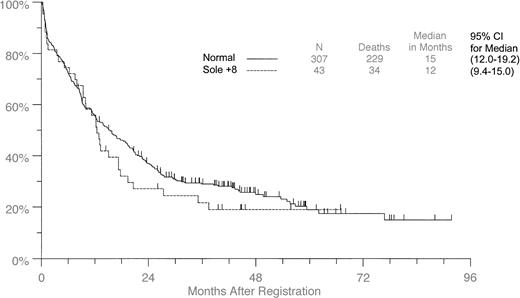
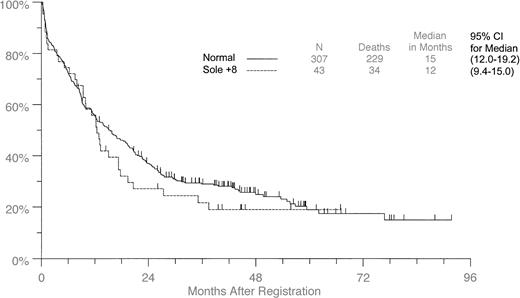
Forty-three of the 849 (5.1%) patients had +8 as the sole aberration. Table 2 compares these patients with 307 patients from the same studies for whom no clonal cytogenetic aberrations were found. Thirty-one of the 43 (72%) patients with +8 alone showed mosaicism with normal metaphase cells in the samples for cytogenetic analysis. Of the variables investigated, only WBC counts and peripheral blast percentages differed significantly. They were lower in the +8 patients than in patients with normal cytogenetics (P = .0012 andP = .0006, respectively) (Table 2). Although the median survival time for the +8 group was 12.5 months (95% CI, 9.4-15.0) compared with 15.4 months (95% CI, 12.0-19.2) for patients with normal karyotype (Figure 2), no significant differences in OS or DFS were observed whether all studies were combined or stratified (OS: combined log-rank, P = .36; stratified log-rank, P = .47) (DFS: combined log-rank,P = .28; stratified log-rank P = .42).
Overall survival in patients with +8 as the sole aberration compared with patients with normal cytogenetics.
Overall survival in patients with +8 as the sole aberration compared with patients with normal cytogenetics.
Comparison of patients with +8 as the sole abnormality and patients with +8 with additional clonal aberrations
Patients with +8 as the sole aberration fared better than patients with +8 in the karyotype with additional clonal abnormalities. CR rates were significantly higher than they were for patients with added aberrations (29 of 43 [67%] vs 25 of 64 [39%]; χ2,P = .004), though DFS did not differ significantly. There was a trend indicating that OS was longer for patients with +8 as the sole aberration, with a median survival of 12.5 months (95% CI, 9.4-15.0) compared with 7.5 months (95% CI, 4.2-10.0).6When stratified by study, this difference was significant (P = .001). The exception to the deleterious effect of +8 in combination with other aberrations was the t(15;17) group of patients, who had OS rates comparable to those of the favorable risk group—2-year survival rates were 62.3% for +8, t(15;17) (95% CI, 36.1-88.5; n = 15) and 65.4% for t(15;17) without +8 (95% CI, 54.1-76.7; n = 73) (Tables 4, 5).
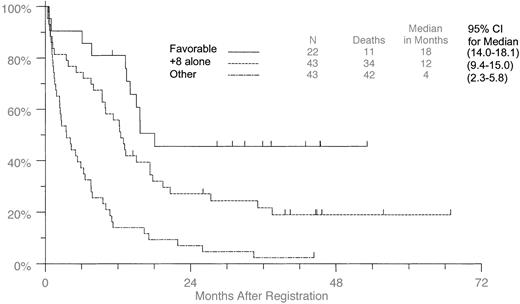
Interaction of +8 with cytogenetic risk groups
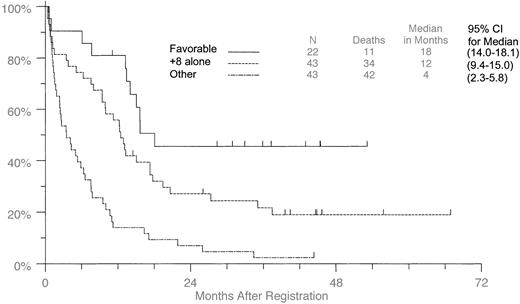
When the patients were separated into favorable (t(8;21), t(15;17), inv(16); [n = 22]) and other (unfavorable and intermediate; n = 43) cytogenetic groups, those with +8 as the sole aberration (Figure 3) had longer survival than the other group but shorter survival than the favorable group. Medians were 18.1 months (95% CI, 14.0-18.1) for the favorable group, 12.5 months (95% CI, 9.4-15.0) for the +8 alone group, and 3.5 months (95% CI, 2.3-5.8) for the “others,” which included 8 patients classified as intermediate and 17 patients with complex aberrations. All variables shown in Table 2, except for performance status and FAB classification, were tested for the interaction between individual cytogenetic risk group and +8 presence. The only significant interaction was for CR rate (P = .03). Comparisons that reached statistical significance in any of the risk group categories are included in Tables 4 and 5, regardless of whether the interaction between risk group and +8 was statistically significant. The median for the favorable risk group is not meaningful because the medians are only just reached in that group. Therefore, for consistency 2-year survival rates rather than medians are used in the analysis of OS.
Overall survival in patients with +8 as the sole aberration compared with patients with additional favorable aberrations.
Patients with +8 were compared with those patients with t(8;21), t(15;17), and inv(16), or with any other aberrations.
Overall survival in patients with +8 as the sole aberration compared with patients with additional favorable aberrations.
Patients with +8 were compared with those patients with t(8;21), t(15;17), and inv(16), or with any other aberrations.
Discussion
Trisomy 8 is found in 10% to 15% of patients with AML in all FAB subgroups, and it is frequent in myelodysplasia and myeloproliferative syndromes. When present, it exists as the sole anomaly in 40% of AML patients, in combination with simple chromosomal changes in 35%, and as a component of a complex karyotypic picture in the remaining 25% of AML patients. A previous broad survey of more than 500 patients with +8 indicated that when +8 was the sole aberration, CR was achieved in 60% to 70% of patients and that the median survival ranged between 13 and 20 months, decreasing to 12 months when all patients with +8 were considered.1 However, individual studies show wide variation in results. For example, the German AML Cooperative study group found that though +8 as the sole aberration (n = 20) conferred an intermediate prognosis (70% CR), when it was combined with “favorable” aberrations such as t(8;21), t(15;17), or inv(16), the prognosis was good (90% CR). In combination with complex aberrations, the prognosis was poorer (67% CR but worse event-free survival).5 In addition, survival was significantly poorer when a high proportion of the mitotic cells were trisomic. In a separate study restricted to patients with APL, no influence from the addition of +8 on prognosis was observed in the presence of t(15;17), nor was there any apparent reduction in WBC count or circulating blast percentage.6
Another recent report indicated no difference in WBC count or blast percentage with +8 alone, approximately 85% achievement of CR, and marked differences for patients with +8 and complex karyotypes (CR = 47%). Despite this, differences in the DFS and OS were not significant between +8 as the sole aberration and in combination with complex changes.3 Similarly, the Cancer and Leukemia Group B (CALGB) reported that +8 alone (n = 42) was associated with poor prognosis (CR, 59%; median survival, 13.1 months), especially in older patients.2,17 In contrast, previous SWOG studies have included +8 and normal karyotypes within the group of cytogenetic findings that confer an intermediate prognosis in AML, and this assumption has been validated by the observed rates of CR and relative risks for death in older13 and younger14patients. In possibly the largest single study of +8 as the sole aberration,4 among 72 patients the median survival was 15 months, placing +8 as an intermediate prognostic factor. Conclusions from different studies may depend on the proportion of patients with normal cytogenetics in the intermediate groups, the different median survival values, and the treatment protocols used, but overall this trisomy has been associated with intermediate to poor prognosis in AML.
In a large study of myelodysplasia, separate from AML,18patients with +8 were classified in the intermediate group, whereas normal cytogenetics were associated with a good prognosis. In fact, patients with trisomy 8 had shorter survival and significantly increased risk for leukemic transformation than patients with other chromosomal aberrations in the intermediate category. Unfortunately, we do not have information regarding the frequency of leukemia secondary to MDS in our patient group.
In our series of studies, separately and combined, patients with trisomy 8 generally had poorer outcomes than the leukemic group in toto and patients with normal cytogenetics. These observations suggest that trisomy 8 may confer poor, rather than intermediate prognoses, in accord with results of a large CALGB study.2 Median survival for the isolated trisomy 8 patients in the CALGB was 13.1 months compared with 12.5 months (95% CI, 9.4-15.0) in this study. It should also be noted that the favorable, intermediate, and unfavorable categories are relative determinations, and enormous variability in response remains within these categories. For example, the definition of poor median survival in the trisomy 8 CALGB study of 13 months2 is similar to the intermediate survival of 12.5 months for trisomy 8 alone in this SWOG study. However, in our study survival was significantly worse in the unfavorable group when +8 was present. As we break down categories into individual cytogenetic aberrations, larger numbers of patients will be required to describe potential differential effects of treatments.
Conversely, in comparison with patients with normal cytogenetics with +8 as the sole aberration or patients with t(15;17) with or without +8, no statistically significant differences were found. However, these comparisons suffer from small sample sizes. Thus, though our data are not inconsistent with the German cooperative group's finding that +8 was not an independent prognostic factor when associated with certain other clonal cytogenetic changes,5even together these studies fail to provide definitive confirmation.
Although the relation of these data to transplantation is of great interest, the available data concerning BMT are incomplete and include only small numbers of patients with +8. In the present study, only S9034 included transplantation as a treatment arm, and too few patients with +8 in that study underwent BMT to enable conclusions regarding the role of BMT.
Our observation of decreased WBC counts and peripheral blast percentages in +8 patients is unique, although large numbers of comparisons made in these analyses make it possible that this result was due to chance. It could reflect an association with unrecognized myelodysplastic disease. Although we13,14 and others5 have not found significant differences in survival between AML with normal cytogenetics and AML with +8 as the sole aberration, there is evidence for altered expression of apoptosis-regulating genes in trisomy 8 AML.19 Virtaneva et al suggested that greater resistance to apoptosis might account for the reported resistance of AML +8 patients to cytarabine chemotherapy2,3; however, differences in apoptosis regulation did not otherwise appear to affect survival. It is also unclear how the down-regulation of apoptosis could result in lower peripheral blood WBC counts. Another attempted explanation of the biologic significance of the extra chromosome was based on the observation of increased copies of the C-MYConcogene that is localized to 8q. Low-level amplification of C-MYC was reported in a number of patients with CML and CML-BC, with and without +8.20 Jennings et al suggested that increases in C-MYC were important to the course of the disease and that trisomy of chromosome 8 was an alternative means for achieving amplification of this gene.
It is evident that the molecular and biologic consequences of this trisomy require further investigation. Our observations, in association with other studies described in this report, suggest that patients with trisomy 8 leukemia have poor overall survival and that this is particularly true of patients with additional unfavorable cytogenetic changes.
The following SWOG institutions and cytogeneticists participated in this study: Cardinal Glennon Hospital, St Louis University, MO, Jacqueline R. Batanian, PhD; City of Hope National Medical Center, Duarte, CA, Marilyn L. Slovak, PhD (CA 30206); Cleveland Clinic Foundation, OH, Gerald Hoeltge, MD; Columbia-Presbyterian Medical Center, New York, NY, V. V. V. S. Murty, PhD; Emory University-Atlanta CCOP, Decatur, GA, Debra F. Saxe, PhD; Henry Ford Hospital, Detroit, MI, Daniel Van Dyke, PhD; Louisiana State University-Shreveport, Leonard A. Prouty, PhD; Loyola University Medical Center, Maywood, IL, Valerie Lindgren, PhD; Oakwood Hospital and Medical Center, Dearborn, MI, Julie L. Zenger-Hain, PhD; Oregon Health Sciences University, Portland, R. Ellen Magenis, MD; Puget Sound DynaCare/Laboratory of Pathology, Seattle, WA, Fred W. Luthardt, PhD; Sacred Heart Medical Center, Spokane, WA, Julie Sanford Hanna, PhD; Scott & White CCOP, Temple, TX, Sheila M. Dobin, PhD; Stanford University, CA, Athena M. Cherry, PhD; Tulane University, New Orleans, LA, Marilyn M. Li, MD; University of Arkansas, Little Rock, Jeffrey R. Sawyer, PhD; University of California at Davis, Sacramento, Jeanna Welborn, MD; University of Chicago, IL, Michelle M. Le Beau, PhD; University of Colorado, Denver, Loris McGavran, PhD; University of Kansas, Kansas City, Diane L. Persons, MD; University of Kentucky, Lexington, Anjana Pettigrew, MD; University of Michigan, Ann Arbor, Diane Roulston, PhD; University of Mississippi, Jackson, Cheng Yu, PhD; University of New Mexico, Albuquerque, Karen Montgomery, PhD; University of South Alabama, Mobile, Cathy Tuck-Muller, PhD; University of Texas at San Antonio, C. Nanette Clare, MD; University of Utah, Salt Lake City, Arthur R. Brothman, PhD; University of Washington Medical Center, Seattle, Thomas H. Norwood, MD; Wayne State University, Detroit, MI, Anwar Mohamed, MD, Sandra R. Wolman, MD; Wesley Medical Center, Wichita CCOP, Wichita, KS, Sechin Cho, MD.
Supported in part by National Institutes of Health grants to the SWOG Leukemia and Cytogenetics Programs (CA-32102).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Sandra R. Wolman, Southwest Oncology Group (SWOG-9007), Operations Office, 14980 Omicron Dr, San Antonio, TX 78245-3217; e-mail: swolman@pathol.faseb.org.