Complement plays an essential role in inflammation and tissue damage. However, it is largely unknown to what extent the system acts as a primary inducer of secondary mediator systems in the inflammatory network of human whole blood. Here we describe a novel in vitro model using the thrombin-specific hirudin analog lepirudin as anticoagulant, which, in contrast to heparin, did not interfere with complement activation. The model was used to study the role of complement inEscherichia coli–induced inflammatory responses. Granulocyte and monocyte oxidative burst was complement dependent as it was reduced by 85% and 70%, respectively, by the CD3 binding peptide compstatin. A similar reduction was found by inhibition of C5, C5a, and C5a receptor (C5aR). Furthermore, anti-CR3 antibodies were as efficient as the C5aR antagonist in reducing granulocyte oxidative burst, whereas blocking CD14 or C3aR had no effect. Up-regulation of granulocyte CR3 was virtually abolished by a C5aR antagonist. Opsonization and phagocytosis was completely inhibited by blocking of C5aR or CR3, whereas blocking of the FcγRs (CD16, CD32, CD64) had no effect. In contrast to oxidative burst and phagocytosis, cytokine secretion was largely complement independent. Thus, anti-CD14 abolished tumor necrosis factor-α, interleukin-6 (IL-6), and IL-10 secretion, whereas IL-8 was equally inhibited by anti-CD14 and compstatin. In conclusion, the present model is particularly useful for studying complement as part of the inflammatory network. The results emphasize a crucial role for C5a-C5aR interaction in E coli–induced up-regulation of CR3 and the subsequent oxidative burst and phagocytosis. Complement inhibition may have therapeutic implications in oxidative burst–induced tissue damage.
Introduction
Complement protects the host against invading microorganisms. However, it is a double-edged sword in the sense that it may induce undesirable inflammation if activated improperly or uncontrolled. Numerous studies have confirmed that complement contributes to tissue damage in conditions like septicemia,1,2 ischemia-reperfusion injury,3,4 capillary leak syndrome,5,6connective tissue disease,7,8 neurologic disease,9 nephritis,10,11 and transplant rejection.12,13 Many of the mechanisms by which complement activates blood cells have been elucidated. These include binding of activated complement fragments to their corresponding receptors14 and effects induced by sublytic attack by the terminal C5b-9 complex.15-17
Complement-mediated activation of granulocytes and monocytes causes oxidative burst with release of reactive oxygen species; production of cytokines; release of arachidonic acid metabolites, histamine, and platelet activating factor; and altered expression of adhesion molecules. However, these various effects of complement have mainly been studied in vitro by using isolated cells stimulated with purified proteins. To investigate the role of complement in the complex inflammatory network, all potential cellular and fluid-phase mediators need to be present and able to interact simultaneously. In vitro, such cross talk can only be achieved by using whole blood. However, whole blood models have been hampered by the adverse effects by anticoagulants on complement activation. Calcium chelators like ethylenediaminetetraacetic acid (EDTA) and citrate inhibit complement activation as well as a number of other biologic processes. Due to lack of other suitable anticoagulants, heparin has been widely used, although it has a number of effects on complement.18Heparin inhibits complement activation at high concentrations, whereas it enhances the activation at low concentrations.19,20 Heparin also has various direct effects on platelets21,22 and leukocytes,23 which excludes it as an optimal anticoagulant in models to study the inflammatory network.
The aim of the present study was to investigate the role of human complement as primary inducer of inflammation by developing an in vitro whole blood model using an anticoagulant without adverse effects on complement. This was achieved by the recombinant hirudin analog lepirudin, a highly specific thrombin inhibitor.24 The C3 inhibitor compstatin, an anti-C5 neutralizing antibody, an anti-C5a antibody, and a small-molecular C5aR antagonist peptide were used to selectively inhibit complement activation. The results indicate thatEscherichia coli–induced oxidative burst and phagocytosis was complement mediated and virtually completely dependent on C5aR-mediated up-regulation of CR3. In contrast, E coli–induced cytokine release was largely complement independent and was efficiently inhibited by blocking CD14.
Materials and methods
All equipment (eg, tubes, tips) and solutions used in the model were endotoxin-free according to information from the manufacturers. Polypropylene tubes were used to obtain low background activation of complement.
Reagents
Sterile phosphate-buffered saline (PBS) was from Life Technologies (Paisley, United Kingdom), lepirudin (Refludan) from Hoechst (Frankfurt am Main, Germany), and heparin from Løvens Kemiske Fabrik (Ballerup, Denmark). Burst test kit, Phago-test kit, opsonized E coli, and fluorescein isothiocyanate (FITC)–labeled E coli, strain LE392 (ATCC 33572), was obtained from ORPEGEN (Pharma, Heidelberg, Germany) and nonopsonized E coli, strain LE392 (ATCC 33572), 1 × 109 bacteria per milliliter, from American Type Culture Collection (ATCC; Manassas, VA). Mouse antihuman CD11b (clone ICRF 44; purified immunoglobulin G1 [IgG1]) was obtained from Serotec (Oxford, United Kingdom), and F(ab′)2 fragments were produced by pepsin digestion. Mouse antihuman CD18 (clone 1B4; purified F(ab′)2) was obtained from ID Labs (London, ON, Canada). Mouse antihuman CD14 (clone 18D11; purified IgG1 and F(ab′)2) was obtained from Diatec (Oslo, Norway). The following mouse antihuman phycoerythrin (PE)–conjugated monoclonal antibodies (mAbs) were used for detection of FcγRs: anti-CD16/FcγRIII (clone 3G8; Immunotech, Marseille, France), anti-CD32/FcγRII (clone K562; Immunotech), and anti-CD64/FcγRI (clone 10.1; Dako, Glostrup, Denmark). The following mouse antihuman mAb F(ab′)2 fragments used for blocking of FcγRs were all obtained from Ancell (Bayport, MN): anti-CD16/FcγRIII (clone 3G8; recognizes both allelic forms of CD16 and blocks binding of complexed IgG to CD16), anti-CD32/FcγRII (clone 7.3; reacts with a domain 2 epitope of all CD32 isoforms and blocks immune complex binding), and anti-CD64/FcγRI (clone 10.1; recognizes the CD64 molecule of 72 kDa from gene FcγRIA and blocks binding of FcγRIA to Ig-opsonized cells). FITC-conjugated rabbit antihuman C3d and FITC-conjugated F(ab′)2 fragment of rabbit antihuman IgG were from Dako.
Complement inhibitors
Compstatin is a 13–amino acid cyclic peptide that binds to and inhibits cleavage of C3. The acetylated form of compstatin (Ac-ICVVQDWGHHRCT-NH2) and the control peptide (IAVVQDWGHHRAT-NH2) have been extensively described.25-27 Mouse antihuman C5 (clone 137-30; purified IgG1) and an isotype-matched control mAb (clone G3-519; purified IgG and F(ab′)2 fragments) were produced and purified under identical conditions in the laboratory of one of the coauthors (M.F.). The production and characterization of the anti-C5a mAb 561, including its antagonistic potency to block C5a binding to its receptor, have been described in detail elsewhere.28,29The synthetic cyclic hexapeptide AcF[OPdChaWR] has previously been characterized as a C5a receptor (C5aR) antagonist.30The peptide was purified using preparative reverse-phase high-performance liquid chromatography (RP-HPLC); purity and molecular weight of eluted fractions were determined by mass spectrometry. The C3a receptor antagonist, SB 290157, was a kind gift from Robert Ames, SmithKline Beecham Pharmaceuticals (King of Prussia, PA), and has been described in detail previously.31
Effect of lepirudin and heparin on complement
Serum from 6 healthy voluntary blood donors was incubated for 60 minutes at 37°C in the presence of lepirudin (5, 50, and 500 μg/mL), heparin (0.2, 2, and 20 IU/mL), or PBS (control). After incubation, EDTA was added (10 mM final concentration) and the samples were stored at −70°C until being analyzed. Furthermore, whole blood (4 mL) from 6 voluntary donors (written informed contest was obtained) was collected into sterile polypropylene tubes (4.5-mL NUNC cryotubes; Nalge Nunc International, Roskilde, Denmark). Immediately after venipuncture, the sample was split and 500 μL blood added to sterile polypropylene tubes (1.8-mL NUNC cryotubes) containing lepirudin (5, 50, or 500 μg/mL) or heparin (0.2, 2, or 20 IU/mL). Then E coli (2 × 107/mL, endotoxin 140 ng/mL) or PBS was added and the tubes rotated (Rock-n-Roller; Labinco, The Netherlands) for 60 minutes at 37°C. Thereafter, further complement activation was blocked by adding EDTA (10 mM). The tubes were centrifuged for 15 minutes at 4000g at 4°C. Plasma was stored at −70°C until being analyzed for complement activation. The samples with lepirudin 5 μg/mL and heparin 0.2 IU/mL clotted during incubation and were excluded.
Standardization of the whole blood model
Based on the results above, lepirudin 50 μg/mL was used as anticoagulant, and a standard temperature of 37°C was chosen in order to approach physiologic conditions. In the present study, the effect of complement on E coli–induced oxidative burst and phagocytosis was investigated in detail. However, to design the model to study several arms of the inflammatory network, changes in leukocyte and platelet cell surface markers, release of their granular proteins, and cytokine production was examined. Incubation up to 4 hours revealed changes in all mediator systems studied, except for interleukin-10 (IL-10), which required 18 hours of incubation. As a control, changes in the basic physiologic conditions were examined using a standard blood gas instrument. During the first 60 minutes pH did not change (stable between 7.3 and 7.4) and was above 7.2 even after 4 hours. The pCO2 did not change significantly during 4 hours of incubation, and base excess was unchanged the first 15 minutes. There were no differences in these parameters when the samples were incubated in a CO2 incubator using 5% (vol/vol) CO2 with the cap open or in a conventional incubator with the cap closed. However, pO2 increased more in the CO2 incubator (from 3 to 14 kPa) than in the conventional incubator (from 3 to 8 kPa) after 4 hours. The increase in pO2 after 15 minutes in the conventional incubator was minor (from 3 to 4 kPa), and this incubator was therefore chosen.
Experimental design
The effect of a panel of different inhibitors on the oxidative burst, phagocytosis, complement activation, and myeloperoxidase (MPO) release was examined as follows: All incubations were performed at 37°C. Whole blood anticoagulated with lepirudin 50 μg/mL was collected and distributed immediately into tubes containing PBS, inhibitor, or control. The samples were preincubated for 4 minutes until PBS (baseline samples) or 1 × 108E coli per milliliter of blood was added (the E coliconcentration was 2 × 107/mL blood in the experiments using compstatin as blocking agent). The T0 baseline sample was processed immediately, and further incubation was performed for the actual time periods as described for the different experiments. After removal of 100 μL blood for the flow cytometric assays, EDTA (10 mM) was added and the tubes were centrifuged for 15 minutes at 4000g (4°C). The plasma was stored at −70°C until analyzed.
Flow cytometry
The oxidative burst and the phagocytic activity was measured using Burst test and the Phago-test, respectively. Immediately after incubation, 100 μL blood was added to sterile polypropylene tubes (Falcon, Becton Dickinson, Franklin Lakes, NJ) and treated according to the kit procedures. Incubation with E coli was 10 minutes for oxidative burst and 20 minutes for phagocytosis. The cells were resuspended in PBS and analyzed on a FACSCalibur flow cytometer (Becton Dickinson, San José, CA). In a forward/side scatter (FSC/SSC) dot plot, gates were set on granulocytes and monocytes to analyze each population with regard to median fluorescence intensity (MFI). Leukocyte surface-bound bacteria in the phagocytosis test were neutralized using quenching solution. In experiments where phagocytosis was blocked, additional experiments omitting quenching solution were performed to evaluate the degree of opsonization.
CD11b and FcγR expression
CD11b was measured after 10 minutes of incubation with E coli at a concentration of 1 × 108 bacteria per milliliter of blood with C5aR antagonist or control peptide. The cells were fixed with 0.5% (vol/vol) paraformaldehyde in an equal volume for 4 minutes at 37°C and then stained with anti-CD11b PE, anti-CD14 FITC (Becton Dickinson, San Jose, CA), and the nuclear dye LDS-751 (FL-3) (Molecular Probes, Eugene, OR). Samples were run on the flow cytometer with threshold at FL-3 to exclude red cells and debris. Granulocytes were gated in an SSC/FL1-dot plot, and CD11b expression was measured as MFI. FcγR expression was measured principally in the same manner using PE-conjugated anti-CD16/FcγRIII, anti-CD32/FcγRII, and anti-CD64/FcγRI antibodies.
Deposition of C3d and IgG on E coli
Preopsonized E coli (1 × 108/mL) was preincubated with PBS or lepirudin plasma for 10 minutes at 37°C and washed twice with PBS containing 0.1% (wt/vol) bovine albumin. Nonoposonized E coli was used as negative control. Samples were then incubated 15 minutes at room temperature with FITC-labeled anti-C3d or anti-IgG (dilution 1:4). Bacteria were gated in an SSC/FSC dot plot, and deposition of C3d or IgG was determined using MFI. At least 10 000 bacteria were counted in each sample.
Enzyme immunoassays
C1rs-C1inhibitor complexes (C1rs-C1inh).
Activation of the classical complement pathway was quantified in an enzyme immunoassays (EIA) described in detail elsewhere,32using the mAb Kok 12 specific to a neoepitope exposed only when C1inh is in complex with its substrates. This antibody was a kind gift from Prof C. E. Hack, Amsterdam, The Netherlands.
C3b-Bb-properdin complexes (C3bBbP).
Activation of the alternative pathway was detected by quantifying the alternative convertase C3bBbP in an EIA. Microtiter plates (Maxisorp; Nunc) were incubated at 4°C overnight with mouse antihuman factor P, clone no. 2 (Quidel, San Diego, CA) diluted 1/1000 in 0.05 M carbonate buffer, pH 9.6. Between each further incubation the plates were washed thrice with PBS containing 0.1% (vol/vol) Tween 20. All incubations were made with 50 μL per well, except for the substrate (100 μL). Standard (see below) was diluted 2-fold from 1/100 to 1/3200, and test samples (containing 10 mM EDTA final concentration) were diluted 1/25 in PBS containing 0.2% Tween 20 and 10 mM EDTA. The plates were incubated for 60 minutes at room temperature. Detection antibody was anti-C3c (Behringwerke, Marburg, Germany) diluted 1/1000 in PBS containing 0.2% (vol/vol) Tween 20. After 45 minutes of incubation at 37°C, horseradish peroxidase–conjugated donkey antirabbit Ig (NA9349; Amersham International, Little Chalfont, United Kingdom), diluted 1/1000 in PBS containing 0.2% Tween 20, was added. After 45 minutes of incubation at 37°C, substrate was added: ABTS (2,2′-azino-di-(3-ethylbenzthiazoline sulfonic acid)), 180 mg/L, diluted in 0.15 M sodium acetate buffer, pH 4.0. H2O2 (10 μL of 3%) was added to 12.5 mL substrate solution immediately before use. The standard was a zymosan-activated human serum pool (ZAS) made by incubating serum with 10 mg/mL zymosan (Sigma Chemical, St Louis, MO) for 60 minutes at 37°C. After centrifugation the supernatant was split and stored in aliquots at −70°C. The ZAS was defined to contain 1000 arbitrary units (AUs) per milliliter of C3bBbP. Optical density was read at 410 nm using 490 nm as reference.
C3bc.
Activation of the final common pathway was quantified in an EIA using the mAb bH6 specific for a neoepitope exposed in C3b, iC3b, and C3c as previously described.33
C5a.
Activation of C5 was quantified in a C5a EIA using the neoepitope-specific mAb 4A2E10E2 as capture antibody and 3G3C4 as secondary antibody as previously described.34 The antibodies were a kind gift from Prof Kåre Bergh, Trondheim, Norway.
The terminal sC5b-9 complex (TCC).
Activation of the terminal pathway was quantified in an EIA using the mAb aE11 specific for C9 incorporated in the sC5b-9 complex as described previously.33
MPO.
MPO was quantified using an EIA as described previously.23
TNF-α, IL-6, IL-8, and IL-10.
These were measured using PeliKine Compact EIA kit (CLB, Amsterdam, The Netherlands). Tumor necrosis factor-α (TNF-α), IL-6, and IL-8 were measured in plasma after incubating whole blood for 2 hours with 4 × 106, 0.8 × 106, and 0.04 × 106E coli per milliliter of blood. IL-10 was measured in plasma after incubating whole blood for 18 hours with 4 × 106 and 0.4 × 106E coli per milliliter of blood.
Complement hemolytic activity
The inhibitory activity of mAb 137-30 against human C5 was tested in a classical pathway hemolytic assay using sensitized chicken red blood cells as described earlier.35
Colony forming units
One or 10 μL of blood obtained immediately after addingE coli and after 20 minutes of incubation was seeded on microbiologic Petri dishes containing blood agar and further incubated 24 hours at 37°C. Bacterial growth was expressed as colony-forming units (CFUs) per milliliter of blood.
Statistics
Data are given as medians with 95% nonparametric confidence intervals (CIs) if otherwise not stated. The Friedman test with subsequent formulas for multiple comparisons was used to compare the interventions.36 A 2-tailed P < .01 was considered statistically significant due to the number of tests performed.
Results
A lepirudin-based human whole blood model for complement studies
Effect of heparin and lepirudin on spontaneous complement activation in serum.
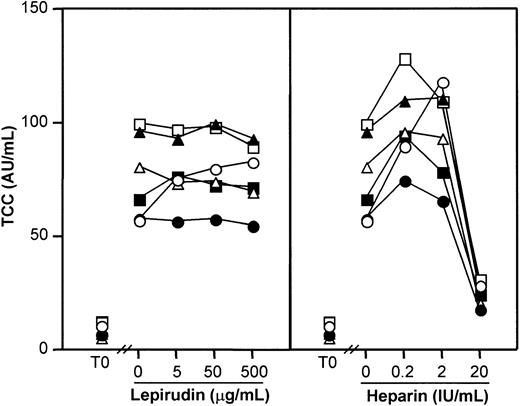
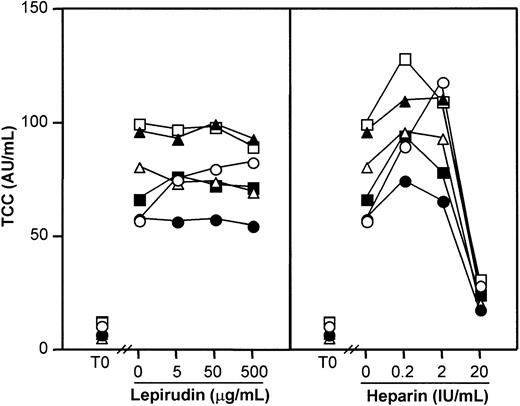
Because anticoagulation may have adverse effects on complement activation, we investigated the thrombin-specific inhibitor lepirudin with respect to effect on complement and compared it with heparin. Serum experiments were first performed including a true negative control (PBS with no anticoagulant) to reveal any effects on spontaneous in vitro complement activation. Heparin and lepirudin were tested in equal doses with respect to their known anticoagulant effect (Figure 1). Complement activation as measured by TCC increased from 9.2 AU/mL (range, 6.0-12.6 AU/mL) at baseline to 77 AU/mL (range, 57-99 AU/mL) after 60 minutes in the PBS control (P < .001). Lepirudin at 3 different doses had no effect (Figure 1, left panel), while heparin 0.2 IU/mL and 2 IU/mL enhanced activation and 20 IU/mL inhibited activation compared with the PBS control (P < .001) (Figure 1, right panel). Similar results were found for C3bBbP and C3bc, whereas heparin increased C1rs-C1inh complexes dose dependently, consistent with the known enhancing effect of heparin on C1inh binding to C1s.
Effect of lepirudin and heparin on spontaneous complement activation in human serum.
Serum was incubated for 60 minutes at 37°C, and complement activation was detected by assay of the terminal sC5b-9 complex (TCC). Each symbol represents results from 1 of 6 healthy blood donors. T0 indicates baseline sample. Lepirudin, left panel; heparin, right panel.
Effect of lepirudin and heparin on spontaneous complement activation in human serum.
Serum was incubated for 60 minutes at 37°C, and complement activation was detected by assay of the terminal sC5b-9 complex (TCC). Each symbol represents results from 1 of 6 healthy blood donors. T0 indicates baseline sample. Lepirudin, left panel; heparin, right panel.
Effect of heparin and lepirudin on complement activation in whole blood.
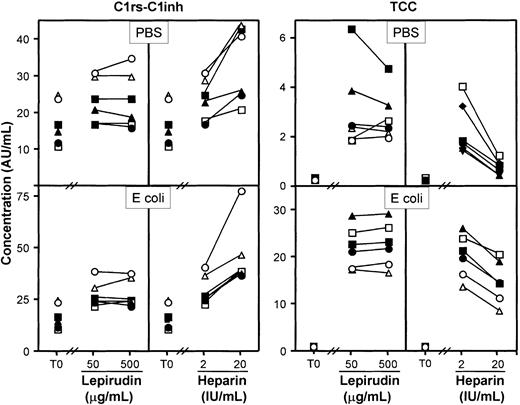
The effect on complement activation was studied by incubating blood for 10 minutes either with PBS (Figure 2, upper panels) or with E coli (2 × 107bacteria per milliliter) (Figure 2, lower panels). Heparin (20 IU/mL) increased C1rs-C1inh complexes markedly, whereas lepirudin had no effect (Figure 2, left panel). TCC formation was substantially inhibited by heparin, whereas lepirudin had no effect (Figure 2, right panel). Similar results as those obtained for TCC were also found for C3bBbP and C3bc. Lepirudin 50 μg/mL was therefore used as anticoagulant in the further experiments. This concentration corresponded to approximately 2 times the dose required to avoid coagulation after 24 hours of incubation.
Effect of lepirudin and heparin on formation of C1rs-C1inh complexes and TCC in human whole blood.
The blood samples were incubated for 60 minutes at 37°C in the presence of PBS (top panels) or E coli (2 × 107bacteria per milliliter; bottom panels). Data are from separate experiments with blood from 6 healthy blood donors. T0 indicates baseline sample; AU, arbitrary units. Each symbol represents results from 1 of 6 donors.
Effect of lepirudin and heparin on formation of C1rs-C1inh complexes and TCC in human whole blood.
The blood samples were incubated for 60 minutes at 37°C in the presence of PBS (top panels) or E coli (2 × 107bacteria per milliliter; bottom panels). Data are from separate experiments with blood from 6 healthy blood donors. T0 indicates baseline sample; AU, arbitrary units. Each symbol represents results from 1 of 6 donors.
Role of complement in E coli–induced oxidative burst in whole blood
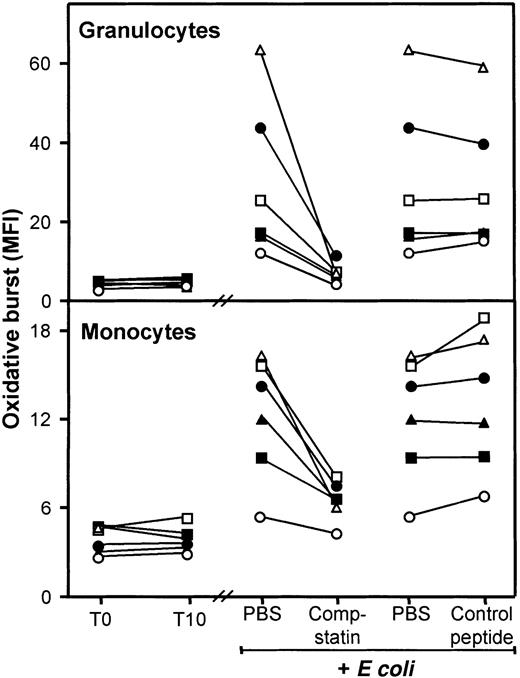
Effect of compstatin on E coli–induced oxidative burst.
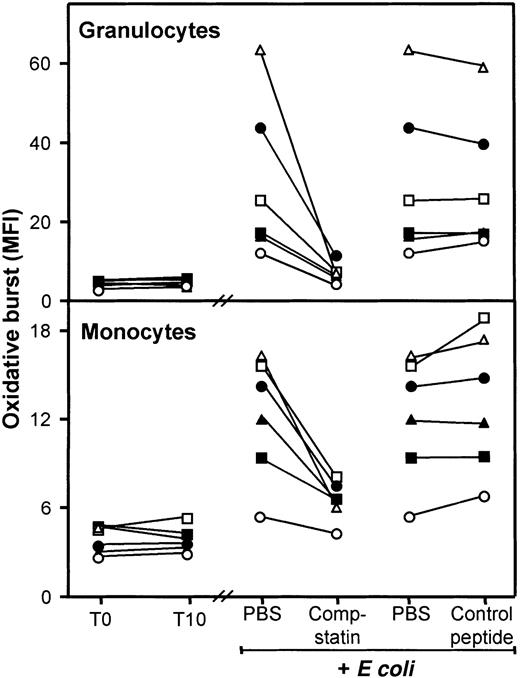
The oxidative burst in granulocytes and monocytes increased rapidly and time dependently with a maximal response after 10 to 15 minutes and thereafter declined. Thus, 10 minutes of incubation was used in the further burst experiments. The oxidative burst in granulocytes increased from baseline MFI 4.5 (range, 3.2-5.3) to MFI 28 (range, 14-58) after incubation with E coli (2 × 107bacteria per milliliter) (Figure 3, upper panel). Compstatin virtually abolished this increase, reducing the oxidative burst to MFI 7.4 (range, 5.2-11), whereas no effect was seen using the control peptide (MFI 28 [range, 16-54]). The difference between compstatin and the control peptide was highly significant (P < .0001). The monocyte oxidative burst also increased from baseline MFI 3.9 (range, 2.8-4.8) to MFI 13 (range, 6.5-16) after incubation with E coli (Figure 3, lower panel). Compstatin again reduced the oxidative burst to MFI 6.7 (range, 4.9-8.0) in contrast to the control peptide, which had no effect (MFI 13.4 [range, 7.7-19]). The difference between compstatin and the control peptide was highly significant (P < .0001). During the 10 minutes of incubation, neither granulocytes nor monocytes showed any spontaneous oxidative burst (Figure 3).
Effect of compstatin on E coli–induced oxidative burst in granulocytes and monocytes in human whole blood.
T0 indicates baseline sample; T10, sample incubated for 10 minutes after addition of PBS. E coli samples (2 × 107 bacteria per milliliter) were incubated for 10 minutes after preincubation with PBS only (samples shown twice), compstatin (200 μM), or control peptide (200 μM). Data are from separate experiments with blood from 6 donors, each represented by a different symbol. The oxidative burst is given as median fluorescence intensity (MFI). Granulocytes, top panel; monocytes, bottom panel.
Effect of compstatin on E coli–induced oxidative burst in granulocytes and monocytes in human whole blood.
T0 indicates baseline sample; T10, sample incubated for 10 minutes after addition of PBS. E coli samples (2 × 107 bacteria per milliliter) were incubated for 10 minutes after preincubation with PBS only (samples shown twice), compstatin (200 μM), or control peptide (200 μM). Data are from separate experiments with blood from 6 donors, each represented by a different symbol. The oxidative burst is given as median fluorescence intensity (MFI). Granulocytes, top panel; monocytes, bottom panel.
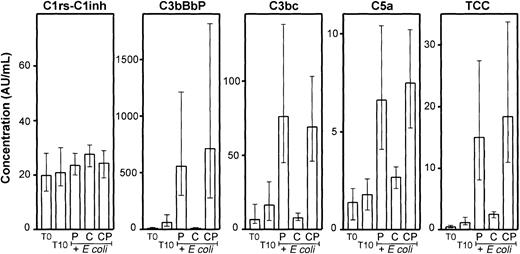
Effect of compstatin on complement activation.
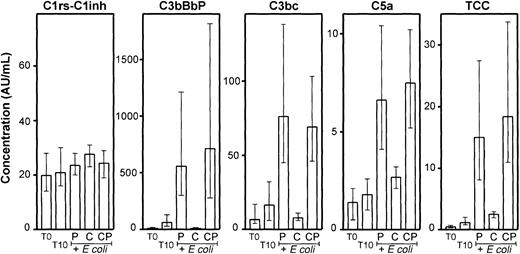
Compstatin efficiently reduced the E coli–induced complement activation in whole blood as measured by C3bBbP, C3bc, C5a, and TCC (Figure 4). The differences between compstatin and the control peptide were highly significant (P < .001). Even the modest spontaneous activation observed for C3bBbP and C3bc during the incubation was completely abolished by compstatin. The effect on the terminal pathway was also substantial and highly significant, although not as complete as for C3 activation. In contrast to the other activation products, only minor changes were found for C1rs-C1inh complexes, which were not inhibited by compstatin.
Effect of compstatin on complement activation.
Activation products from the classical (C1rs-C1inh complexes), alternative (C3bBbP), final common (C3bc), and terminal (C5a and TCC) pathways were measured in plasma from the same whole blood experiments as described in Figure 3. T0 indicates baseline sample; T10, sample incubated for 10 minutes after addition of PBS. E coli samples (2 × 107 bacteria per milliliter) were incubated for 10 minutes after preincubation with equal volumes of PBS (P), 200 μM compstatin (C), or 200 μM control peptide (CP). Median and range of the 6 experiments are indicated. AU indicates arbitrary units.
Effect of compstatin on complement activation.
Activation products from the classical (C1rs-C1inh complexes), alternative (C3bBbP), final common (C3bc), and terminal (C5a and TCC) pathways were measured in plasma from the same whole blood experiments as described in Figure 3. T0 indicates baseline sample; T10, sample incubated for 10 minutes after addition of PBS. E coli samples (2 × 107 bacteria per milliliter) were incubated for 10 minutes after preincubation with equal volumes of PBS (P), 200 μM compstatin (C), or 200 μM control peptide (CP). Median and range of the 6 experiments are indicated. AU indicates arbitrary units.
Effect of neutralizing C5 on the oxidative burst.
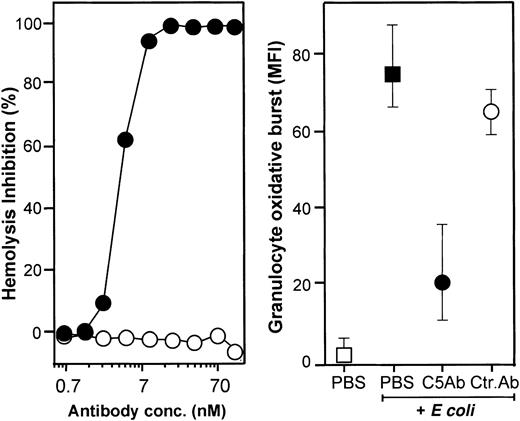
The effect of C5 activation on the oxidative burst was investigated using an anti-C5 antibody (clone 137-30) shown to completely inhibit lysis of antibody-sensitized chicken erythrocytes by human serum (Figure 5, left panel). Clone 137-30 (final concentration 0.7 μM) inhibited E coli–induced granulocyte oxidative burst by 70% (Figure 5, right panel). In comparison, monocyte oxidative burst was inhibited by approximately 50%.
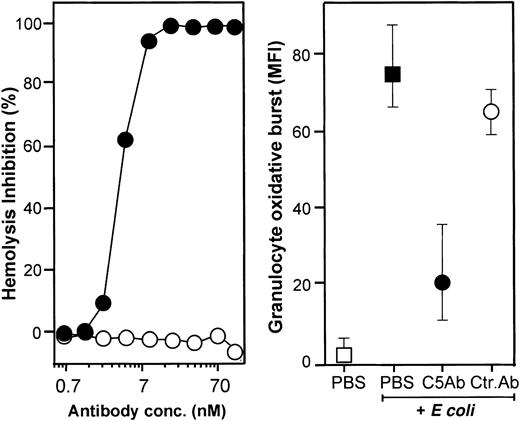
Effect of neutralizing C5 on hemolysis and on granulocyte oxidative burst.
The monoclonal anti-C5 antibody 137-30 completely inhibited lysis of antibody-sensitized chicken erythrocytes in a dose-dependent manner (left panel, log scale). The same antibody reduced granulocyte oxidative burst by 73% using a final concentration of 0.7 μM (right panel; median and 20-80 percentile of 6 experiments). Granulocyte oxidative burst is given as median fluorescence intensity (MFI). ● indicates anti-C5 (clone 137-30); ○, isotype-matched control antibody (clone G3-519); ▪, whole blood incubated 10 minutes with E coli (1 × 108bacteria per milliliter); and ■, baseline value (T0).
Effect of neutralizing C5 on hemolysis and on granulocyte oxidative burst.
The monoclonal anti-C5 antibody 137-30 completely inhibited lysis of antibody-sensitized chicken erythrocytes in a dose-dependent manner (left panel, log scale). The same antibody reduced granulocyte oxidative burst by 73% using a final concentration of 0.7 μM (right panel; median and 20-80 percentile of 6 experiments). Granulocyte oxidative burst is given as median fluorescence intensity (MFI). ● indicates anti-C5 (clone 137-30); ○, isotype-matched control antibody (clone G3-519); ▪, whole blood incubated 10 minutes with E coli (1 × 108bacteria per milliliter); and ■, baseline value (T0).
Effect of C5a and C5aR on E coli–induced oxidative burst.
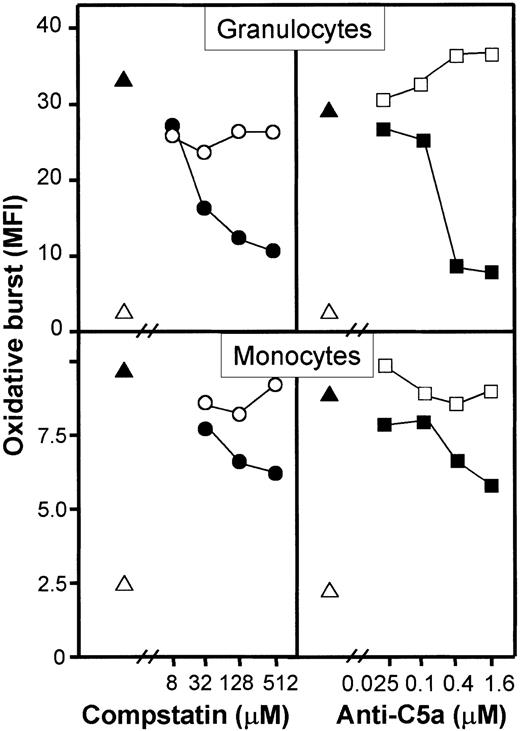
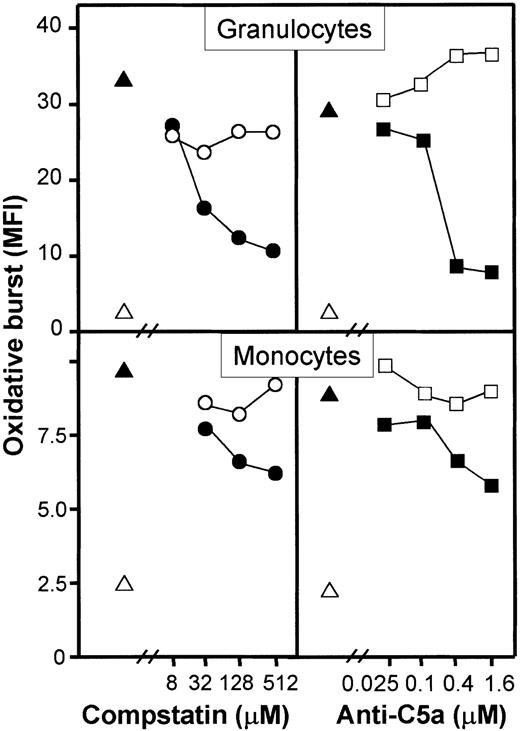
A monoclonal anti-C5a antibody (clone 561) inhibited granulocyte oxidative burst dose dependently and to a degree comparable with compstatin (Figure 6, upper panels). Monocyte oxidative burst was also inhibited by the same reagents but to a lower degree as compared with granulocytes (Figure 6, lower panels). The synthetic cyclic hexapeptide AcF[OPdChaWR], a previously described C5aR antagonist, inhibited granulocyte oxidative burst in a dose-dependent manner (Figure 7, left panel). By detailed titration the threshold for maximal effect of the peptide was found at approximately 1 μM. The relative inhibition was independent of the amount of E coli used (Figure 7, right panel). A similar effect, although less pronounced, was seen in monocytes. The C3aR antagonist had no effect on the oxidative burst. Thus, the complement-mediated E coli–induced oxidative burst, particularly in human granulocytes but also in monocytes, is highly dependent on the C5a-C5aR interaction.
Dose-response effect of compstatin and anti-C5a antibody on E coli–induced oxidative burst in granulocytes and monocytes.
One representative of 5 experiments is shown. The oxidative burst is given as median fluorescence intensity (MFI). ▵ indicates baseline value (T0); ▴, whole blood incubated 10 minutes with E coli (1 × 108 bacteria per milliliter), ●,E coli plus compstatin; ○, E coli plus control peptide, ▪, E coli plus anti-C5a; ■ isotype-matched control antibody.
Dose-response effect of compstatin and anti-C5a antibody on E coli–induced oxidative burst in granulocytes and monocytes.
One representative of 5 experiments is shown. The oxidative burst is given as median fluorescence intensity (MFI). ▵ indicates baseline value (T0); ▴, whole blood incubated 10 minutes with E coli (1 × 108 bacteria per milliliter), ●,E coli plus compstatin; ○, E coli plus control peptide, ▪, E coli plus anti-C5a; ■ isotype-matched control antibody.
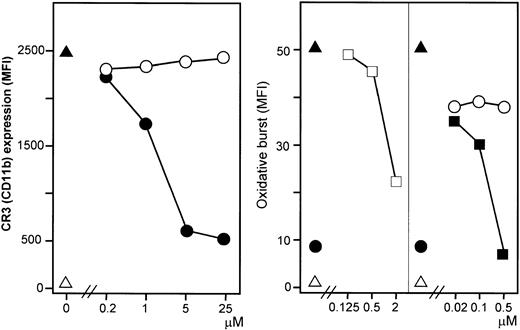
Effect of the C5aR antagonist AcF[OPdChaWR] on
E coli–induced oxidative burst in granulocytes.The left panel shows dose-response effect of the C5aR antagonist using 1 × 108E coli per milliliter (1 representative of 5 experiments is shown). The right panel shows the effect of 2 μM C5aR antagonist on E coli–induced oxidative burst by reducing the concentration ofE coli (median and range of triplicate). The oxidative burst is given as median fluorescence intensity (MFI). ▵ indicates baseline value (T0); ▴, whole blood incubated 10 minutes withE coli (1 × 108 bacteria per milliliter); ●, E coli plus C5aR antagonist; ○, E coli plus control peptide; and ■, E coli plus PBS.
Effect of the C5aR antagonist AcF[OPdChaWR] on
E coli–induced oxidative burst in granulocytes.The left panel shows dose-response effect of the C5aR antagonist using 1 × 108E coli per milliliter (1 representative of 5 experiments is shown). The right panel shows the effect of 2 μM C5aR antagonist on E coli–induced oxidative burst by reducing the concentration ofE coli (median and range of triplicate). The oxidative burst is given as median fluorescence intensity (MFI). ▵ indicates baseline value (T0); ▴, whole blood incubated 10 minutes withE coli (1 × 108 bacteria per milliliter); ●, E coli plus C5aR antagonist; ○, E coli plus control peptide; and ■, E coli plus PBS.
Role of complement in E coli–induced MPO release
Spontaneous MPO release increased from 185 μg/L (range, 112-246 μg/L) at baseline to 404 μg/L (range, 267-636 μg/L) after 10 minutes of incubation (P < .01). E coli(2 × 107 bacteria per milliliter) significantly increased MPO release to 1002 μg/L (range, 605-1372 μg/L) (P < .0001). Compstatin reduced MPO to 687 μg/L (range, 461-1052 μg/L), whereas no effect was seen for the control peptide (1176 μg/L [range, 798-1601 μg/L]). The difference between compstatin and the control peptide was highly significant (P < .0001).
Role of complement in E coli–induced CR3 and FcγR expression
Effect of C5aR antagonist on CD11b expression.
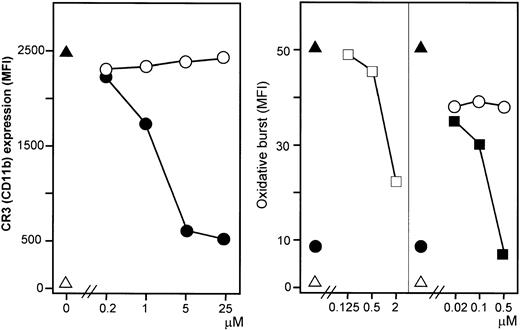
E coli (1 × 108 bacteria per milliliter) substantially increased granulocyte CD11b expression. This increase was efficiently and dose dependently inhibited by the C5aR antagonist (Figure 8, left panel). A modest E coli–induced monocyte CD11b expression was also found.
Effect of C5aR antagonist on CR3 (CD11b) expression and effect of anti-CR3 antibodies on the oxidative burst.
The C5aR antagonist inhibited E coli–induced CD11b expression dose dependently in contrast to the control peptide (left panel). ▵ indicates baseline value (T0); ▴, whole blood incubated 10 minutes with E coli(1 × 108 bacteria per milliliter); ●, C5aR antagonist; and ○, control peptide. F(ab′)2 fragments of anti-CR3 (anti-CD18 and anti-CD11b) inhibited granulocyte oxidative burst dose dependently, in contrast to a control F(ab′)2(right panel). ▵ indicates baseline value (T0); ▴, whole blood incubated 10 minutes with E coli(1 × 108 bacteria per milliliter); ■, anti-CD18; ▪, anti-CD11b; ●, C5aR antagonist (2 μM); and ○, control mAb. One representative of 5 experiments is shown.
Effect of C5aR antagonist on CR3 (CD11b) expression and effect of anti-CR3 antibodies on the oxidative burst.
The C5aR antagonist inhibited E coli–induced CD11b expression dose dependently in contrast to the control peptide (left panel). ▵ indicates baseline value (T0); ▴, whole blood incubated 10 minutes with E coli(1 × 108 bacteria per milliliter); ●, C5aR antagonist; and ○, control peptide. F(ab′)2 fragments of anti-CR3 (anti-CD18 and anti-CD11b) inhibited granulocyte oxidative burst dose dependently, in contrast to a control F(ab′)2(right panel). ▵ indicates baseline value (T0); ▴, whole blood incubated 10 minutes with E coli(1 × 108 bacteria per milliliter); ■, anti-CD18; ▪, anti-CD11b; ●, C5aR antagonist (2 μM); and ○, control mAb. One representative of 5 experiments is shown.
Effect of C5aR antagonist on FcγR (CD16, CD32, CD64) expression.
Granulocytes expressed abundant amounts of CD16/FcγRIII constitutively (MFI 4384 compared with 7 in the isotype control).E coli (1 × 108 bacteria per milliliter) decreased CD16 expression by 50% (MFI 2218). This decrease was fully complement dependent because incubation with the C5aR antagonist (10 μM) restored CD16 expression (MFI 4294). CD16 was neither expressed on monocytes constitutively nor after E coli incubation. CD32/FcγRII was moderately expressed constitutively both on granulocytes (MFI 200-300) and monocytes (MFI 300-400). The expression did not change after incubation with E coli. CD64/FcγRI was neither expressed on granulocytes constitutively nor after incubation with E coli. A modest constitutive expression of CD64 on monocytes was not influenced by E coli.
Role of CR3 (CD11b/CD18) in E coli–induced oxidative burst
The powerful effect of blocking C5aR both on the oxidative burst and on CR3 expression led us to investigate a possible role for CR3 in induction of E coli–induced burst. F(ab′)2fragments of anti-CD18 and anti-CD11b inhibited granulocyte oxidative burst dose dependently (Figure 8, right panel). The inhibition with anti-CD11b was as efficient as for the C5aR antagonist. Similar results were obtained for monocytes, although less pronounced. Inhibition of the oxidative burst was not seen by blocking CD14, although anti-CD14 efficiently reduced cytokine secretion (see below).
Role of complement in phagocytosis of E coli
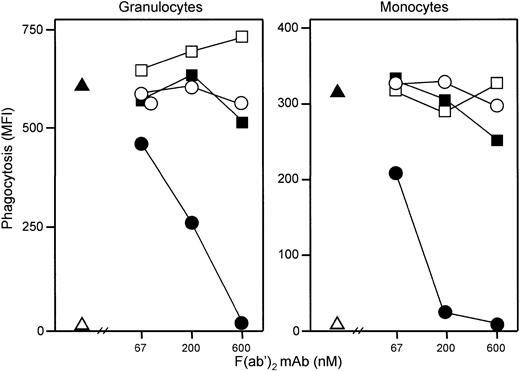
Effect of C5aR antagonist on phagocytosis.
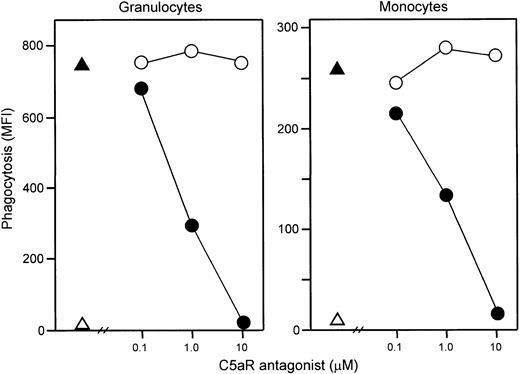
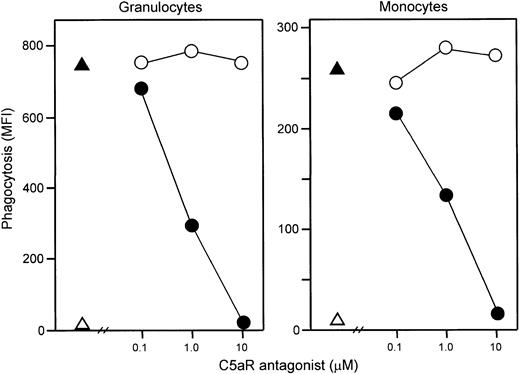
Addition of E coli (1 × 108 bacteria per milliliter) led to an immediate and efficient phagocytosis. Samples obtained just after addition of the bacteria to the whole blood revealed 5 × 105 CFUs per milliliter, whereas after 20 minutes of incubation CFUs could not be detected. C5aR antagonist completely abolished both granulocyte and monocyte phagocytosis as measured by flow cytometry (Figure9).
Effect of C5aR antagonist on phagocytosis.
The C5aR antagonist dose dependently inhibited granulocyte (left) and monocyte (right) phagocytosis of E coli. Phagocytosis was completely abolished at 10 μM C5aR antagonist, whereas control peptide had no effect. One representative of 5 experiments is shown. ▵ indicates baseline value (T0); ▴, whole blood incubated 20 minutes with E coli (1 × 108 bacteria per milliliter); ●, E coli plus C5aR antagonist; and ○,E coli plus control peptide.
Effect of C5aR antagonist on phagocytosis.
The C5aR antagonist dose dependently inhibited granulocyte (left) and monocyte (right) phagocytosis of E coli. Phagocytosis was completely abolished at 10 μM C5aR antagonist, whereas control peptide had no effect. One representative of 5 experiments is shown. ▵ indicates baseline value (T0); ▴, whole blood incubated 20 minutes with E coli (1 × 108 bacteria per milliliter); ●, E coli plus C5aR antagonist; and ○,E coli plus control peptide.
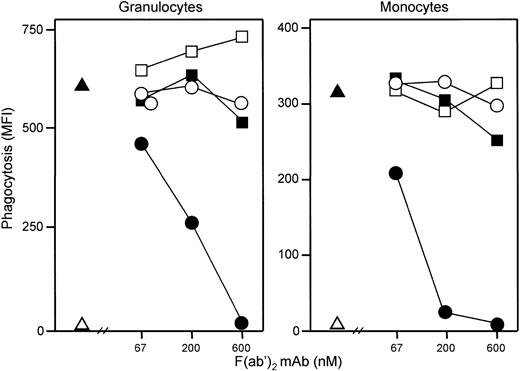
Effect of anti-CD11b and anti-FcγR on phagocytosis.
Both granulocyte and monocyte phagocytosis was completely abolished by anti-CD11b, whereas anti-CD16, anti-CD32, and anti-CD64 had no effect (Figure 10). A quenching solution neutralizing the fluorescence from bacteria bound to the cell surface is regularly used in the Phago-test. Experiments performed without adding this solution demonstrated that E coli did not bind to the leukocytes when the phagocytosis was inhibited by C5aR antagonist. A modest C3d and IgG deposition was detected on opsonizedE coli (MFI 7 and 16, respectively, compared with 3 as background staining on nonopsonized E coli). In comparison, incubation of the bacteria in lepirudin plasma for 10 minutes (comparable situation to the whole blood model) increased C3d deposition to MFI 669 and IgG deposition to MFI 103.
Effect of anti-CR3 and anti-FcγR on phagocytosis.
Anti-CR3 (CD11b) antibody dose dependently inhibited granulocyte (left) and monocyte (right) phagocytosis of E coli preopsonized with C3 and IgG, in contrast to anti-FcγRI (CD64), anti-FcγRII (CD32), and anti-FcγRIII (CD16) antibodies, which had no effect. All antibodies were F(ab′)2 fragments of mouse mAbs blocking the function of the receptors (see “Materials and methods”). One representative of 4 experiments is shown. ▵ represents baseline value (T0); ▴, whole blood incubated 20 minutes with E coli (1 × 108 bacteria per milliliter); ●, anti-CD11b; ○, anti-CD16; ■, anti-CD32; and ▪, anti-CD64.
Effect of anti-CR3 and anti-FcγR on phagocytosis.
Anti-CR3 (CD11b) antibody dose dependently inhibited granulocyte (left) and monocyte (right) phagocytosis of E coli preopsonized with C3 and IgG, in contrast to anti-FcγRI (CD64), anti-FcγRII (CD32), and anti-FcγRIII (CD16) antibodies, which had no effect. All antibodies were F(ab′)2 fragments of mouse mAbs blocking the function of the receptors (see “Materials and methods”). One representative of 4 experiments is shown. ▵ represents baseline value (T0); ▴, whole blood incubated 20 minutes with E coli (1 × 108 bacteria per milliliter); ●, anti-CD11b; ○, anti-CD16; ■, anti-CD32; and ▪, anti-CD64.
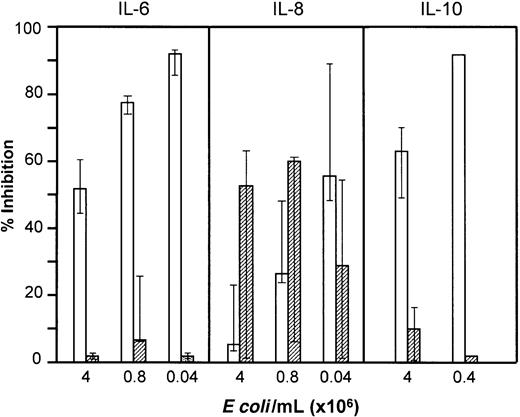
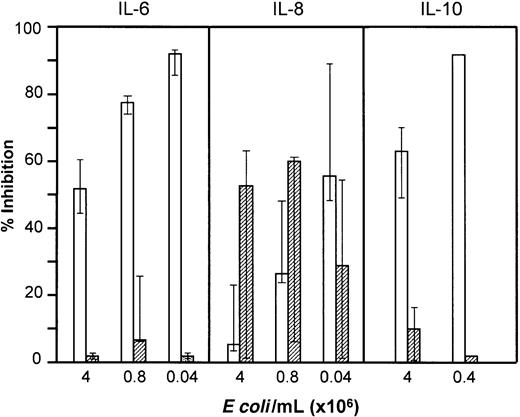
Role of complement in E coli–induced cytokine formation
Plasma concentrations of TNF-α, IL-6, and IL-8 increased substantially after 2 hours and IL-10 after 18 hours of incubation with E coli. Cytokine production was induced by smaller amounts of E coli than required for oxidiative burst (Figure 11). Spontaneous cytokine release during incubation with PBS was not seen for TNF-α, IL-6, or IL-10, whereas a modest release of IL-8 was observed. Inhibition of complement had no effect on E coli–induced secretion of TNF-α, IL-6, or IL-10, whereas IL-8 formation was partly complement dependent. In contrast, anti-CD14 very efficiently inhibited TNF-α, IL-6, and IL-10 secretion, whereas the effect on IL-8 was less pronounced. By combining compstatin and anti-CD14, IL-8 secretion was abolished. Inhibition of E coli–induced IL-6, IL-8, and IL-10 secretion by compstatin and anti-CD14 is shown in Figure 11. The results for TNF-α were virtually identical to those for IL-6. The C5aR antagonist had the same effect as compstatin on the cytokines.
Effect of anti-CD14 and compstatin onE coli–induced cytokine formation.
IL-6 and IL-8 (median and 25-75 percentile of 5 experiments) and IL-10 (median and range of 3 experiments for higher dose; 1 experiment with lower dose) were measured in plasma after incubation of whole blood withE coli for 2 and 18 hours, respectively. The effect of anti-CD14 (clone 18D11, F(ab′)2 fragments, 0.7 μM) (open columns) and compstatin (50 μM) (hatched columns) is presented as percent inhibition of cytokine formation compared with control peptide or control F(ab′)2 antibody.
Effect of anti-CD14 and compstatin onE coli–induced cytokine formation.
IL-6 and IL-8 (median and 25-75 percentile of 5 experiments) and IL-10 (median and range of 3 experiments for higher dose; 1 experiment with lower dose) were measured in plasma after incubation of whole blood withE coli for 2 and 18 hours, respectively. The effect of anti-CD14 (clone 18D11, F(ab′)2 fragments, 0.7 μM) (open columns) and compstatin (50 μM) (hatched columns) is presented as percent inhibition of cytokine formation compared with control peptide or control F(ab′)2 antibody.
Discussion
The inappropriate effects of commonly used anticoagulants on the various inflammatory systems have been a major limitation when using whole blood to study inflammatory reactions. A main goal for an optimal whole blood model is to keep all potential effector systems functional and able to cross talk by mutual interaction and still avoid coagulation. Anticoagulants containing calcium chelators (eg, EDTA and citrate) inhibit complement activation as well as a number of other plasma and cell inflammation markers and therefore cannot be applied. Heparin has been used, although it is known that various effects on complement may influence the results. We confirm in the present study that low concentrations of heparin enhance, whereas high concentrations inhibit, complement activation.18-20 The known enhancing effect of heparin on C1inh binding to C1r and C1s18,37 was also evident from the present study. Heparin binds to a number of plasma proteins and also has adverse effects on leukocytes21,22 and platelets,23disqualifying it as a suitable anticoagulant for whole blood studies on inflammation.
In contrast to heparin, hirudin is a specific thrombin inhibitor.24 Thus, the coagulation cascade is kept functional upstream to thrombin formation. Hirudin has been used successfully in a whole blood model to study monocyte and platelet activation,21 and lepirudin per se has no effect on IL-6 or tissue factor formation.38 We demonstrate for the first time that the hirudin analog lepirudin has no adverse effects on complement activation and can be recommended as anticoagulant in models to study the role of complement in inflammation. The limitation of the model is the effect of lepirudin on thrombin. Thrombin is known to be an inflammatory mediator,39 and hirudin-based peptides were found to inhibit thrombin-induced inflammatory effects on endothelial cells.40 Because whole blood models need anticoagulation, it is impossible to circumvent this problem completely. However, we suggest that lepirudin presently is the best alternative in complement activation studies because its effect is limited to thrombin inhibition.
The oxidative burst reflects formation of reactive oxygen species such as the superoxide anion and hydrogen peroxide. Flow cytometric detection of the oxidative burst has the advantage that single-cell populations can be examined individually in whole blood. This assay was found to be very stable and reproducible. No spontaneous oxidative burst took place during the incubation period. In all individuals tested the oxidative burst response was largely dependent on complement. The oxidative burst of granulocytes was generally stronger than for monocytes; however, granulocyte burst was also more efficiently reduced by complement inhibition. Reactive oxygen species are highly toxic and may damage host tissue and interfere with homeostasis. They are known to activate the transcription factor nuclear factor–κB (NF-κB),41 which regulates a number of genes, encoding for cytokines, adhesion molecules, and other mediators of inflammation. Because we show that complement is a major primary inducer of E coli–mediated oxidative burst, inhibition of complement may have therapeutic implications in clinical settings in which the oxidative burst contributes to the disease process, such as in Gram-negative septicemia. Of particular importance is that blocking the C5a-C5aR interaction inhibited CR3 up-regulation and that blocking of CR3 efficiently inhibited the oxidative burst. CR3 is one of the most important adhesion molecules expressed during inflammation and has a number of biologic functions,42which may be attenuated by inhibition of the C5a-C5aR interaction.
Phagocytosis and oxidative burst are 2 closely linked leukocyte defense events. Blocking the C5aR inhibited phagocytosis virtually completely both in granulocytes and monocytes. Furthermore, opsonization of the bacteria to the cell surface was also abolished. The opsonization and subsequent phagocytosis was fully dependent on C5aR-induced CR3 up-regulation because blocking of CR3 also abolished phagocytosis. TheE coli–induced marked down-regulation of granulocyte CD16 (FcγRIII) was also shown to be completely C5aR dependent. However, the FcγRs were not involved in the opsonization and phagocytosis ofE coli. Thus, blocking of the FcγRs CD16, CD32, and CD64 had no effect on phagocytosis despite the presence of IgG on the preopsonized bacteria. The anti-FcγR antibodies used bound to the receptors and are documented to block the function of the various forms of the receptors (see “Materials and methods”). When the bacteria were incubated in lepirudin plasma, IgG deposition increased modestly, whereas the deposition of C3 was markedly enhanced. These data indicate that iC3b binding to CR3 is by far the most important mechanism in opsonization and phagocytosis of E coli in human whole blood and that this mechanism is fully dependent on C5a-C5aR interaction. A crucial role for the C5aR in the bacterial defense in the respiratory tract has previously been demonstrated in C5aR-deficient mice.43 Recently it was also shown that group A streptococci could be phagocytosed via CR3 in a C5aR-dependent manner but, in contrast to our findings with E coli, exogenous C5a had to be added.44
The concentrations of E coli used in this study are similar to lethal and sublethal doses used in primate models of septicemia.45 Furthermore, our data confirm that the CFU count greatly underestimates the bacterial load in human whole blood,46 most likely due to rapid phagocytosis of bacteria by leukocytes, as demonstrated in the present study by the rapid decline in CFUs during E coli incubation in human whole blood. This suggests that the experimental conditions in the present in vitro model may be pathophysiologically relevant for an in vivo situation.
Since Weisman et al3 in 1990 demonstrated that complement inhibition by recombinant soluble complement receptor 1 (sCR1) markedly reduced the tissue damage during experimental myocardial infarction, numerous studies have highlighted complement inhibition as an important issue in various pathophysiologic and clinical conditions. Thus, studies of specific inhibition of complement using either human regulatory proteins, antibodies to complement components or peptides to block activation or receptors, have demonstrated that complement is a key mediator of tissue injury and inflammation.47However, limited data are available concerning the role of complement as a primary inducer of the other branches of the inflammatory network. The C5aR antagonist we used in the present study has been shown to reduce the adverse effects of endotoxic shock and immune complex–mediated tissue damage (reverse-passive arthus reaction).48 Similarly, antibodies neutralizing C5a have been found to protect against multiorgan failure and death in a rat cecal ligation and puncture (CLP) model of septicemia.49 50 These favorable effects may be explained by preservation of neutrophil function, attenuation of CR3 expression, and reduced production of reactive oxygen species, although inhibition of other secondary mediators triggered by complement activation may also be involved.
Complement activation is known to induce cytokine production in models using isolated cells, and the present C5aR antagonist was recently shown to inhibit lipopolysaccharide (LPS)-dependent C5aR-induced IL-1β, TNF-α, and IL-6 production in monocytes.51 In our whole blood model we found a partly complement-dependent induction of IL-8, whereas TNF-α, IL-6, and IL-10 production was complement independent and efficiently blocked by anti-CD14, indicating a differential regulation of the cytokine production. The data for TNF-α, IL-6, and IL-10 are consistent with the well-known CD14/Toll-like receptor-dependent cytokine induction. The doses of E coli needed to induce cytokine formation were lower than those required for the oxidative burst, and it may be speculated that complement-induced oxidative burst depends more on whole bacteria than the LPS-induced cytokine formation.
Taken together, our results show that the lepirudin-based human whole blood model is useful for studying the roles of complement activation within the inflammatory network. We have demonstrated that E coli–induced oxidative burst and phagocytosis is dependent on C5aR-mediated up-regulation of CR3. Inhibition of complement-mediated oxidative burst could be a therapeutic approach to attenuate tissue damage.
The authors thank Lynn Spruce for peptide synthesis and Dr William T. Moore for mass spectrometric analysis of the peptides. Berit Brusletto is greatly acknowledged for quantifying endotoxin in theE coli suspension and Arumugam Kugendradas for performing the MPO assays.
Supported by the Norwegian Council on Cardiovascular Disease; the Health and Rehabilitation Foundation; Tanox, Houston, TX; Odd Fellow Foundation; Gythfeldt's Legacy; and NIH grant GM62134.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Tom Eirik Mollnes, Institute of Immunology, The National Hospital, N-0027 Oslo, Norway; e-mail:t.e.mollnes@labmed.uio.no.

![Fig. 7. Effect of the C5aR antagonist AcF[OPdChaWR] on. / E coli–induced oxidative burst in granulocytes.The left panel shows dose-response effect of the C5aR antagonist using 1 × 108 E coli per milliliter (1 representative of 5 experiments is shown). The right panel shows the effect of 2 μM C5aR antagonist on E coli–induced oxidative burst by reducing the concentration ofE coli (median and range of triplicate). The oxidative burst is given as median fluorescence intensity (MFI). ▵ indicates baseline value (T0); ▴, whole blood incubated 10 minutes withE coli (1 × 108 bacteria per milliliter); ●, E coli plus C5aR antagonist; ○, E coli plus control peptide; and ■, E coli plus PBS.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/100/5/10.1182_blood.v100.5.1869.h81702001869_1869_1877/3/m_h81723034007.jpeg?Expires=1767812698&Signature=1Ts~nn--vRpyQCEx6RoHIY2A49lzG7sa93HhcBfUQ2ze42y3Fqi2dIes2MqzDFewe9zLYpVhPuigRqzfWubumPcyo-CIMmePO-kQC8nV95f3CaV2FGamgQTySNpQGlN~FIMjMuvMjzHvPXX~od12PV0wVx9L4ayV8IY8tgmvIAC3vynfkwyN7wIIE-B2m~-M-OlgoPn-gXmF9iHppcVbGCzjzp8x8RJWeMDWm4TCxZcrEJpIl2MZNNnjxZ4p17rivPsPZFgCioOl95ABzkw9Pk-ONb4ai9-DoKJ4MG9VDNq4LqVrQGuylo3Z5QVmDxIk931QOQ2MCC8UsJuAbFdf9w__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)


![Fig. 7. Effect of the C5aR antagonist AcF[OPdChaWR] on. / E coli–induced oxidative burst in granulocytes.The left panel shows dose-response effect of the C5aR antagonist using 1 × 108 E coli per milliliter (1 representative of 5 experiments is shown). The right panel shows the effect of 2 μM C5aR antagonist on E coli–induced oxidative burst by reducing the concentration ofE coli (median and range of triplicate). The oxidative burst is given as median fluorescence intensity (MFI). ▵ indicates baseline value (T0); ▴, whole blood incubated 10 minutes withE coli (1 × 108 bacteria per milliliter); ●, E coli plus C5aR antagonist; ○, E coli plus control peptide; and ■, E coli plus PBS.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/100/5/10.1182_blood.v100.5.1869.h81702001869_1869_1877/3/m_h81723034007.jpeg?Expires=1768321198&Signature=THfr92y~dEDT6WIr880dff4g1amzd2bmr6Q9EhyOZ3rT2JQVqWuVaWUbyVRXs3W~CVO2HNYp87~fzPuG9fx7tY3uJNg0dMrdNphJHLbspmrj2FaPPdxM31Xh1KhReWwdgI4hIcCHoGBsrgVXrqkQcB1HdKUp7UgTd~6VZffzjVTEPhGSE6BGvyXX3fFE82Hy00WcTgVE-YqNQKAt1TJ9YdIyPK6hrJHvb6-yBGFRPOGDfeBanCg1sxPD~f2vNUp~MNf~QJuJRzrbbG7asg2ua~HnOJNYHpUqWQvrzaIN6-YJXNCp2OWgbvCQFz-UdprlfEFdg9-ZvOKskXCX6q4Uhg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)