Lymphoblastoid cell lines (LCLs) are human B cells latently infected and immortalized by Epstein-Barr virus (EBV). Presenting viral antigens, they efficiently induce EBV-specific T-cell responses in vitro. Analogous ways to generate T-cell cultures specific for other antigens of interest are highly desirable. Previously, we constructed a mini-EBV plasmid that consists of less than half the EBV genome, is unable to cause virus production, but still immortalizes B cells in vitro. Mini-EBV–immortalized B-cell lines (mini-LCLs) are efficiently produced by infection of B cells with viruslike particles carrying only mini-EBV DNA. Mini-EBV plasmids can be engineered to express an additional gene in immortalized B cells. Here we present a mini-EBV coding for a potent CD8+ T-cell antigen, the matrix phosphoprotein pp65 of human cytomegalovirus (CMV). By means of this pp65 mini-EBV, pp65-expressing mini-LCLs could be readily established from healthy donors in a one-step procedure. We used these pp65 mini-LCLs to reactivate and expand effector T cells from autologous peripheral blood cells in vitro. When generated from cytomegalovirus (CMV)–seropositive donors, these effector T-cell cultures displayed strong pp65-specific HLA-restricted cytotoxicity. A large fraction of CD8+ T cells with pp65 epitope specificity was present in such cultures, as demonstrated by direct staining with HLA/peptide tetramers. We conclude that the pp65 mini-EBV is an attractive tool for CMV-specific adoptive immunotherapy. Mini-EBVs could also facilitate the generation of T cells specific for various other antigens of interest.
Introduction
Epstein-Barr virus (EBV), a ubiquitous human herpesvirus, has the unique ability to infect and subsequently immortalize human B cells in vitro with high efficiency, leading to the outgrowth of permanent lymphoblastoid cell lines (LCLs).1LCLs are valuable immunologic tools for several reasons. The majority of cells in an LCL are latently infected and constitutively express 9 EBV latent genes.2,3 Most of the EBV latent proteins elicit T-cell responses in vivo. Thus LCLs, which have good antigen-processing function, present immunogenic EBV peptides in complexes with HLA class I molecules at their surface. In addition, LCLs express costimulatory molecules like B7.1 (CD80) and B7.2 (CD86) and adhesion molecules like intercellular adhesion molecule 1 (ICAM-1; CD54) and leukocyte function-associated antigen 3 (LFA-3; CD58), which improve interaction with T cells.4 As a result, LCL cells efficiently reactivate and expand EBV-specific T cells from cultured peripheral blood mononuclear cells (PBMCs) of EBV+donors.5 LCL-stimulated T-cell cultures have been valuable tools to investigate the EBV-specific T-cell response. Moreover, clinical protocols using LCL-expanded EBV-specific T cells for adoptive transfer have been shown to be beneficial in bone marrow transplant recipients at risk for EBV lymphoproliferative disease,6and their use for solid organ transplant recipients and patients suffering from EBV-related malignancies is under clinical evaluation.7 8
Due to the ease of their generation and cultivation, LCLs have also been widely used as target cells or stimulator cells to investigate CD8+ T-cell responses against other antigens. For such purposes, it is possible to load LCL cells with the antigenic peptide, which binds to major histocompatibility complex (MHC) molecules on the cellular surface, to supply the antigen as an exogenous protein for reprocessing within the cell,9 to infect LCL cells with wild-type or recombinant viruses expressing the antigen of choice,10-12 or (albeit inefficiently) to transfect LCLs with antigen-coding plasmid vectors.13-15 All of these protocols have drawbacks, however, stemming either from the difficulty of making pure antigen preparations or from the delivery of irrelevant viral proteins within viral vector preparations. Such protocols require performing at least 2 subsequent procedures, establishing the LCL and providing the antigen. In addition, an inherent problem when using conventional LCLs in clinical protocols is the reactivation of the lytic cycle in some of the LCL cells, leading to the release of significant quantities of infectious EBV.16
Therefore, we sought for a way to directly generate B-cell lines that are free of infectious virus and constitutively express foreign antigens of interest. Previously, we showed that it is possible to generate immortalized cell lines, so-called mini-LCLs, by transfection of primary B cells with a mini-EBV plasmid. Thus, the B- cell–transforming functions can be provided by plasmid DNA containing no more than 71 kb EBV genomic sequences, that is, 41% of the EBV genome, encompassing the 11 latent genes, but lacking many of the lytic genes essential for virus replication.13,17 Furthermore, the development of an EBV-packaging cell line allowed such a transformation-competent, replication-deficient mini-EBV genome to be provided in the form of a helper virus-free virion preparation that efficiently infects primary B cells, yielding mini-LCLs.18Mini-EBVs can accommodate and express additional genes,4and so we planned to introduce a foreign antigen into a mini-EBV plasmid, to generate virus-free mini-LCLs expressing this antigen, and to test the ability of these cells to induce antigen-specific T-cell responses. As a model foreign antigen, we chose the major matrix protein pp65 (UL83) of human cytomegalovirus (CMV), for a variety of reasons: the majority of healthy adults are CMV seropositive, the pp65-specific CD8+ T-cell response is strong and well characterized at the epitope level for a number of HLA alleles,19 and we recently developed MHC-peptide tetrameric complexes allowing direct staining of T cells specific for a number of immunodominant pp65 epitopes.20,21 In addition, CMV is a significant clinical problem in immunocompromised patients; therefore, it is important to optimize methods for the generation of CMV-specific T-cell preparations in vitro because adoptive therapy with such preparations may be of great clinical benefit.22 23
Materials and methods
Standard cell culture medium was RPMI-1640 with 10% fetal calf serum, penicillin (100 U/mL), and streptomycin (100 μg/mL; all components from Biochrom, Berlin, Germany). Medium was supplemented as follows: for T-cell culture, with interleukin 2 (IL-2) as specified; for B-cell immortalization, with cyclosporin A as specified; for cultivation of the packaging cell line TR−2/293, with hygromycin (100 μg/mL).
Mini-EBVs
The pp65 mini-EBV plasmid was constructed by inserting an expression cassette encoding pp65 into the mini-EBV plasmid 1478.A using the chromosomal building technique, as described.4The pp65 coding sequence from CMV strain AD169 was first inserted into the cloning site of the expression plasmid pSG-5 (Stratagene, La Jolla, CA). The pp65 expression cassette including CMV promotor, β-globin intron, and polyadenylation signal was then excised, inserted into shuttle plasmid p1242.1, and finally transferred to plasmid 1478.A by a series of homologous and site-directed recombination events. The correct structure of the resulting plasmid, termed pp65 mini-EBV, was confirmed by restriction digests.
Infectious virions carrying mini-EBV DNA were produced by transfection of mini-EBV DNA into the first-generation EBV-packaging cell line TR−2/293. This cell line stably carries a nonpackageable EBV genome.18 Packaging cells were grown to semiconfluency; then, per 10-cm dish, 12 μg mini-EBV DNA and 6 μg plasmid p509, carrying an expression cassette for the EBV lytic transactivator BZLF1, was transfected into cells using Fugene reagent (Roche, Indianapolis, IN). Supernatants were repeatedly harvested and replaced by fresh culture medium on days 3, 4, and 5 after transfection. Supernatants were concentrated 10-fold by pelleting virions at 15 000g for 2 hours and resuspending in culture medium. Virion concentrates were stored frozen at −80°C.
Mini-LCLs
Mini-LCLs were generated by infection of B cells with virion-packaged mini-EBV. Mononuclear cells were freshly isolated from peripheral blood samples from healthy human donors by centrifugation of diluted heparinized blood on a Ficoll cushion and harvesting interphase cells. Part of these PBMCs was cryoconserved for later use in T-cell reactivation. From each donor, about 10 million cells were used to generate pp65 mini-LCLs and control cell lines. Half a million cells in 100 μL medium were seeded per well of a 96-microwell flat bottom plate, and 50 μL of either concentrated pp65 mini-EBV, concentrated control mini-EBV, supernatant of the EBV strain B95.8 producer cell line (positive EBV immortalization control), or culture medium (endogenous infection control) was added. These cultures were maintained by replacing half of the supernating medium by fresh medium every 4 to 7 days. For the first 4 weeks of cultivation, medium was supplemented with cyclosporin A (0.5 μg/mL). Outgrowth of immortalized cells was first visible after 3 to 6 weeks, when cell aggregates of spherical shape appeared. Cells were then carefully expanded. Sufficient cells to start a first T-cell restimulation cycle were usually obtained 5 to 8 weeks after in vitro mini-EBV infection.
Each mini-LCL was checked by polymerase chain reaction (PCR)4 for the presence of a mini-EBV-specific sequence (chloramphenicol acetyltransferase [cam]) and absence of a wild-type EBV-specific sequence (viral glycoprotein gp85) using primers cam-up (5′-TTC TGC CGA CAT GGA AGC CAT C-3′), cam-down (5′-GGA GTG AAT ACC ACG ACG ATT TCC-3′), gp85c (5′-TGG TCA GCA GCA GAT AGT GAA CG-3′), and gp85d (5′-TGT GGA TGG GTT TCT TGG GC-3′), performing 30 cycles of amplification with 45 seconds each of denaturation at 96°C, primer annealing at 59°C, and DNA synthesis at 72°C.
Mini-LCLs were checked for pp65 expression by intracellular staining. Cells were fixed at 4°C for 30 minutes with 0.25% paraformaldehyde/phosphate-buffered saline (PBS), permeabilized at 37°C for 15 minutes with 0.2% Tween-20/PBS, stained on ice with anti-pp65 monoclonal antibody (clone 981, purchased from Biodesign [Saco, ME], or clone 65-33, courtesy of William Britt [Birmingham, AL]), which was detected with fluorescein isothiocyanate (FITC)–conjugated antimouse secondary antibody.
Reactivation and analysis of T cells
T cells were derived from peripheral blood of the following donors: F14 (HLA-A1, A2, B13, B62), F16 (A1, A23, B8, B44), F22 (A11, A26, B49, B53), donor no. 1 (A2, A32, B27), donor no. 2 (A2, A11, B35, B44), donor no. 3 (A1, A2, B16, B40), donor no. 4 (A2, A24, B27.05, B35), and donor no. 5 (A2, A24, B44, B51). Donor no. 1 was CMV-seronegative and EBV-seropositive, donor no. 2 was CMV-seropositive and EBV-seronegative, and donor nos. 3, 4, and 5 were seropositive for both viruses.
T cells were reactivated from cryoconserved or freshly isolated autologous PBMCs by restimulation with the irradiated autologous pp65 mini-LCL or control mini-LCL. The protocol was adapted from standard procedures.7 Per well of a 24-well plate, 2 million PBMCs and 5 × 104 irradiated mini-LCL cells (50 Gy) were cocultivated in 2 mL medium. First on days 8 to 10, and later in intervals of 7 to 10 days, cells were pooled, counted, and replated at 1 million cells/2 mL medium per well, adding freshly irradiated mini-LCL cells as stimulators at an effector-stimulator ratio of 4:1. Cells were refed or expanded at least every 3 days, either exchanging half of the supernatant by fresh medium or adding an equal volume of fresh medium. From day 15 onward, culture medium was supplemented with IL-2 (6 U/mL; Boehringer Mannheim).
The HLA/peptide tetrameric complexes representing CMV and EBV epitopes were prepared as described.25 26 T cells were stained by incubating with phycoerythrin (PE)–labeled tetramer for 20 minutes at 37°C and counterstaining with FITC-labeled CD8 antibody and peridinin chlorophyll protein (PerCP)–labeled CD3 antibody (Pharmingen/Becton Dickinson, San Diego, CA) on ice for 30 minutes. Cells were washed and analyzed immediately on a Coulter Epics flow cytometer. For analysis, viable lymphocytes were gated in a forward/sideward scatter dot plot. Data analysis was performed using CellQuest software (Becton Dickinson). Tetramers representing six pp65 or EBV epitopes were used in this study. Tetramers and epitopes are designated by the first few letters of the epitope's single-letter coded amino acid sequence. Their short designations, amino acid sequences, antigens of derivation, positions of the epitope in the antigen's amino acid sequence, and HLA restrictions are as follows: NLV (NLVPMVATV, pp65 [495-503], A2-restricted); IPS (IPSINVHHY, pp65 [123-131], B35-restricted); CLG (CLGGLLTMV, LMP2 [426-434], A2-restricted); RRIY (RRIYDLIEL, EBNA3C [258-266], B27-restricted); YPL (YPLHEQHGM, EBNA3A [458-466], B35-restricted); and HPV (HPVGEADYFEY, EBNA1 [407-417], B35-restricted).
In general, cytotoxic activities of T cells were analyzed in chromium release assays. Target cells (mini-LCLs or fibroblasts, the latter after infection with CMV or recombinant vaccinia) were labeled with 40 μCi (1.48 MBq) 51CrOfor 60 minutes at 37°C, washed, and coincubated with effector cells. Per well of a v-shaped bottom 96-well plate, 2500 chromium-loaded target cells and a defined excess number of effector cells were coincubated, in triplicate, in a volume of 200 μL medium. After 4 hours at 37°C, 100 μL of the supernatants was harvested and mixed with scintillation liquid (MicroScint-40, Packard, Meriden, CT); and radioactivity was measured in a scintillation counter (TopCount, Packard). Results were expressed as percent specific lysis, in relation to spontaneous release (no effectors added to target cells) defined as 0% specific lysis and maximum release (0.5% Triton X-100 added to target cells) defined as 100% specific lysis.
In some instances, cytotoxicity was assessed by TDA (2,2′:6′,2"-terpyridine-6,6"-dicarboxylate) release.27 Target cells were labeled with the chelate ligand BATDA (bis[acetoxymethyl]-TDA), which is deacetylated inside the cell, yielding TDA, and retained until cell lysis occurs. Released TDA is converted into its europium(III) complex and quantified by time-resolved fluorometry. Labeling and assaying were performed according to the manufacturer's instructions (Wallac, Turku, Finland).
Stocks of CMV strain AD169 were prepared by infection of MRC-5 human fetal lung fibroblasts at 0.1 viruses per cell. When most cells appeared cytopathic, supernatants were harvested, cleared from cellular debris by centrifugation, and stored at −80°C. Virus titer was determined by infection of MRC-5 fibroblasts in 96-well plates with virus stocks at limiting dilution. Infected wells were visually identified after the infection had spread over the whole well (no earlier than 2 weeks after infection). CMV-infected target cells for cytotoxicity assays were prepared by infection of human fibroblasts at 5 to 10 infectious units per cell for 12 hours prior to labeling with chromium.
A recombinant vaccinia virus expressing pp65 was constructed as described28 using vaccinia virus vRB12 and transfer vector vRB21 (both kindly provided by Bernard Moss, Bethesda, MD). Target cells for cytotoxicity assays (fibroblasts or mini-LCLs) were infected at 10 viruses per cell for 15 hours prior to labeling with chromium. As a control, the recombinant vaccinia10vacc-TK was used in parallel.
Results
Construction of a pp65 mini-EBV plasmid and B-cell immortalization
To construct a pp65 mini-EBV, we used the chromosomal building technique24 to insert a pp65 expression cassette into the mini-EBV plasmid p1478.A. This mini-EBV had been shown to immortalize B cells, yielding virus-free mini-LCLs.4 A map of the pp65 mini-EBV is presented in Figure 1A. By various restriction digests, this plasmid was confirmed to be identical to 1478.A, except that it carried the pp65 expression cassette. We used the first-generation EBV-packaging cell line18TR2−/293 to produce infectious mini-EBV virions, transfecting either pp65 mini-EBV DNA or control mini-EBV DNA (plasmid 1478.A) into producer cells, together with an expression plasmid for an EBV lytic cycle inducer. Virion-containing culture supernatants of this packaging cell line were used to infect primary human B cells from PBMC preparations. After 3 to 5 weeks of cultivation, proliferating cells occurred, which could be continually recultivated and expanded. This way, pp65 mini-LCLs were established from PBMCs of 8 of 9 healthy donors and control mini-LCLs from 9 of 9 donors.
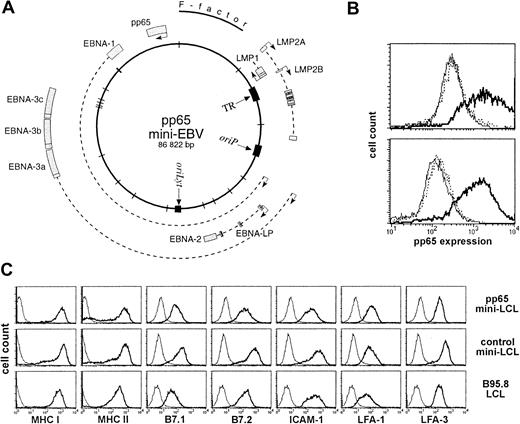
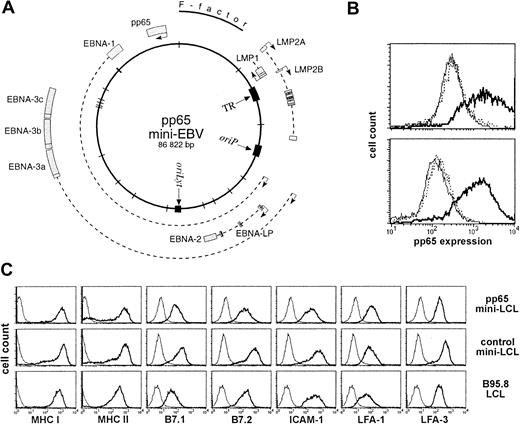
A mini-EBV plasmid for immortalization of B cells and expression of pp65.
(A) Map of the pp65 mini-EBV plasmid. The pp65 gene is constitutively expressed from an SV40 promoter. (B) Expression of pp65 in mini-EBV–immortalized B-cell lines (mini-LCLs). A pp65 mini-LCL (solid line), a control mini-LCL (thin line), and an EBV B95.8 LCL (dotted line) from the same donor were fixed, permeabilized, stained with monoclonal pp65 antibody 981, and analyzed on a flow cytometer. Upper plot, cell lines from donor a41; lower plot, cell lines from donor F22. (C) Surface expression of MHC class I and II, B7.1 and 2, LFA-1, LFA-3, and ICAM-1 on a pp65 mini-LCL, a control mini-LCL, and a B95.8 LCL from donor no. 4.
A mini-EBV plasmid for immortalization of B cells and expression of pp65.
(A) Map of the pp65 mini-EBV plasmid. The pp65 gene is constitutively expressed from an SV40 promoter. (B) Expression of pp65 in mini-EBV–immortalized B-cell lines (mini-LCLs). A pp65 mini-LCL (solid line), a control mini-LCL (thin line), and an EBV B95.8 LCL (dotted line) from the same donor were fixed, permeabilized, stained with monoclonal pp65 antibody 981, and analyzed on a flow cytometer. Upper plot, cell lines from donor a41; lower plot, cell lines from donor F22. (C) Surface expression of MHC class I and II, B7.1 and 2, LFA-1, LFA-3, and ICAM-1 on a pp65 mini-LCL, a control mini-LCL, and a B95.8 LCL from donor no. 4.
Characterization of pp65 mini-LCLs
To confirm that these cell lines were mini-LCLs, we verified by PCR the presence of DNA sequences specific for mini-EBV (cam) and the absence of EBV sequences specific for wild-type EBV (glycoprotein gp85).4 The pp65 expression of pp65 mini-LCLs was confirmed by intracellular staining. Figure 1B shows the flow cytometry profiles of pp65 mini-LCLs from 2 donors in comparison to control mini-LCLs and standard EBV strain B95.8 LCLs from the same individuals. Some of the pp65 mini-LCLs were kept in culture for more than 6 months, and no change nor loss of pp65 expression was observed. The expression of MHC, costimulatory, and adhesion molecules on the surface of pp65 mini-LCL cells was strong and comparable to control mini-LCLs and wild-type EBV LCLs (Figure 1C).
Stimulation of T cells with pp65 mini-LCLs
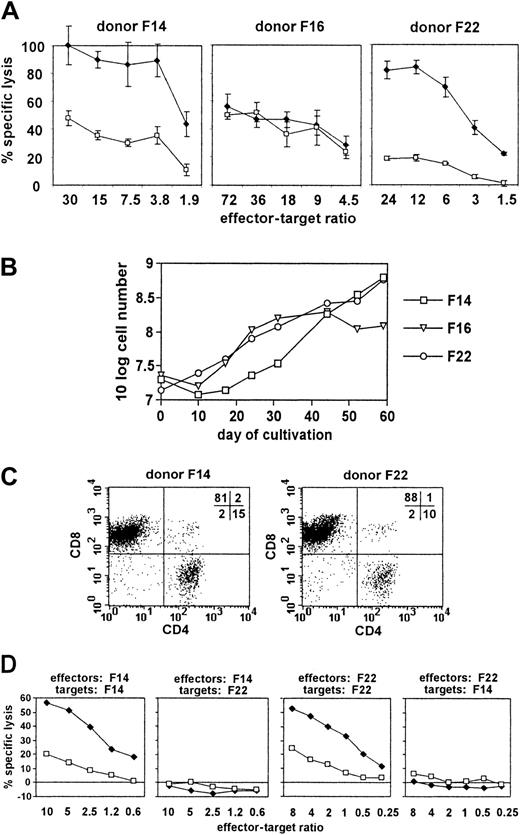
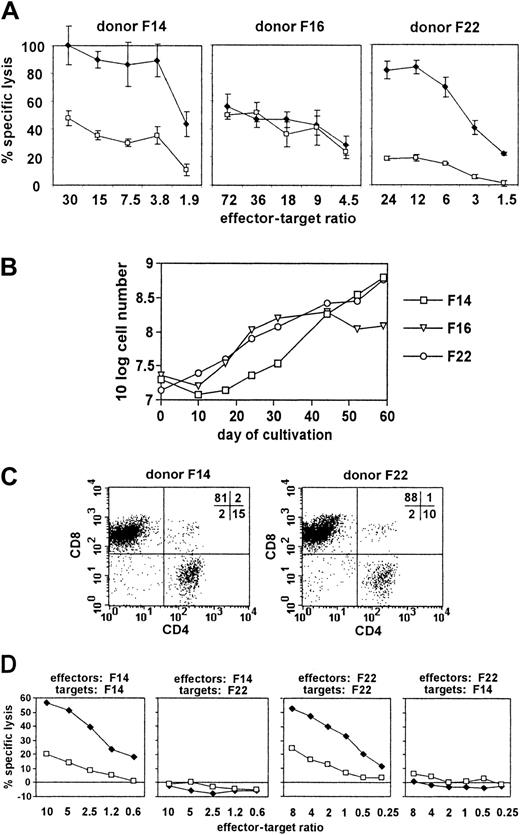
Next, we attempted to stimulate T cells in vitro with autologous pp65 mini-LCLs. Sufficient stimulator cells were usually available 5 to 8 weeks after mini-EBV infection. We adapted a protocol that is currently used to generate EBV-specific polyclonal T-cell populations from PBMCs by LCL restimulation for application in adoptive immunotherapy of EBV-associated malignant disease.7 In our initial experiments, we used peripheral blood buffy coats from 3 randomly chosen anonymous blood donors. We generated pp65 mini-LCLs and control mini-LCLs from these 3 donors, used the pp65 mini-LCLs to restimulate autologous PBMCs, and analyzed the cytotoxic activity of the resulting cell populations against both kinds of mini-LCLs in an autologous setting (Figure 2A). Cultures from 2 of the 3 randomly chosen donors, F14 and F22, displayed a much more pronounced reactivity against the autologous pp65 mini-LCL than against the autologous control mini-LCL. The cells from the third donor, F16, however, lysed pp65 and control mini-LCLs without difference. We maintained and expanded the cytotoxic cell cultures by periodic restimulation with pp65 mini-LCLs, and observed that the cultures of those 2 donors suspected of a pp65-specific reactivity could be readily and continually expanded over a period of 60 days (Figure 2B). Flow cytometric analysis made clear that the cultures were composed of T cells, mainly of the CD8+ phenotype (Figure2C). HLA typing of F14 and F22 showed these 2 donors not to share any HLA class I alleles. To check for a possible class I–restricted cytotoxic reactivity, we compared the lysis of mini-LCLs from F14 and F22 by effector cells from the same and the other donor (Figure 2D). We found in both cases that autologous pp65 mini-LCLs were subject to strong cytotoxicity, autologous control mini-LCLs were attacked less strongly, and allogeneic pp65 mini-LCLs as well as allogeneic control mini-LCLs were not lysed at all. These preliminary results induced us to hypothesize that (1) restimulation of PBMCs by pp65 mini-LCLs might result in cytotoxic T-cell cultures with predominant HLA class I–restricted pp65 specificity in some cases; (2) this result might be favored when the donor is likely to have a CMV-specific T-cell memory, that is, is CMV-seropositive; (3) these pp65-specific T cells might be readily proliferating in such cultures; and (4) the pp65-specific reactivity of the T-cell cultures might cause a stronger cytotoxicity against pp65 mini-LCLs than against control mini-LCLs. To verify these hypotheses and facilitate a closer analysis, we turned toward an HLA-typed panel of healthy donors with characterized CMV and EBV serostatus.
Characterization of pp65 mini-LCL–stimulated T cells from 3 randomly selected healthy donors.
(A) Cytotoxic activity of pp65 mini-LCL–stimulated PBMC cultures from donors F14, F16, and F22 against autologous pp65 mini-LCLs (♦) or control mini-LCLs (■) as determined in a TDA release assay on day 17 of culture. (B) Proliferation of these cultures. (C) Flow cytometry analysis of the expression of CD4 and CD8 in cultures from donors F14 and F22 on day 33 of culture. (D) Cytotoxic activity of pp65 mini-LCL–stimulated cultures from donors F14 and F22 against autologous or allogeneic pp65 mini-LCLs (♦) or control mini-LCLs (■). Chromium release assays were performed on day 42 (F14 effectors) or on day 62 (F22 effectors) of culture.
Characterization of pp65 mini-LCL–stimulated T cells from 3 randomly selected healthy donors.
(A) Cytotoxic activity of pp65 mini-LCL–stimulated PBMC cultures from donors F14, F16, and F22 against autologous pp65 mini-LCLs (♦) or control mini-LCLs (■) as determined in a TDA release assay on day 17 of culture. (B) Proliferation of these cultures. (C) Flow cytometry analysis of the expression of CD4 and CD8 in cultures from donors F14 and F22 on day 33 of culture. (D) Cytotoxic activity of pp65 mini-LCL–stimulated cultures from donors F14 and F22 against autologous or allogeneic pp65 mini-LCLs (♦) or control mini-LCLs (■). Chromium release assays were performed on day 42 (F14 effectors) or on day 62 (F22 effectors) of culture.
Analysis of T-cell cultures from a CMV-seronegative, EBV-seropositive donor
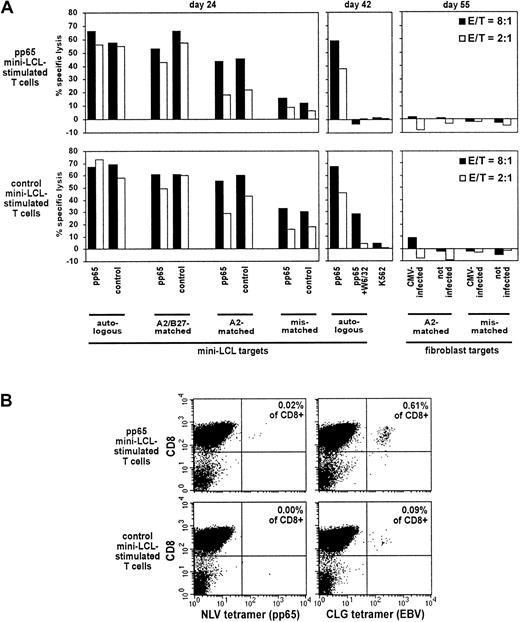
We generated T-cell cultures by restimulation of PBMCs from CMV-seronegative, EBV-seropositive donor no. 1 (HLA-A2, B27, B32) by restimulation with the autologous pp65 mini-LCL or with the autologous control mini-LCL. We analyzed the cytotoxic reactivity of both T-cell populations against a panel of autologous, HLA class I–matched and class I–mismatched pp65 mini-LCLs and control mini-LCLs (Figure3A). We observed a decrease in lysis with decreasing HLA matching. This applied to pp65 mini-LCL–stimulated T cells as well as to the control effector population. Among each pair of a pp65 and a control mini-LCL from a given donor, however, lysis was equal, suggesting a cytotoxic activity directed against antigens equally presented on both kinds of target cells, most likely EBV latent antigens. Cytotoxic activity was largely inhibited by the class I–specific antibody, W6/32, which further argued in favor of an HLA class I–restricted lysis. To check for cytotoxicity toward CMV antigens in cells free of EBV antigens, we used HLA-A2–matched and HLA-A2–mismatched CMV-infected fibroblasts as targets. This experiment did not disclose any cytotoxic reactivity, indicating that pp65-specific cytotoxic T cells restricted through HLA-A2 were absent.
Properties of pp65 mini-LCL–stimulated and control mini-LCL–stimulated T cells from the CMV−, EBV+ donor no. 1.
(A) The cytotoxic activity of pp65 mini-LCL–stimulated polyclonal T cells (upper row) and control mini-LCL–stimulated T cells (lower row) from donor no. 1 against diverse target cells was assayed by chromium release on days 24, 42, and 55, respectively. (B) Tetramer analysis of the T-cell cultures using a pp65 epitope tetramer (NLV) and an EBV epitope tetramer (CLG), both HLA-A2-restricted, performed on day 35 of culture. E/T indicates effector-target ratio.
Properties of pp65 mini-LCL–stimulated and control mini-LCL–stimulated T cells from the CMV−, EBV+ donor no. 1.
(A) The cytotoxic activity of pp65 mini-LCL–stimulated polyclonal T cells (upper row) and control mini-LCL–stimulated T cells (lower row) from donor no. 1 against diverse target cells was assayed by chromium release on days 24, 42, and 55, respectively. (B) Tetramer analysis of the T-cell cultures using a pp65 epitope tetramer (NLV) and an EBV epitope tetramer (CLG), both HLA-A2-restricted, performed on day 35 of culture. E/T indicates effector-target ratio.
For phenotypic analysis of the T-cell cultures, we used HLA/peptide tetrameric complexes specific for the HLA-A2–restricted pp65 epitope NLV, often observed to be immunodominant in CMV-infected A2+ individuals,20 and the EBV latent membrane protein LMP2 epitope CLG, known to elicit reactivities that are usually subdominant but widely detected.29 We found low numbers of CLG-specific cells in the T-cell cultures from donor no. 1 (Figure 3B). However, NLV-specific cells were absent, strengthening the conclusion that the cytotoxic T-cell cultures generated from this CMV− donor were not pp65 specific.
Analysis of T-cell cultures from a CMV-seropositive, EBV-seronegative donor
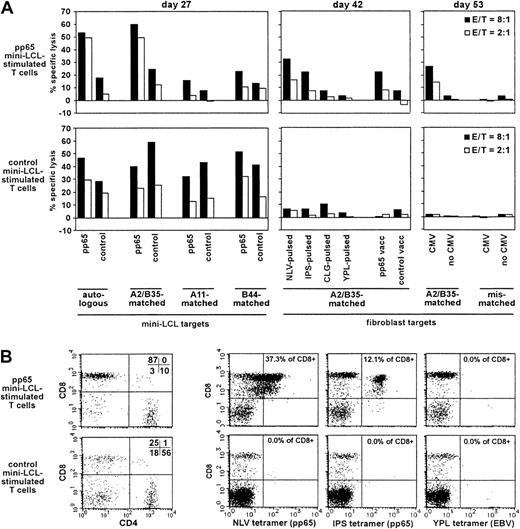
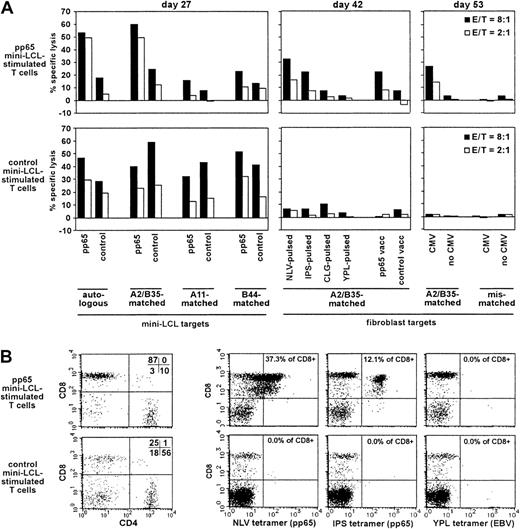
We presumed that the detection of pp65-specific T cells generated with our procedure should be easiest when departing from primary cell populations unlikely to contain responsive EBV-specific memory T cells, which might outnumber or overgrow the pp65-specific cells on restimulation. Therefore, we generated and analyzed T-cell cultures from the CMV-seropositive, EBV-seronegative donor no. 2 (HLA-A2, A11, B35, B44). The pp65 mini-LCL–restimulated cultures exhibited a strong cytotoxic activity against pp65-expressing mini-LCLs, which were autologous or HLA-A2/B35–matched (Figure4A). Reactivity against mini-LCLs lacking these class I alleles was much weaker, as was reactivity against mini-LCLs lacking pp65. Consistent with these observations, HLA-A2/B35–matched, but not mismatched, fibroblasts that expressed pp65 due to previous infection with a recombinant pp65-vaccinia or with CMV itself were lysed by these T cells. Experiments with peptide-loaded fibroblasts indicated which epitopes were targeted by this pp65-specific reactivity, namely, the CMV epitopes NLV (HLA-A2 restricted) and IPS (HLA-B35 restricted). In contrast, target cells loaded with EBV epitopes (CLG, HLA-A2, and YPL, HLA-B35) did not attract significant cytotoxic activity. Analogous experiments were performed with a control mini-EBV–restimulated T-cell population. Fibroblasts expressing pp65 after CMV or recombinant vaccinia infection, or loaded with CMV or EBV peptide epitopes, were not lysed by these control T cells. Mini-LCL targets, however, were subject to lysis independently of HLA type or pp65 expression, a result concordant with the non–HLA-restricted cytotoxic reactivity often observed in standard EBV LCL-restimulated cultures from EBV-seronegative donors, accompanied by the expansion of nonspecifically reactive CD4+ T cells.5 Remarkably, this nonspecific reactivity was not evident in the pp65-specific culture from the same donor. In fact, the pp65 mini-LCL–stimulated T-cell culture consisted mainly of CD8+ cells, whereas CD4+ cells predominated in the control T-cell population (Figure 4B). Consistent with the cytotoxicity data, NLV and IPS tetramer-staining T cells could readily be detected in the pp65 mini-LCL–stimulated T-cell culture, making up for almost 50% of the cells, but were not detected in the control population. Neither T-cell culture contained T cells binding a tetramer for the EBV latent epitope YPL, which is often immunodominant in EBV-immune individuals.30
Analysis of pp65 mini-LCL–stimulated and control mini-LCL–stimulated T cells from the CMV+, EBV− donor no. 2.
(A) The cytotoxic activity of pp65-mini-LCL–stimulated polyclonal T cells (upper row) and control mini-LCL–stimulated T cells (lower row) from donor no. 2 against diverse target cells was assayed by chromium release on days 27, 42, and 53, respectively. (B) Analysis of the 2 T-cell populations with CD4- and CD8-specific antibodies and NLV, IPS, and YPL tetramers, performed on day 52 of cultivation.
Analysis of pp65 mini-LCL–stimulated and control mini-LCL–stimulated T cells from the CMV+, EBV− donor no. 2.
(A) The cytotoxic activity of pp65-mini-LCL–stimulated polyclonal T cells (upper row) and control mini-LCL–stimulated T cells (lower row) from donor no. 2 against diverse target cells was assayed by chromium release on days 27, 42, and 53, respectively. (B) Analysis of the 2 T-cell populations with CD4- and CD8-specific antibodies and NLV, IPS, and YPL tetramers, performed on day 52 of cultivation.
Characteristics of T-cell cultures from CMV+/EBV+ donors
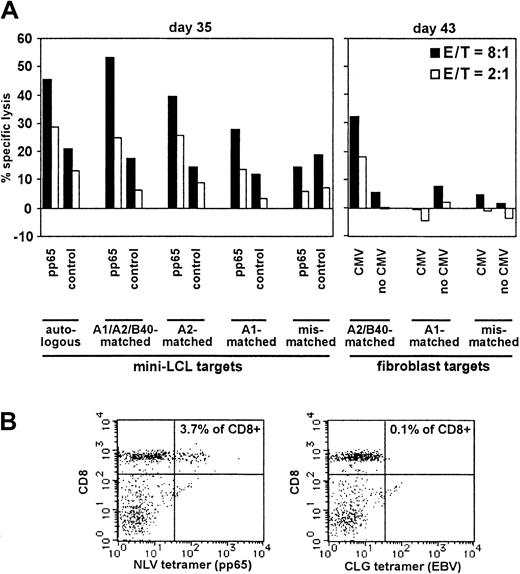
These results encouraged us to proceed to donors seropositive for both CMV and EBV. We included 3 HLA-A2 donors in these studies. From donor no. 3 we generated a pp65 mini-LCL–restimulated T-cell culture. From donor nos. 4 and 5, both T-cell cultures restimulated with pp65-expressing and with control mini-LCLs were analyzed. Results for the 3 donors are displayed in Figures 5,6, and 7, respectively. Donor no. 3 (HLA-A1, A2, B16, B40) displayed an enhanced cytotoxic activity against pp65-expressing mini-LCLs as compared to control mini-LCLs (Figure 5), quite similar to the results with the EBV− donor no. 2, and pp65 specificity was confirmed by lysis of CMV-infected fibroblasts. The results with HLA-matched and mismatched targets suggested that lysis was largely HLA restricted. Staining with the NLV tetramer showed that these reactivities might be attributed, at least partially, to T cells specific for this epitope. CLG-tetramer binding cells, however, were hardly above background, consistent with the weaker lysis of targets expressing EBV antigens only.
Analysis of pp65 mini-LCL–stimulated T cells from a donor (no. 3) seropositive for both CMV and EBV.
(A) The T-cell population was tested for cytotoxic activity against diverse target cells by chromium release on days 35 and 43, as indicated. (B) The same T-cell culture was analyzed for NLV and CLG tetramer staining (day 32 of cultivation).
Analysis of pp65 mini-LCL–stimulated T cells from a donor (no. 3) seropositive for both CMV and EBV.
(A) The T-cell population was tested for cytotoxic activity against diverse target cells by chromium release on days 35 and 43, as indicated. (B) The same T-cell culture was analyzed for NLV and CLG tetramer staining (day 32 of cultivation).
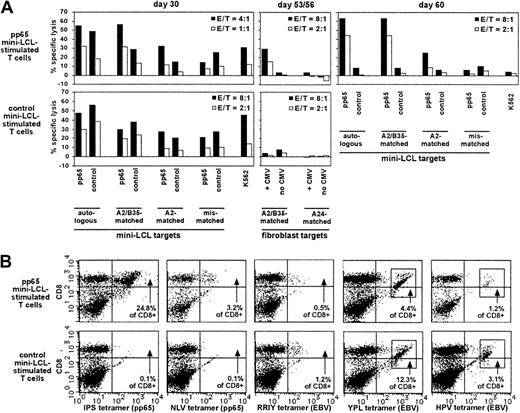
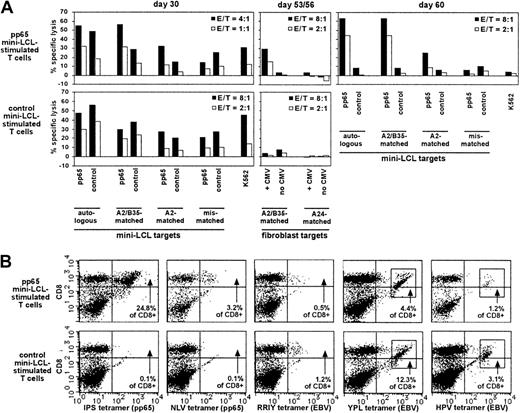
Analysis of pp65 mini-LCL–stimulated and control mini-LCL–stimulated T cells from the CMV+, EBV+ donor no. 4.
(A) Cytotoxic activities of the T-cell cultures, assayed on cultivation days 30, 53, 56, and 60 by chromium release. (B) Both T-cell populations were analyzed by tetramer staining on day 34. Tetramers used were IPS (pp65, B35-restricted), NLV (pp65, A2-restricted), RRIY (EBV EBNA3C, B27-restricted), YPL (EBV EBNA3A, B35-restricted), and HPV (EBV EBNA1, B35-restricted).
Analysis of pp65 mini-LCL–stimulated and control mini-LCL–stimulated T cells from the CMV+, EBV+ donor no. 4.
(A) Cytotoxic activities of the T-cell cultures, assayed on cultivation days 30, 53, 56, and 60 by chromium release. (B) Both T-cell populations were analyzed by tetramer staining on day 34. Tetramers used were IPS (pp65, B35-restricted), NLV (pp65, A2-restricted), RRIY (EBV EBNA3C, B27-restricted), YPL (EBV EBNA3A, B35-restricted), and HPV (EBV EBNA1, B35-restricted).
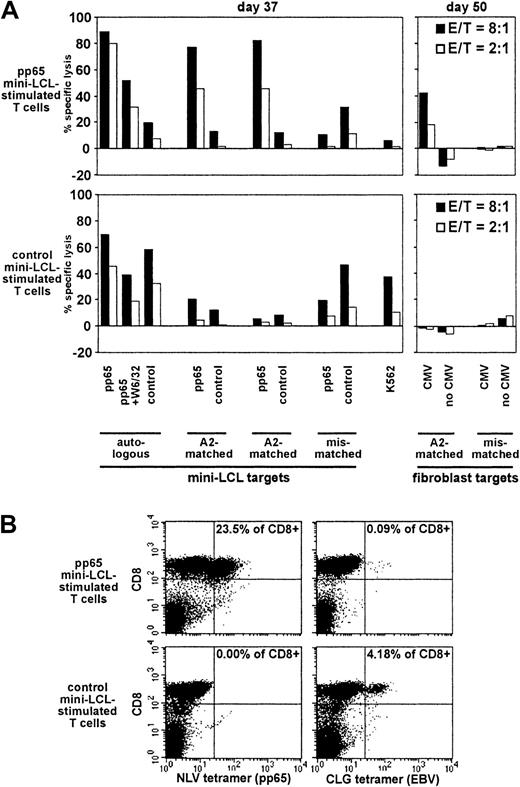
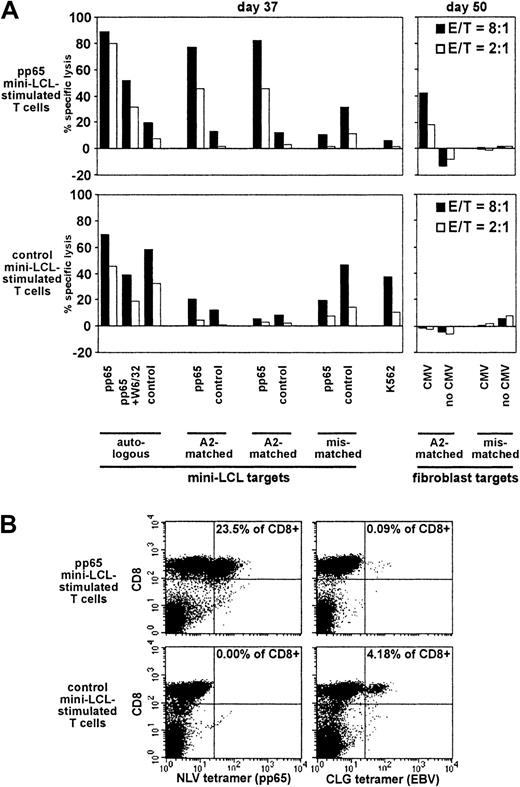
Analysis of pp65 mini-LCL–stimulated and control mini-LCL–stimulated T cells from the CMV+, EBV+ donor no. 5.
(A) Cytotoxic activities were assayed on cultivation days 37 and 50 by chromium release. (B) The T-cell populations were analyzed by tetramer staining on day 49.
Analysis of pp65 mini-LCL–stimulated and control mini-LCL–stimulated T cells from the CMV+, EBV+ donor no. 5.
(A) Cytotoxic activities were assayed on cultivation days 37 and 50 by chromium release. (B) The T-cell populations were analyzed by tetramer staining on day 49.
Simultaneous expansion of T cells with different epitope specificities
Donor no. 4's HLA type (HLA-A2, A24, B27.05, B35) encompassed a number of alleles, for which immunodominant EBV as well as CMV epitopes were known. Therefore, we could analyze this donor's T-cell cultures with tetramers for 2 different pp65 epitope specificities (the HLA-A2–restricted NLV and the HLA-B35–restricted IPS epitope) and 3 different EBV latent epitope specificities (the HLA-B27–restricted RRIY, and the HLA-B35–restricted YPL and HPV epitopes). On day 34 of culture, the pp65 mini-LCL–stimulated culture contained T cells specific for each of these 5 epitopes, in different frequencies, the largest number of cells recognizing the CMV epitope IPS, followed by the EBV epitope YPL (Figure 6B). In the control T-cell culture, all 3 EBV epitope specificities but none of the CMV specificities were represented. A follow-up of the pp65 culture up to day 56 showed that CMV epitope IPS-specific T cells continued to increase in relative number, and finally amounted to 49.8% of total cells, whereas the fraction of NLV-specific cells remained constant, and the proportion of cells with each of the 3 EBV epitope specificities RRIY, YPL, and HPV decreased to 0.4%, 0.2%, and 0.0%, respectively (not shown). The cytotoxicity data were consistent with these results (Figure 6A); earlier in culture, cytotoxic reactivity seemed to be only in part directed toward pp65, and there were EBV-specific and non–HLA-restricted components. In later passage, however, the T-cell culture had a reactivity exclusively directed against pp65, as shown by experiments on days 53 to 60, using CMV-infected fibroblasts and mini-LCLs as targets. This T-cell population could be continually expanded during the observation period of 60 days, although proliferation rates slowly decreased; departing from 2.0 × 107 PBMCs on day 0, an extrapolated 3.0 × 109 cells were obtained on day 60.
Selective expansion of pp65-specific cells
Donor no. 5 was an additional CMV-seropositive, EBV-seropositive individual (HLA-A2, A24, B44, B51) whose pp65 mini-LCL–stimulated cultures displayed strong cytotoxic activity against cells expressing pp65 in the context of HLA-A2 (Figure 7A), consistent with high frequencies of NLV epitope-specific cells (Figure 7B). For this donor, we analyzed the time course of expansion of NLV and CLG tetramer-binding cells (Table 1). Most interestingly, for the pp65 mini-LCL–stimulated T-cell culture, an increase in relative numbers of NLV-specific cells and a decrease in relative numbers of CLG-specific cells during the observation period was evident. In the control mini-EBV–stimulated culture, however, CLG-specific cell numbers increased with time and, as expected, NLV-specific cells were absent. Other remarkable differences between the 2 populations were that the pp65 mini-LCL–stimulated T-cell culture experienced a stronger increase in absolute cell numbers and a sharper decrease in numbers of CD56+CD3−natural killer (NK) cells.
Discussion
Here we present a new method to reactivate T cells specific for MHC-restricted antigens. The novelty of our approach lies in the use of a new kind of antigen-presenting cell, called mini-LCL, to restimulate and expand specific T cells. Mini-LCLs are B cells immortalized by a mini-EBV vector lacking the viral functions necessary for lytic EBV replication, preventing the accidental release of infectious virus. However, they share with standard EBV-immortalized B cells their indefinite proliferation ability and the activated B-cell phenotype causing the efficient reactivation of antigen-specific T cells.
We attempted to reactivate and expand CMV pp65-specific T cells by restimulation of peripheral blood cells with mini-LCLs immortalized by a pp65 mini-EBV, thus constitutively expressing this immunodominant CMV antigen. We consistently observed the reactivation and expansion of pp65-specific cytotoxic T cells when the donor was CMV+. All of the 4 cultures from CMV+ donors displayed HLA-restricted CMV-specific cytotoxicities. By tetramer staining, the expansion of T cells specific for an immunodominant HLA-A2–restricted pp65 epitope was demonstrated in cultures from all of the 4 CMV+ HLA-A2 individuals, and T cells specific for an HLA-B35–restricted pp65 epitope were found in cultures from both CMV+ B35 donors. In addition, we observed that during the first weeks of cultivation EBV epitope specificities were simultaneously reactivated in cultures from CMV+/EBV+ donors, yielding T-cell cultures specific for both viruses. This was strikingly evident in data from donor no. 4, where immunodominant CMV as well as EBV epitopes could be studied and the expansion of T cells specific for 5 epitopes restricted through 3 different HLA class I alleles was shown. However, in cultures from the CMV−/EBV+donor no. 1, no pp65 tetramer-staining cells and no cytotoxic reactivity against CMV-infected cells was present. Both the pp65 mini-LCL–stimulated and the control mini-LCL–stimulated cultures from this donor displayed a strong cytotoxic reactivity against mini-LCLs. This reactivity was HLA class I restricted and did not discriminate between pp65-expressing and -nonexpressing targets. Therefore, this reactivity was most likely caused by EBV-specific CD8+ T cells. In consequence, pp65-specific T cells, EBV-specific T cells, or both can be expanded by short-term pp65 mini-LCL restimulation, according to the immune status of the donor.
With continued cultivation, CMV epitope-specific T cells were expanded further and finally dominated the cultures from CMV+donors. With cultures from 3 donors (donor nos. 2, 4, and 5), we performed tetramer analyses on day 49 or later and found that T cells specific for the 1 or 2 CMV epitopes accessible to analysis accounted for 15.3%, 43.0%, and 49.8% of total cells. In contrast, EBV-specific T cells were gradually lost from the cultures, though the EBV latent antigens expressed in mini-LCLs are known to be immunodominant in EBV+ individuals. In this context, Gilbert et al31 showed that pp65 can specifically inhibit the processing of a coexpressed CMV antigen, IE1, for HLA class I presentation by infected fibroblasts. However, a similar effect of pp65 on the presentation of EBV antigens remains speculative. Because we observed an equal lysis of pp65 mini-LCLs and control mini-LCLs by the T-cell populations from CMV−/EBV+ donor no. 1 and by control mini-LCL–stimulated T-cell populations from the other EBV-immune donors, we assume that EBV antigen presentation by mini-LCLs was intact. Expansion of CMV-specific T cells might have been favored by their greater responsiveness to stimulation or by an enhanced expression and presentation of pp65, being expressed from a constitutive synthetic promotor.
In cultures from some of our donors, we observed a certain level of nonspecific cytotoxic reactivity, associated with lysis of K562 and of mismatched LCLs. It is well known that the expansion of EBV-specific cytotoxic T cells from EBV+ donors by standard LCL restimulation is usually accompanied by some cytotoxic activity exhibited by non–HLA-restricted effectors like NK cells, leading to lysis of mismatched LCLs.6 This has not hindered the use of such T-cell preparations in adoptive therapy to target EBV-associated disease, and no severe side effects have been reported. However, it remains an important aim to minimize activation of non–HLA-restricted or potentially alloreactive cells. Some authors have recommended delaying the addition of IL-2 until day 21.8 If considered necessary, the depletion of CD16/56+ NK cells was shown to be helpful to reduce non–HLA-restricted reactivities.6
Because CMV-specific T cells are efficiently generated by pp65 mini-LCL stimulation, it is worth considering the use of this system for adoptive T-cell therapy of CMV-related disease. Adoptive transfer of antigen-specific T cells was introduced into clinical practice several years ago with the prophylactic administration of CMV-specific T-cell clones after bone marrow transplantation. Although CMV-specific cellular immunity could successfully be restored by this procedure,22,23 therapy with CMV-specific T cells has not been widely applied so far. In the original study, CMV-specific T cells were reactivated by stimulation of blood cells with CMV-infected fibroblasts, the only cell type readily infectable with CMV in vitro. Fibroblasts are not professional antigen-presenting cells, though, and this procedure required generation of an autologous fibroblast line, use of infectious CMV, and cloning of the polyclonal T-cell population.23 These practical difficulties might constitute one of the reasons why CMV-specific adoptive T-cell transfer has not yet found its way into everyday clinical practice.
Increasing interest in adoptive therapy of CMV-related disease is reflected by the recent presentation of several protocols to generate pp65- or CMV-specific T cells using dendritic cells (DCs) as antigen presenters, among them methods that rely on exogenous loading of a defined pp65 epitope peptide32,33 or on the uptake and processing of a viral antigen preparation by DCs.34 These methods obviate the use of infectious CMV and cloning. However, they rely on the in vitro generation of monocyte-derived DCs, a procedure that is difficult to upscale due to their limited proliferation in culture. Methods using defined peptide epitopes tend to be efficient but are limited to donors carrying the appropriate HLA allele.
An alternative method to reactivate pp65-specific polyclonal T cells was presented by Sun et al11 who generated pp65-specific T-cell cultures by restimulation with wild-type EBV LCLs expressing pp65 from a retroviral murine stem cell virus (MSCV) vector. Similar to our results, these studies described pp65-specific cytotoxic activity in each of their T-cell preparations from CMV+, but not CMV− donors. Subsequently, the authors beautifully demonstrated35 that pp65 MSCV LCLs were able to reactivate not only pp65-specific T cells but also the complete spectrum of EBV latent antigen specificities characteristic of the individual donor. What might have helped to ensure bispecificity of such cultures, however, is that pp65 was expressed in only about 50% to 90% of the cells in pp65 MSCV LCLs,11 possibly favoring stimulation of EBV-specific cells by the remaining subpopulation of stimulators expressing EBV antigens only. Besides, Sun et al11regularly analyzed their T-cell cultures at day 21 of cultivation, earlier than we generally did. It seems possible that competition processes favoring the expansion of pp65-specific T cells were not yet operative at this early stage of cultivation. In the therapeutic setting after stem cell transplantation, to minimize the risk of accidentally introducing residual alloreactive T cells, it might be advisable to use T-cell populations that have undergone more rounds of selection of virus-specific cells. In addition, a larger number of specific T cells will be available at later time points. In contrast to the method presented by Sun et al,11our strategy obviates the need for retrovirus infection, selection, and additional measures to suppress lytic EBV replication, because the mini-LCL needed for T-cell restimulation is generated from primary blood B cells in one single step and inherently incapable of sustaining lytic EBV replication.
We were able to generate CMV-specific T-cell cultures only from positive donors, indicating that CMV-specific cells were efficiently reactivated, but not primed, by our approach. Although DCs are considered to be best suited to activate naive T cells, DC-based methods have rarely been successful in generating CMV-specific T cells from CMV-nonimmune donors.32-34 However, there are reports describing priming of EBV-specific T cells by standard LCLs.36 Because the continuous proliferation of pp65 mini-LCLs, as opposed to DCs, allows for prolonged restimulation protocols, which might be necessary to efficiently amplify primary T-cell responses, it will be interesting to readdress this issue using mini-LCLs as stimulators.
The capacity of mini-EBVs for additional genetic information is large. Up to 80 kilobase pairs of foreign DNA sequences can be added without impairing the efficient packaging of mini-EBVs into EBV virions.37 Therefore, it will be possible to assemble several genes encoding T-cell antigens on the same mini-EBV construct, for example, to express several important CMV antigens in one mini-LCL. Because a minority of healthy CMV+ individuals lack immunity against pp65, but compensate for this deficiency by recognizing other well-characterized CMV antigens,38 such a mini-LCL cell could be universally applied in anti-CMV immunotherapy. Finally, it will be attractive to express antigens specific for other infectious agents or tumors in mini-LCLs to generate an unlimited supply of cells efficiently presenting the antigens in question.
We are indebted to Barbara Mosetter, Marie Roskrow, Dolores Schendel, and Georg Wittmann for help, advice, and discussions. We thank all voluntary blood donors.
Supported by Wilhelm-Sander-Stiftung, Deutsche Krebshilfe and Deutsche Forschungsgemeinschaft (Sonderforschungsbereich 455). A.M.was supported by a European Molecular Biology Organization short-term fellowship.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Andreas Moosmann, HNO-Forschung, Klinikum Grosshadern, Marchioninistr. 15, 81377 München, Germany; e-mail:moosmann@hno.med.uni-muenchen.de.