Abstract
Little is known about the prognostic role of multidrug resistance (MDR) in adults with newly diagnosed acute lymphoblastic leukemia (ALL). In the context of the GIMEMA ALL0496 protocol, we evaluated the impact of MDR1 (protein expression and function) on the achievement of complete remission (CR) and clinical outcome. Flow cytometric analysis of MDR1 expression (D) and function (rhodamine-123 efflux) was obtained in 203 and 158 patients, respectively. MDR1 expression was detected in 44 (21.7%) of 203 patients, and function was found in 23 (14.6%) of 158 (14.6%) patients. Expression of the multidrug resistance-associated protein 1 (MRP1) and lung-resistance protein (LRP) evaluated in 43 samples was found in 13 and 26 patients, respectively. Among the 200 patients evaluable for the clinical correlation study, 125 (79.6%) of 157 without MDR1 expression achieved CR compared with 23 (53.5%) of 43 with MDR1 expression (P = .001). At univariate analysis, MDR1 expression was significantly associated with CR when considered as a dichotomized (P = .001) or continuous (P = .01) variable. At multivariate analysis, dichotomized evaluation of MDR1 expression independently predicted CR (P = .004) with age (P = .03) and CD34 (P = .03); as a continuous variable, MDR1 expression (P = .03) was the only significant factor other than CD34 (P = .01). MDR1 function failed to predict achievement of CR or of MRP1 and LRP expression. MDR1 expression did not correlate with CR duration, nor did it predict for survival duration. These results demonstrate that MDR1 expression in de novo adult ALL is an independent predictor of CR achievement.
Introduction
Multidrug resistance (MDR) contributes to therapeutic failure in several hematologic malignancies,1-3 particularly when treated with regimens containing drugs such as anthracyclines, vinca alkaloids, or epipodophyllins, which are modulated in their intracellular retention by the P-glycoprotein 170 (P-gp) product of the mdr-1gene.4 Although many of the drugs included in the standard induction treatment of acute lymphoblastic leukemia (ALL) are known to be modulated by MDR, its prognostic role in adult ALL has yet to be settled.5-8 This is largely a consequence of the lack of multicenter studies on MDR involving uniformly treated patients adhering to the consensus technical guidelines9-11 for the detection of P-gp. The heterogenous methodologic approach is contributed by the different MDR assays utilized by differences in flow cytometric data analysis, and by the use of arbitrary cut-off points.
Additional proteins that have a role in MDR mechanisms of accelerated drug efflux and are potentially implicated in leukemic drug resistance, that is the multidrug resistance-associated protein 1 (MRP1) and the lung-resistance protein (LRP), have been reported.12,13 MRP1 is a protein distinct from MDR1 because of its substrate specificity (amphophilic anions or glutathione-conjugated) and the types of drugs that can restore chemotherapy sensitivity (butathionine, which depletes intracellular glutathione content).14,15 LRP is a component of the major vault protein complex that appears to be involved in nuclear-cytoplasmic trafficking and in promoting drug redistribution away from the nucleus.15 16
Studies on the treatment of adult ALL have shown only modest improvements over the last 2 decades, with the actual cure rate still ranging between 15% and 40%.17-21 Leukemic cells resistant to induction chemotherapy lead to persistence of disease or relapse and ultimately to death of the patient. Unfavorable prognostic factors in adult ALL include high WBC count, older age, late achievement of complete remission (CR), presence of the Philadelphia (Ph) chromosome, and t(4;11) translocation.22-25 Although the prognostic role of the immunophenotypic profile remains uncertain,23 that of P-gp and other MDR-associated proteins is still largely unknown. In pediatric ALL, the prognostic relevance of MDR, initially proposed by us and others,13,26 has recently been confirmed in a large prospective study that showed an independent adverse effect of MDR on remission duration.27 In addition, the unfavorable significance of MDR1 overexpression has been clearly demonstrated in acute myeloid leukemia (AML),12,28-31 especially in older patients, in whom it has been significantly associated with a lower CR rate.32
In the present work, we investigated the frequency and biologic–clinical significance of MDR1 expression and function and of MDR-associated proteins in de novo adult ALL. Our analysis was performed in the context of the ALL0496 study of the GIMEMA group (Gruppo Italiano Malattie Ematologiche dell'Adulto). This prospective, multicenter study enrolled a large series of uniformly treated adult ALL patients. The ALLVR589 treatment protocol,33 which had provided encouraging results at a single institution,34was used. The ALL0496 study required a central handling of fresh samples at presentation. Thus, taking advantage of the diagnostic immunophenotypic data performed in each center and of the central cytogenetic and molecular analyses, we investigated the prognostic influence of MDR1 on achievement of CR and clinical outcome with respect to other clinical and biologic factors. Our results suggest that MDR1 expression is an independent predictor of CR in patients with de novo adult ALL.
Patients, materials, and methods
Patients and samples
Bone marrow or peripheral blood samples for MDR1 studies were obtained at initial diagnosis before therapy from adult ALL patients enrolled in the prospective GIMEMA multicenter ALL0496 study. The study was performed on 286 patients enrolled and evaluable at the interim analysis for response and with a minimum follow-up of 6 months. Diagnosis of these patients was established in each center according to established morphologic, cytochemical, and immunophenotypic criteria.18 33 The study included (as a mandatory eligibility requirement) the central overnight dispatch handling of fresh samples at the Department of Biotecnologie Cellulari ed Ematologia at La Sapienza University in Rome. Because of cell limitation, sufficient amounts of fresh material for MDR expression and function were obtained in 203 and 158 patients, respectively. Samples were processed in a single laboratory in Rome within 24 hours of arrival—1 day after collection from the external centers, the same day for patients enrolled in Rome (ALL samples tested on the same day and the day after showed no differences in MDR1 expression and function; data not shown). A hierarchic cell distribution of samples was adopted according to clinical priorities; cytogenetic and molecular genetic analyses were preferentially performed, whereas MDR represented a second priority. Only in patients with less than 10 × 106/mL cells, the minimum amount needed to perform cytogenetic and molecular genetic analyses, was the MDR test preferentially performed. In fact, cytogenetic and molecular analyses (performed in 3 Italian laboratories for cytogenetics and 3 for molecular biology) referred to 195 and 199 of the 203 samples, respectively.
According to the GIMEMA ALL0496 study protocol, all patients underwent 3 induction cycles consisting of vincristine (1.4 mg/m2 weekly for 5 weeks), daunorubicin (30 mg/m2 per day for 3 days), L-asparaginase (6000 IU/m2 every other day from day 10 to day 30), and prednisone (60 mg/m2 per day for 30 days), followed by consolidation therapy as reported elsewhere.33 CR was defined as the achievement of normal peripheral blood values (polymorphonuclear, greater than 1.5 × 109/L; Hb, greater than 10 g/dL; platelet count, greater than 100 000 × 109/L; absence of circulating blast cells) and less than 5% blast cells in the bone marrow aspirate in the presence of a normal cellularity.21,34 35 CR was evaluated after the 3 induction cycles. To obtain unequivocal homogeneous data analysis, only patients with a minimum follow-up of 6 months were included in the present study. The correlation between MDR1 expression and function and the clinical follow-up was possible in 200 of 203 and in 156 of 158 patients, respectively, because of major protocol violations that emerged in 3 patients at the time of data analysis. MRP and LRP data were available on 43 patients.
MDR1 expression and function
Blast cells from fresh centralized material were enriched by Ficoll-Hypaque (1.077 g/mL; Sigma, Milan, Italy) gradient centrifugation. Mean blast percentage was 86.3% (range, 19%-100%). Assessment of cell number and viability was performed by trypan-blue exclusion; only 0.5% of samples could not be further processed because of poor viability (less than 80% of viable cells). MDR1 expression and function were assessed by 2 flow cytometric tests. MDR1 expression was evaluated using a previously described procedure32 with slight modifications. In brief, leukemic cells were stained with the MRK16 antibody (Kamiya Biomedical, Seattle, WA), which reacts with a cell surface epitope of P-gp 170. Cells were incubated with 2% goat serum (Sigma Chemical, Milan, Italy) for 15 minutes at 37°C and then were stained with 10 μg/5 × 105 MRK16 cells for 45 minutes at 4°C. Appropriately matched isotype controls (mouse IgG2a) at the same protein concentration as the relevant antibody were used in all analyses. After 2 washes, cells were stained first with biotin–goat antimouse IgG2a antibody (Southern Biotech, Birmingham, AL) for 30 minutes at 4°C and then with phycoerythrin (PE)-conjugated streptavidin (Becton Dickinson, Mountain View, CA) for 20 minutes at 4°C, at the manufacturer's recommended concentrations. Immediately after staining, samples were measured by flow cytometry (as described below). The biotin-avidin detection system was used to augment the signal obtained with the MRK16 staining.32 The MDR1-positive 8226/DOX and CEM/VBL cell lines and the parental 8226/S and CEM cells (kindly provided by Dr W. S. Dalton, H. Lee Moffit Cancer Center and Research Institute, Tampa, FL) were used as controls in the standardization of this analysis.7,32 P-gp 170 expression was analyzed using the Kolmogorov-Smirnov statistic test (D), which allows the objective and accurate identification of small differences in fluorescence intensity.11 MRK16 staining intensity was also categorized for descriptive purposes: samples with D < 0.05 and those with negative control fluorescence brighter than MRK16-stained cells (D = 0) were considered negative, whereas those with a D ≥ 0.05 were considered positive.
MDR1 function was investigated in 158 of 203 ALL samples using the rhodamine-123 (Rhd-123) efflux test, according to a previously described procedure26 with slight modifications. Briefly, blasts were incubated with 200 ng/mL Rhd-123 (Sigma Chemical) in RPMI 1640 medium plus 10% fetal calf serum for 30 minutes at 37°C to allow uptake. Cells were then pelleted, washed twice, and resuspended in fresh medium with or without the MDR modulator verapamil (Sigma Chemical) at 20 mg/mL for 60 minutes at 37°C.36 Rhd-123 uptake at baseline and efflux (with or without the MDR modulator) was immediately measured by flow cytometry (as described below). MDR function was evaluated by analyzing cellular fluorescence of gated leukemic blasts in the presence or absence of the MDR modulator and was expressed as a ratio. MDR1 function was considered negative for Rhd-123 efflux ratio values less than 1.10 and positive for values of 1.10 or greater.
To eliminate bias derived from the choice of cut-off points, assessment of the relationships between clinical outcome and MDR1 expression, and between clinical outcome and function, was also performed considering both values as a continuous variable.
The few (11 of 203) samples that had less than 70% blasts were double stained with antibodies directed against the most relevant leukemic antigens. In particular, following the staining for assessment of MDR1 expression and function as described above, blasts were incubated with CD19, Leu-12 (Becton Dickinson) or CD34, HPCA II (Becton Dickinson) for 60 minutes at 4°C using avidin–fluorescein isothiocyanate (FITC) instead of PE-conjugated streptavidin to allow double staining with the PE-conjugated anti–surface antigen antibody.
Multidrug resistance–associated protein 1 and lung resistance–related protein expression
MRP1 and LRP expression were detected using a previously described procedure12 with slight modifications. Briefly, 1× 106/mL cells were fixed and permeabilized using the Fix & Perm kit (Caltag Laboratories, Burlingame, CA) to allow intracellular epitope staining. Cells were then incubated for 30 minutes in the dark at room temperature with the monoclonal antibodies MRPm6 and LRP56 (MONOSAN, Uden, The Netherlands) at a concentration of 0.5 and 1 μg/mL, respectively. An irrelevant mouse antibody of the appropriate subclass was used as a negative control to determine background fluorescence. After 2 washes, cells were incubated with FITC-conjugated goat F(ab')2 fragment mouse IgG (H-L) for 30 minutes in the dark at room temperature. After washing, cells were immediately measured by flow cytometry. Results were analyzed using the Kolmogorov-Smirnov statistic test (D). Staining intensity was categorized for descriptive purposes and considered positive for D = 0.10 or greater for MRP1 and LRP. MRP1- and LRP-positive SW-1573/2R120 and GLC4/ADR cell lines and the parental cells (kindly provided by Dr H. J. Broxterman, Academisch Ziekenhuis Vrije Universiteit, Amsterdam, Netherlands) were used as controls in the standardization of this analysis.37 38
Flow cytometry
Flow cytometric analysis was conducted using a FACScan flow cytometer (Becton Dickinson) operated at 488 nm, which detects green (Rhd-123, avidin-FITC/MRK16, FITC/MRP1, FITC/LRP) and red (PE-streptavidin/MRK16 and PE/CD surface antigens) fluorescence. Data acquisition and analysis were performed with the CellFit and Lysys II software (Becton Dickinson). Forward and side scatter signals were collected using linear scales; leukemic cells were gated according to these parameters and lineage-specific antibodies. Fluorescence signals were collected on a logarithmic scale.
Statistical analysis
MDR1 expression (Kolmogorov-Smirnov analysis; D) and function (Rhd-123 efflux ratio) were represented as continuous variables or were dichotomized as positive versus negative variables. Univariate analysis for baseline clinical and biologic characteristics between groups was performed using χ2 analysis. To test factors as predictors of response, χ2 analysis and the logistic model were used for univariate analysis and multivariate analysis, respectively.39 Differences between the curves were analyzed with the log-rank test for univariate analysis40 and the Cox regression model41 for multivariate analysis. Overall survival (OS) was measured from diagnosis to death from any cause, with observations censored for patients at the last follow-up. Event-free survival (EFS) was measured from the time of diagnosis to first event (resistant disease, relapse, or death from any cause), whichever came first, or to last follow-up. Continuous CR (CCR) was measured from the date of achievement of CR to the date of relapse or last follow-up. Curves were constructed using the Kaplan-Meier method.42 SAS software (SAS Institute, Cary, NC) was used for the analysis.43
Results
Clinical characteristics
Table 1 reports the clinical and pathologic characteristics of the 203 patients with available cell material for MDR studies. This population was among the 286 patients enrolled in the GIMEMA ALL0496 study and evaluable at the present interim analysis. As reported in “Patients, materials, and methods,” the clinical correlation study was performed in 200 of 203 patients for MDR expression and in 156 of 158 patients for MDR function. Clinical characteristics and outcome of the 286 and 200 patients did not differ, as confirmed by the distribution of the following major variables: mean age (30.0 vs 32.9 years), B phenotype (81.5% vs 79.0%), Ph positivity (26.9% vs 26.0%), and CR achievement (75.2% vs 74.0%).
MDR1 protein expression and function
MDR1 protein expression was measured by MRK16 binding. Results were expressed as D values between 0.00 and 0.77. An example of the flow cytometric analysis is shown in Figure1. MDR1 expression was detected in 44 (21.7%) of 203 patients. In 158 of 203 samples, MDR1 function was also measured by the Rhd-123 efflux test (Figure2), which results in a ratio between 1.0 and 3.66. In 23 (14.6%) of 158 patients, Rhd-123 efflux was inhibited by the MDR modulator verapamil. A significant association between the 2 parameters was apparent when the data were dichotomized in positive and negative values (P = .001). Most patients with negative MDR1 expression were also negative for MDR1 function (115 of 128; 89.8%), whereas only 10 (33.3%) of 30 patients were positive for both (Table 2). In line with reports in adult AML patients,12 we also found patients with absent MDR1 expression and positive function and vice versa.
Flow cytometric detection of MDR1/P-gp expression.
Flow cytometric histograms of MDR1 expression in the VBL cell line by single staining with the MRK16 mAb (A), analyzed according to the Kolmogorov-Smirnov test (B). Dot plots of a representative ALL sample in which leukemic cells were identified by double staining with IgG2a/CD19 (C) and MRK16/CD19 (D). Histogram (E) made according to the method of Kolmogorov-Smirnov36 to compare fluorescence of the IgG2a isotypic control (black) with the MRK16 (white) mAb.
Flow cytometric detection of MDR1/P-gp expression.
Flow cytometric histograms of MDR1 expression in the VBL cell line by single staining with the MRK16 mAb (A), analyzed according to the Kolmogorov-Smirnov test (B). Dot plots of a representative ALL sample in which leukemic cells were identified by double staining with IgG2a/CD19 (C) and MRK16/CD19 (D). Histogram (E) made according to the method of Kolmogorov-Smirnov36 to compare fluorescence of the IgG2a isotypic control (black) with the MRK16 (white) mAb.
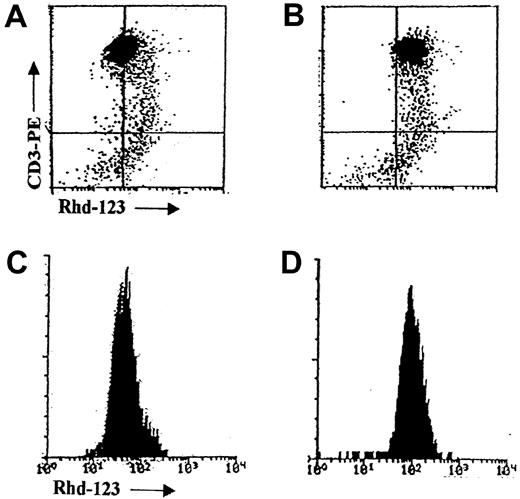
Flow cytometric detection of MDR1 function by rhodamine-123 efflux.
Flow cytometric dot plots and histograms of MDR1 function in a representative T-ALL sample as measured by the Rhd-123 efflux test without (A, C) and with (B, D) the verapamil MDR modulator.
Flow cytometric detection of MDR1 function by rhodamine-123 efflux.
Flow cytometric dot plots and histograms of MDR1 function in a representative T-ALL sample as measured by the Rhd-123 efflux test without (A, C) and with (B, D) the verapamil MDR modulator.
Relationship between MDR1 expression–function and achievement of CR
Dichotomized univariate analysis of MDR1 expression and function.
Table 3 shows the frequency distribution of the clinical and biologic characteristics investigated with respect to MDR1 expression. No significant difference was found between MDR1 expression and age, WBC values, or immunophenotypic, cytogenetic, or molecular characteristics. It is noteworthy that the presence of t(4;11), as assessed by cytogenetic and molecular analyses, was never found associated with MDR1 positivity (0 of 11 and 0 of 13 patients, respectively). Table 4 reports the CR rates with respect to the clinical and biologic characteristics investigated, including MDR1 expression and function expressed as discrete variables (in terms of negativity and positivity) in univariate analysis. In particular, patients who were negative for MDR1 expression had a significantly higher CR rate than those who were positive (79.6% vs 53.5%; P = .001). MDR1 function, in contrast, did not reach a significant correlation with CR (77.4% vs 60.9%; P = .09). Negative MDR1 expression in univariate analysis was the most significant predictor of achievement of CR and was more significant than CD34 positivity or age (P = .001 vs P = .004 and P = .031, respectively). It should be noted that among the 20 patients with positive MDR1 expression who did not achieve CR, 11 (55.0%) were clinically resistant, 7 died during induction, and 2 patients did not complete the induction therapy because of toxicity.
As shown in Figure 3, an association between negative MDR1 expression and achievement of CR was observed in the B- and T-cell subgroups (P = .044 andP = .001, respectively). Interestingly, among the 42 patients in the T-cell subgroup, the 4 patients who were positive for CD34 and MDR1 expression all failed to achieve CR, compared to only 1 of the 21 patients who were negative for both parameters (P = .001). With regard to the influence of the Ph chromosome, among the 148 Ph-negative patients (at molecular and cytogenetic analyses), CR was achieved in 98 (86%) of 114 of the MDR1 negative patients (P = .001). Among the 51 Ph-positive patients, CR was achieved in 4 (50%) of 8 of the MDR1-positive patients (P = .049). With regard to MDR1 function, the only significant relationship that emerged among the various pathologic subgroups regarded the T-cell subset, in which function positivity for MDR1 was strongly associated with absence of CR (P = .001).
Association between MDR1/P-gp expression and CR achievement in B- and T-ALL.
CR rates among B-lineage (A) and T-lineage (B) ALL patients with negative (black bar) or positive (white bar) MDR1 expression. Ninety-seven of 126 MDR-negative B-ALL patients achieved CR compared with 19 of 32 MDR-positive patients; 28 of 31 MDR-negative T-ALL patients achieved CR compared with 4 of 11 MDR-positive patients.
Association between MDR1/P-gp expression and CR achievement in B- and T-ALL.
CR rates among B-lineage (A) and T-lineage (B) ALL patients with negative (black bar) or positive (white bar) MDR1 expression. Ninety-seven of 126 MDR-negative B-ALL patients achieved CR compared with 19 of 32 MDR-positive patients; 28 of 31 MDR-negative T-ALL patients achieved CR compared with 4 of 11 MDR-positive patients.
Univariate analysis of MDR1 expression and function expressed as continuous variables.
When considered as a continuous variable, MDR1 expression was inversely correlated with CR rate (P = .010; OR, 15.261; 95% CI, 1.903-122.362). When considered in the same way, MDR1 function did not reach significance as a predictor of CR (P = .0988).
Multivariate analysis of MDR1 expression and function.
Multivariate analysis was performed to evaluate the impact of MDR1 expression and function—expressed as either dichotomized or continuous variables—on achievement of CR in a model containing the following pathologic parameters: age, WBC count, phenotype, CD34 expression, BCR/ABL, and t(4;11). Among the 174 patients for whom all the above data were available, when expressed as a dichotomized variable, MDR1 expression was an independent predictor of CR (P = .0036), together with age (P = .0342) and CD34 expression (P = .0282) (Table 5). Furthermore, when considered as a continuous variable, MDR1 expression retained its status as an independent predictor of CR (P = .0342), along with CD34 expression (P = .0126) (Table 5). By contrast, when tested among the 138 patients for whom the necessary data were available, MDR1 function (expressed as a dichotomized or a continuous variable) was not an independent predictor of CR (significant independent factors were age [P = .0324], phenotype [P = .0223], and CD34 [P = .0073]).
Relationship between MDR1 expression–function and CCR, EFS, and OS.
Among 148 evaluable ALL patients, 82 were in CCR, and the probability of remaining in CR at 2 years was 40%. Among patients in CCR, 67 (81.7%) were MDR1 negative. Sixty-six of 148 ALL patients had relapses; among these, 8 (12.1%) of 66 were MDR1 positive (P = .303). The only 2 parameters that influenced CCR were, in fact, BCR/ABL (P = .0002) and t(4;11) (P = .004). Multivariate analysis confirmed these results, showing that BCR/ABL (P = .0001), phenotype (P = .0022), CD34 (P = .0101), MDR1 (P = .0255), and t(4;11) (P = .0276) influenced EFS. CCR, in contrast, was affected only by BCR/ABL (P = .0002), t(4;11) (P = .0040), and phenotype (P = .0280). MDR function failed to show significance on EFS and CCR. In addition, MDR1 expression failed to predict OS duration (Figure 4).
OS according to MDR1 expression in ALL.
OS duration in MDR1-positive and MDR1-negative patients treated according to the GIMEMA ALL0496 protocol.
OS according to MDR1 expression in ALL.
OS duration in MDR1-positive and MDR1-negative patients treated according to the GIMEMA ALL0496 protocol.
MRP1 and LRP expression: achievement of CR
MRP1 flow cytometric expression ranged between 0 and 0.92; MRP1 positivity was found in 13 (30.2%) of 43 patients. LRP ranged between 0 and 0.79; LRP positivity was found in 26 (60.5%) of 43 patients. No significant difference was found between MRP1 and LRP expression and age, WBC values, or immunophenotypic, cytogenetic, or molecular characteristics. A correlation between the 2 parameters showed that most patients with negative MRP1 expression were also negative for LRP expression (22 of 30; 73.3%) and that only 8 (57.1%) of 14 patients were positive for both. Achievement of CR was not influenced by MRP1 or LRP expression. A trend was only found between MRP1 negativity and CR achievement (26 of 30 patients; 86.7%), whereas induction therapy failed in 5 (38.5%) of 13 MRP1-positive patients (P = .063).
Discussion
The design of the GIMEMA multicenter trial ALL0496 has enabled evaluation of the role of different prognostic parameters in adult ALL. An effort has been made to improve our understanding of the biologic features of this disease and to identify prognostic factors that could, ultimately, lead to the design of potential new therapeutic strategies for defined subgroups of patients. In the present study, our attention focused on MDR1 expression and function. In view of the well-known high inter-laboratory variability in MDR data,11 we centralized the MDR studies of the national trial in a single laboratory at our institution in Rome. As already reported,9,11 overnight shipment of clinical samples for MDR studies allowed adequate quality for protein and efflux studies. Similarly, comparability between samples accepted overnight and during the same day was, as in other studies,9,11 validated by internal tests (data not shown). Samples were processed and analyzed according to the consensus guidelines on MDR studies.10,11,44 To evaluate MDR1 function, verapamil was chosen as the specific MDR1 modulator; unlike cyclosporin A, it does not exert cytotoxic effects on T-ALL cells.36,45 The Kolmogorov-Smirnov test was used to evaluate MDR1 expression, as recommended for clinical sample analysis.11,36,46 Results were analyzed as continuous and dichotomized variables, as recommended.9 11
Multicenter studies on the prevalence of MDR1 protein expression and function in large series of adult ALL patients are lacking. Among the recently published multicenter trials,18-24 several clinical and biologic factors were evaluated as prognostic features, but none included in the analysis MDR expression or function data. Few reports6,8,27 from single-center studies have evaluated in adult ALL patients the prognostic role of MDR1. Only 2 studies6,27 (one also included pediatric patients) pointed to the unfavorable role of P-gp expression on CR achievement and duration, but no correlation was reported in the other report.8 Our multicenter study documents for the first time that approximately one third of de novo adult ALL patients expressing MDR1 at the protein level have a significantly lower likelihood of achieving CR. In fact, in the context of the therapeutic protocol used, MDR1 protein expression (as opposed to function) predicted CR achievement when considered either as a dichotomized or a continuous variable. Indeed, when a multivariate model including age, WBC, phenotype, CD34 expression, BCR/ABL, and t(4;11) was applied to patients with a minimum follow-up of 6 months, MDR1 expression proved to be an independent predictor of CR; the only other significant factors were age and CD34 expression, and this was true of the latter only when MDR1 expression was considered a dichotomized variable. Thus far, MDR1 expression has not affected the duration of CR, suggesting a role for this mechanism in the initial debulking phase rather than in the subsequent depletion of leukemic cells during the postinduction phase. In fact, other biologic factors—high WBC counts, BCR/ABL, and of t(4;11)—may play a role in ALL disease recurrence. Furthermore, MDR expression failed to affect OS duration. It may be hypothesized that MDR-positive patients, though not achieving first CR, may respond to second-line regimens based on non–cross-reactive drugs. In fact, different drugs (intermediate- to high-dose Ara-C) included in our postinduction treatment may reduce the value of MDR resistance on refractory patients, as suggested for AML.12
No significant association was found between MDR1 expression and any of the other clinical or biologic features considered. Interestingly, MDR1 expression was never found in the 13 patients with t(4;11), furnishing an additional reason for the good initial CR rate of this genetically defined subgroup of ALL.25 A similar observation was made in the small group of patients characterized by 6q deletion reported in this study. In addition and in agreement with an earlier report,47 patients expressing a T-cell phenotype showed a slightly higher frequency of MDR1 positivity than those with a B-cell subset (33% vs 25%). Induction treatment failed in the 4 T-ALL patients who expressed MDR1 and CD34. Not surprisingly, MDR1 protein function, evaluated by Rhd123 efflux, generally had less predictive significance than MDR1 expression. Apart from the bias caused by the lower number of samples available for evaluation, the comparison between MDR expression and function on CR achievement resulted in our study on adult ALL cells in a better correlation of the first because this assay represents a specific test for the detection of the MDR1-encoded protein P-gp 170, whereas the functional test may detect other efflux proteins (eg, mitochondrial ones) and MDR-related proteins (ie, MRP), with a more relevant role in the lymphoid blast cell; this result may thus explain the lack of correlation between the functional test and CR achievement. A previous report48 has documented a lack of strict correlation between function and expression in ALL, compared with AML, suggesting that such discrepancy may characterize ALL cells. In our report, we found discordance between the 2 tests in 20% of patients.
The use of a 0.05 cut-off threshold for MDR1 expression was arbitrarily chosen in our study (and in others); the use of a higher cut-off (D = 0.10) point further confirmed the significant (P = .001) correlation between MDR expression and CR achievement, suggesting that the use of the first cut-off value allowed a more sensitive test in the presence of an equal significance level. Because the frequency of cases with positive MDR1 function might have been influenced by the choice of cut-off points, the validation of our study was finally obtained by continuous variable analysis of MDR expression.
The present study clearly indicates that the detection of MDR1 expression appears to represent a significant prognostic indicator in adult ALL. However, it should be underscored that our results cannot automatically be extended to adult ALL patients treated with different protocols. The main rationale of the GIMEMA ALL0496 treatment protocol was to achieve more rapid destruction of leukemic cells before the onset of resistance through the administration of high-dose daunorubicin in the early phase of induction, followed by high-dose Ara-C as early postremission therapy. A previous study49has demonstrated in AML cells the MDR1 gene up-regulation caused by anthracycline exposure and reverted by adding the cyclosporine analogue PSC 833. The intensive use of daunorubicin in our protocol could conceivably have accentuated in de novo ALL the unfavorable prognostic significance of MDR1 expression. A potential application of including MDR1 detection in the diagnostic biologic screening of ALL could be, therefore, to help choose the therapeutic approach for individual patients within this and other protocols similarly characterized by an unfavorable impact of MDR expression. Selective use of anti-MDR strategies (modulators in association with daunorubicin, anti–MDR-blocking antibodies) or the adoption of protocols based on drugs that are not P-gp substrates may offer therapeutic advantages for CR achievement for adult ALL patients who express the MDR1 protein.
Enrollment of a higher number of patients and longer follow-up time will allow definition of the role of P-gp on CR duration. In addition, the prognostic impact in adult ALL of other MDR-related proteins, such as MRP1 and LRP, must be further evaluated. A recent study50 in lymphoproliferative disorders, including adult acute T-ALL, has shown a correlation between shorter survival and LRP mRNA expression.
In summary, these results demonstrate that MDR1 expression in adult de novo ALL is an independent predictor of CR achievement. Our study shows the successful applicability of MDR detection in fresh samples from a multicenter trial. The inclusion of this parameter in the screening of other biologic features, usually already accepted as disease–therapy indicators in adult ALL, may result in the design of a broader, biologically based, risk-adapted therapeutic strategy for the management of adult ALL.
We thank Francesco Mazzola for technical assistance and Robin M. T. Cooke for manuscript editing.
Prepublished online as Blood First Edition Paper, April 30, 2002; DOI 10.1182/blood-2001-12-0371.
Supported by Istituto Superiore di Sanità, Rome, Ministero dell'Istruzione, dell'Università e della Ricerca (MIUR), Associazione Italiana contro le Leucemie, sezione di Roma (ROMAIL) and sezione di Salerno, Associazione Italiana per le Ricerca sul Cancro (AIRC), Italy.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Agostino Tafuri, Department of Biotecnologie Cellulari ed Ematologia, University La Sapienza of Rome, Via Benevento 6, 00161 Rome, Italy; e-mail:agotaf@bce.med.uniroma1.it.