Abstract
2-Methoxyestradiol (2ME2) an estrogen derivative, induces growth arrest and apoptosis in leukemic cells and is also antiangiogenic. In this study, we demonstrate that 2ME2 inhibits growth and induces apoptosis in multiple myeloma (MM) cell lines and patient cells. Significantly, 2ME2 also inhibits growth and induces apoptosis in MM cells resistant to conventional therapies including melphalan (LR-5), doxorubicin (Dox-40 and Dox-6), and dexamethasone (MM.1R). In contrast to its effects on MM cells, 2ME2 does not reduce the survival of normal peripheral blood lymphocytes. Moreover, 2ME2 enhances Dex-induced apoptosis, and its effect is not blocked by interleukin-6 (IL-6). We next examined the effect of 2ME2 on MM cells in the bone marrow (BM) milieu. 2ME2 decreases survival of BM stromal cells (BMSCs), as well as secretion of vascular endothelial growth factor (VEGF), and IL-6 triggered by the adhesion of MM cells to BMSCs. We show that apoptosis induced by 2ME2 is mediated by the release of mitochondrial cytochrome-c (cyto-c) and Smac, followed by the activation of caspases-8, -9, and -3. Finally, 2ME2 inhibits MM cell growth, prolongs survival, and decreases angiogenesis in a murine model. These studies, therefore, demonstrate that 2ME2 mediates anti-MM activity directly on MM cells and in the BM microenvironment. They provide a framework for the use of 2ME2, either alone or in combination with Dex, to overcome drug resistance and to improve outcome in MM.
Introduction
2-Methoxyestradiol (2ME2) a natural metabolite of estradiol, is a potent antitumor and antiangiogenic agent in leukemic cells.1-7 Although 2ME2 has potent antiproliferative activity in various tumorigenic and nontumorigenic cell lines, the mechanisms mediating the biologic effects of 2ME2 remain unclear. We and others8-11 have demonstrated that multiple myeloma (MM) cells express estrogen receptors (ERs) and that antiestrogen compounds, such as tamoxifen and ICI 182 780, induce apoptosis in MM cells. Treatment of MM cells with estrogen (E2) alone did not alter the growth of MM cells.9 Moreover, despite its being a natural derivative of estradiol (E2), 2ME2 binds poorly to the ERs; therefore, the antiproliferative effects of 2ME2 are not mediated by ERs.12 13 These findings, coupled with our studies, suggest that 2ME2-mediated effects on MM cells are independent of ER.
Previous studies using xenografts and metastatic disease models in mice have suggested that 2ME2 targets not only the tumor cell but also the tumor vasculature.2,14 Recent reports of increased bone marrow (BM) angiogenesis in multiple myeloma (MM),15-17coupled with the known antiangiogenic properties of 2ME2,2 14 provide a potential rationale for evaluating 2ME2 as a novel therapeutic in MM.
In the present study, we characterize the in vitro effects of 2ME2 against MM cells in the BM microenvironment and its in vivo anti-MM activity in an animal model. We demonstrate that 2ME2 induces apoptosis in MM cells resistant to conventional therapies, and we delineate the signaling cascades mediating its anti-MM activity. Significantly, we show that 2ME2 directly decreases cytokine secretion in bone marrow stromal cells (BMSCs), thereby modulating the tumor BM environment. These data, along with our demonstration of its anti-MM and antiangiogenesis effects in vivo, provide the framework for clinical trials of 2ME2 to improve patient outcome in MM.
Materials and methods
Cell culture and reagents
Dex-sensitive MM.1S and Dex-resistant MM. and 1R human MM cell lines were kindly provided by Dr Steven Rosen (Northwestern University, Chicago, IL).18 Dox-6, Dox-40, and LR-5 drug-resistant RPMI 8226 human MM cell lines were kindly provided by Dr William Dalton (Moffit Cancer Center, Tampa, FL).19 U266 MM cells were obtained from the American Type Culture Collection (Rockville, MD). All cell lines were grown in RPMI 1640 medium supplemented with 10% heat-inactivated fetal bovine serum (FBS), 100 U/mL penicillin, 100 μg/mL streptomycin, and 2 mM l-glutamine. Drug-resistant cell lines were cultured with either Dox or Dex to confirm their lack of drug sensitivity. Mononuclear cells were prepared from MM patient BM samples by Ficoll-Hypaque density gradient centrifugation. The MM cells were freshly isolated from patients who had relapses after multiple prior therapies, including therapy with dexamethasone, melphalan, or thalidomide. Informed consent was obtained from all patients in accordance with the Helsinki protocol. Tumor cells (CD138+97.0% ± 2.0%) were isolated by CD138+ selection method using CD138 (Syndecan-1) Micro Beads and Auto MACS magnetic cell sorter machine, according to the manufacturer's instructions (Miltenyi Biotec, Auburn, CA). CD138+ myeloma cells were viable (94%-97%) for 2 to 3 weeks in vitro. Cells were treated with 0.05 μM Dex (Sigma Chemical, St Louis, MO) or 2ME2 (1-20 μM).
Proliferation assays
BrdU incorporation assay was performed using the BrdU proliferation assay kit, according to manufacturer's instructions (Calbiochem, San Diego, CA) with some modifications.20Briefly, cells were seeded onto 96-well plates in RPMI 1640 medium containing 10% FBS. Medium was replaced 24 hours later with RPMI 1640 medium containing 2% FBS, with or without 2ME2. Plates were incubated for 48 hours and 72 hours, pulsed for 2 to 6 hours with BrdU primary and secondary antibodies, and developed using a colorimetric reaction. Absorbance at 460 nm was measured using a Molecular Device plate reader.
MTT assays
Cell viability was assessed by 3-(4,5-dimethylthiozol-2-yl)-2,5-diphenyltetrazolium bromide (MTT; Chemicon International, Temecula, CA) assay according to the manufacturer's instructions (Roche Molecular Biochemical), with some modifications. Cells were seeded in 96-well plates in RPMI 1640 medium containing 5% FBS. 2ME2 or Dex was added 24 hours later and incubated for 72 hours. Cell viability was evaluated as previously described.21 22
Enzyme linked immunosorbent assays
Conditioned media were generated from 24-hour cultures of MM patient-derived BMSCs, MM.1S cells, and BMSCs + MM.1S cells, either untreated or treated with 3 μM 2ME2; IL-6 and VEGF levels were measured using enzyme-linked immunosorbent assay (ELISA; R&D Systems, Minneapolis, MN), as previously described.23 BMSC viability at 24 hours was greater than 80%, as determined by trypan blue exclusion assay. Briefly, 96-well plates were coated with either anti–human VEGF or IL-6 antibodies overnight, washed, and then blocked with 300 μL phosphate-buffered saline (PBS), 1% bovine serum albumin (BSA), 5% sucrose, and 0.05% NaN3 for 2 hours. After washing, 100 μL sample or standards diluted in Tris-Cl, 0.1% BSA, and 0.05% Tween 20 (pH 7.3) was added to the wells and incubated for 2 hours at room temperature. The cells were rinsed, and either biotinylated anti–human VEGF or IL-6 antibodies were added for 2 hours at room temperature. After a further washing step, the wells were incubated with streptavidin–horseradish peroxidase for 20 minutes at room temperature and rinsed. The reaction was started by the addition of 100 μL H2O2 and tetramethylbenzidine for 30 minutes at room temperature. After stopping the reaction with 1 μM H2SO4, the optical density of each well was detected by means of a microtiter plate reader at 450 nm, with correction at 540 nm.
Western blotting
Total cell lysates and cytosolic extracts were prepared as previously described.24 Briefly, equal amounts of proteins were resolved by 10% sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to nitrocellulose membranes. Proteins were then transferred to nitrocellulose filters, blocked by incubation in 5% dry milk in PBST (0.05% Tween-20 in PBS), and probed with anti–cyto-c, anti-Smac (kindly provided by Dr Xiaodong Wang, University of Texas Southwestern Medical Center, Dallas), anti–caspase-3 (Santa Cruz, CA), anti–caspase-8 and -9 (Transduction Laboratories, Detroit, MI), or anti-SHP2 (Santa Cruz, CA) antibodies. Blots were then developed by enhanced chemiluminescence (ECL; Amersham, Arlington Heights, IL). Preparation of cell lysates for PARP immunoblot analysis was performed as described using C-2-10 anti-PARP monoclonal antibody.25
Quantification of apoptosis
Dual fluorescence staining with DNA-binding fluorochrome Hoechst 33342 (HO) and propidium iodide (PI) was used to quantitate the percentage of apoptotic (PI−HO+) cells using flow cytometry (Vantage; Becton Dickinson), as previously described.26 DNA fragmentation assays were also performed, as in prior studies.27
Preparation of S-100 cytosolic fractions from MM.1S cells
MM.1S MM cells were washed twice with PBS, and the pellet was suspended in 5 ml ice-cold buffer A (20 mM HEPES [N-2-hydroxyethylpiperazine-N'-2-ethanesulfonic acid], pH 7.5, 1.5 mM MgCl2, 10 mM KCl, 1 mM EDTA [ethylenediaminetetraacetic acid], 1 mM EGTA [ethyleneglycotetraacetic acid], 1 mM dithiothreitol, 0.1 mM phenylmethylsulfonyl fluoride, 10 μg/mL leupeptin and aprotinin and pepstatin A) containing 250 nM sucrose. Cells were homogenized by douncing 3 times in a Dounce homogenizer with a sandpaper-polished pestle. Cytosolic fractions were isolated as previously described.24 Immunoblots were scanned using an LKB producer (Bromma, Sweden) Ultrascan XL laser densitometer and analyzed with the Gelscan software package. Signal intensity was determined in a linear range and normalized to that for SHP2.
Drug preparation and in vivo evaluation in murine tumor model
For animal studies, 2ME2 was kindly provided by Entremed (Rockville, MD). 2ME2 (100 mg/kg) was suspended in 0.5% carboxymethylcellulose (Sigma). Six-week-old triple immune-deficient beige nude xid (BNX) mice were obtained from Frederick Cancer Research and Development Center (Frederick, MD). All animal studies were conducted according to protocols approved by the Animal Ethics Committee of the Dana Farber Cancer Institute. Mice were observed daily for signs of toxicity. Terminal bleeding was performed under anesthesia using isoflurane inhalation, and animals were killed by CO2asphyxiation.
To determine the in vivo anti-MM activity of 2ME2, mice were inoculated subcutaneously in the flank with 3 × 107 RPMI 8226 MM cells in 100 μL RPMI 1640 medium, together with 100 μL Matrigel (Becton Dickinson, Bedford, MA). Drug treatment was started after the development of measurable tumor. The drug (100 mg/kg) was given daily using an orogastric feeding tube, with carboxymethylcellulose 0.5% as a control. Serial caliper measurements of perpendicular diameters were taken every other day to calculate tumor volume using the following formula: 4/24 × (shortest diameter)2 × (longest diameter). Animals were killed if the tumor was 2 cm or larger or it was necrotic. Tumor was then harvested for hematoxylin and eosin staining and microvessel density (MVD) evaluation. For tumor growth studies, 10 mice were used in each group.
CD31 immunostaining
Tumor tissues were fixed in 10% neutral buffered formalin and embedded in paraffin, according to standard histologic procedures. After deparaffinization, tissue sections were pretreated with proteinase K (Roche Molecular Biochemical) at 37°C for 30 minutes before staining with rat anti–mouse CD-31 antibody (PharMingen, San Diego, CA). Positive staining was detected using secondary biotinylated rabbit anti–rat antibody, followed by incubation with streptavidin–horseradish peroxidase (DAKO, Carpenteria, CA). 3-Amino-9-ethylene carbazole (DAKO) was used as the chromogenic substrate, and sections were counterstained with Gill hematoxylin (Fisher Scientific, Fair Lawn, NJ). MVD was determined by light microscopy, as previously described.28 MVD assessment was conducted without knowledge of mouse treatment history. Areas of most intense neovascularization were counted at high magnification (× 400), and 2 investigators using double-headed light microscopy analyzed at least 5 separate fields. The mean MVD was compared among the treatment groups and analyzed using the Student ttest.
Statistical analysis
Nonparametric tests and mixed models were used to analyze the data. These included the Wilcoxon signed rank test to compare proliferation in untreated and treated patient cells and the Jonchkeere-Terpstra (J-T) trend test for assessing the viability of MM cell lines resistant to conventional therapy and of healthy donors. The mixed model was used to adjust for repeated measures in the analysis of the 2ME2 patient MM-derived BMSCs. Analysis of in vivo data included a Wilcoxon rank-sum test on the change in the tumor volume and a log-rank test to compare the overall survival.
Results and Discussion
2ME2 induces apoptosis in MM.1S cells
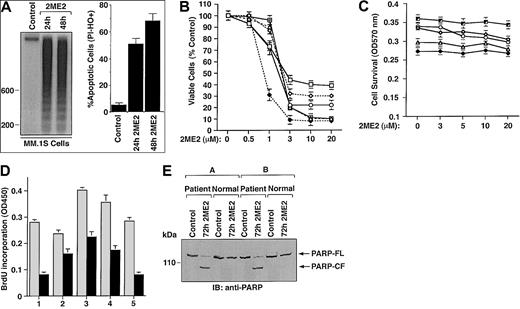
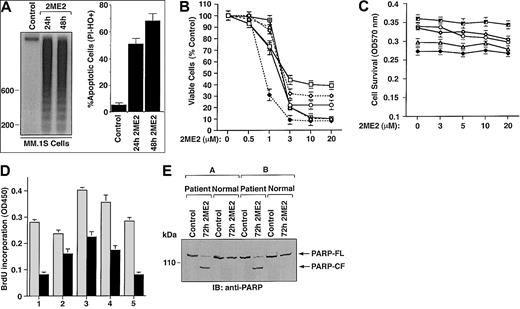
To examine whether 2ME2 induces apoptosis in the MM.1S MM cell line, we performed DNA fragmentation and flow cytometric analyses. MM.1S MM cells were cultured in the presence or absence of 2ME2 (3 μM) for 24 and 48 hours. DNA cleavage was analyzed by agarose gel electrophoresis of the α32P-ATP labeled genomic DNA (Figure 1A, left panel). Low to undetectable DNA cleavage was observed in the untreated cells; in contrast, 2ME2 induced significant cleavage of DNA into oligonucleosomal fragments of approximately 200 base pairs. This pattern was more pronounced following treatment with 2ME2 at 48 hours. The finding that 2ME2 induces fragmentation of DNA into multiples of nucleosome-sized fragments confirms changes in chromatin structure observed during apoptosis or programmed cell death.29Apoptosis induced by 2ME2 was further confirmed by flow cytometric analysis using dual PI and Hoechst staining. Treatment of cells with 2ME2 (3 μM) significantly (P < .005) increased the percentage of apoptotic cells (Figure 1A, right panel).
Selective cytotoxicity of 2ME2 against human MM cells.
(A) MM.1S cells were treated with 2ME2 (3 μM) for the indicated times and analyzed for apoptosis by DNA fragmentation (left panel is a representative of 3 separate experiments with similar results) and flow cytometric analysis of PI− and HO+ apoptotic cells (right panel is the mean ± SD from 3 independent experiments;P < .005). (B) MTT assay was performed after incubation of MM cell lines (MM.1S, ▵; RPMI-8226, ○; LR-5, ♦; Dox-6, ⋄; Dox-40, ■; MM.1R, ) with the indicated doses of 2ME2 for 72 hours. Results are mean ± SD from 5 independent experiments; P < .0001 for all cell lines. (C) Effect of treatment with 2ME2 (0-20 μM) for 72 hours on normal lymphocyte viability, assessed by MTT assay. Results are the mean ± SD of 5 independent experiments; P = 0.23 from J-T test for trend. (D) MM cells (CD138+) from 5 patients (patients 1-5) were treated with 2ME2 (9 μM) for 72 hours, followed by BrdU assay. Values are the mean ± SD of triplicate samples (P = .06); experiments were repeated 3 times with similar results. (E) 2ME2 induces proteolytic cleavage of PARP in patient MM cells. CD138+ cell from 2 MM patients and normal lymphocytes from 2 healthy donors were treated with 2ME2 (9 μM) for 72 hours and harvested, and total protein lysates were subjected to SDS-PAGE analysis. Immunoblot analysis of the lysates was performed with anti-PARP antibody. FL indicates full length; CF, cleaved fragment.
) with the indicated doses of 2ME2 for 72 hours. Results are mean ± SD from 5 independent experiments; P < .0001 for all cell lines. (C) Effect of treatment with 2ME2 (0-20 μM) for 72 hours on normal lymphocyte viability, assessed by MTT assay. Results are the mean ± SD of 5 independent experiments; P = 0.23 from J-T test for trend. (D) MM cells (CD138+) from 5 patients (patients 1-5) were treated with 2ME2 (9 μM) for 72 hours, followed by BrdU assay. Values are the mean ± SD of triplicate samples (P = .06); experiments were repeated 3 times with similar results. (E) 2ME2 induces proteolytic cleavage of PARP in patient MM cells. CD138+ cell from 2 MM patients and normal lymphocytes from 2 healthy donors were treated with 2ME2 (9 μM) for 72 hours and harvested, and total protein lysates were subjected to SDS-PAGE analysis. Immunoblot analysis of the lysates was performed with anti-PARP antibody. FL indicates full length; CF, cleaved fragment.
Selective cytotoxicity of 2ME2 against human MM cells.
(A) MM.1S cells were treated with 2ME2 (3 μM) for the indicated times and analyzed for apoptosis by DNA fragmentation (left panel is a representative of 3 separate experiments with similar results) and flow cytometric analysis of PI− and HO+ apoptotic cells (right panel is the mean ± SD from 3 independent experiments;P < .005). (B) MTT assay was performed after incubation of MM cell lines (MM.1S, ▵; RPMI-8226, ○; LR-5, ♦; Dox-6, ⋄; Dox-40, ■; MM.1R, ) with the indicated doses of 2ME2 for 72 hours. Results are mean ± SD from 5 independent experiments; P < .0001 for all cell lines. (C) Effect of treatment with 2ME2 (0-20 μM) for 72 hours on normal lymphocyte viability, assessed by MTT assay. Results are the mean ± SD of 5 independent experiments; P = 0.23 from J-T test for trend. (D) MM cells (CD138+) from 5 patients (patients 1-5) were treated with 2ME2 (9 μM) for 72 hours, followed by BrdU assay. Values are the mean ± SD of triplicate samples (P = .06); experiments were repeated 3 times with similar results. (E) 2ME2 induces proteolytic cleavage of PARP in patient MM cells. CD138+ cell from 2 MM patients and normal lymphocytes from 2 healthy donors were treated with 2ME2 (9 μM) for 72 hours and harvested, and total protein lysates were subjected to SDS-PAGE analysis. Immunoblot analysis of the lysates was performed with anti-PARP antibody. FL indicates full length; CF, cleaved fragment.
) with the indicated doses of 2ME2 for 72 hours. Results are mean ± SD from 5 independent experiments; P < .0001 for all cell lines. (C) Effect of treatment with 2ME2 (0-20 μM) for 72 hours on normal lymphocyte viability, assessed by MTT assay. Results are the mean ± SD of 5 independent experiments; P = 0.23 from J-T test for trend. (D) MM cells (CD138+) from 5 patients (patients 1-5) were treated with 2ME2 (9 μM) for 72 hours, followed by BrdU assay. Values are the mean ± SD of triplicate samples (P = .06); experiments were repeated 3 times with similar results. (E) 2ME2 induces proteolytic cleavage of PARP in patient MM cells. CD138+ cell from 2 MM patients and normal lymphocytes from 2 healthy donors were treated with 2ME2 (9 μM) for 72 hours and harvested, and total protein lysates were subjected to SDS-PAGE analysis. Immunoblot analysis of the lysates was performed with anti-PARP antibody. FL indicates full length; CF, cleaved fragment.
Effect of 2ME2 on MM cells resistant to conventional therapy
Cytotoxic effects of 2ME2 on various MM cell lines, including cell lines resistant to conventional therapy (RPMI 8226, Dox-40, Dox-6, LR5, and MM.1R) was determined using an MTT assay, in the presence or absence of drug at various concentrations (0.5 μM, 1 μM, 3 μM, 10 μM, and 20 μM.). 2ME2 induced a significant (P < .0001) decrease in cell viability in a dose-dependent manner for all cell lines (Figure 1B). A 50% decrease in viable cells was noted at 1 to 3 μM of 2ME2 in most of the cell lines. IC50 of 2ME2 for LR-5 cells was 0.5 to 1.0 μM. We next examined the effect of 2ME2 on apoptosis in these cells. PI staining demonstrated a dose-dependent increase in the percentage of apoptotic (sub-G1) cells in response to 2ME2 (P < .001); however, estrogen (E2) alone did not induce apoptosis in these cells (data not shown). This finding is consistent with our prior study9 showing that exposure to E2 does not affect growth kinetics or induce apoptosis of these cells.
Effect of 2ME2 on normal lymphocytes
Normal lymphocytes from 5 healthy donors were also treated with various doses (0.5 μM, 1.0 μM, 3.0 μM, 10 μM, and 20 μM) of 2ME2 and were analyzed for the inhibition of proliferation and apoptosis by BrdU and PI staining, respectively. In contrast to MM cells, survival of normal lymphocytes from 5 healthy donors was not altered significantly (P = .23 from the J-T trend test), even at higher doses (3-20 μM) of 2ME2 (Figure 1C). Moreover, no significant (P = .31) apoptosis of normal lymphocytes was observed in response to 2ME2 (data not shown). Taken together, these findings indicate that 2ME2 has selective anti-MM activity.
Effect of 2ME2 on patient MM cells
We next determined the effects of 2ME2 on MM cells freshly isolated from patients who had relapsed after multiple prior therapies, including therapy with dexamethasone, melphalan, or thalidomide. Tumor cells were purified from bone marrow aspirates by CD138+ selection using CD138 (Syndecan-1) Micro Beads and Auto MACS magnetic cell sorting. Treatment of MM cells from 5 patients (patients 1-5) with 2ME2 (9 μM) significantly (P = .06) decreased growth, as measured by BrdU incorporation (Figure 1D). To further determine whether growth inhibition correlates with apoptosis in patient MM cells, we next determined the effects of 2ME2 (9 μM) on poly (ADP-ribose) polymerase (PARP) cleavage, a known signature event during apoptosis.30,31 2ME2 (9 μM) induced PARP cleavage in patient MM cells, consistent with apoptosis, but failed to induce PARP cleavage in normal lymphocytes (Figure 1E). These findings correlate with our earlier results (Figure 1C) and another study32demonstrating lack of cytotoxicity of 2ME2 (9 μM) on normal lymphocytes. Taken together, our results suggest that 2ME2 inhibits growth and induces apoptosis in patient MM cells.
Effect of 2ME2 on patient MM-derived bone marrow stromal cells
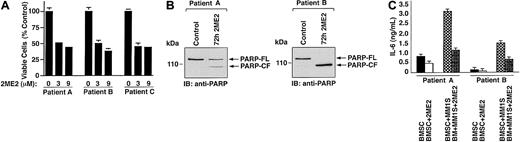
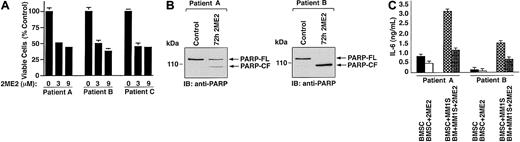
In MM, tumor cells are predominantly localized in the BM microenvironment because of their adherence to extracellular matrix proteins and to BMSCs. This interaction between MM cells and BMSCs triggers the production of cytokines mediating autocrine and paracrine growth and survival of MM cells and protection against drug-induced apoptosis.33 Therefore, we next examined the effect of 2ME2 on patient MM-derived BMSCs from 5 MM patients. Treatment of BMSCs with 2ME2 significantly (slope = −5.5; P = .002) decreases viability (Figures2A, patients A-C). To determine whether 2ME2-induced growth inhibition correlates with apoptosis in patient BMSCs, we next examined the effects of 2ME2 (9 μM) on PARP cleavage. 2ME2 (3 μM) induced PARP cleavage, consistent with apoptosis (Figure 2B, patient A and B). Taken together, these results suggest that 2ME2 not only acts directly on MM cells, but also affects the BM microenvironment.
Effect of 2ME2 on BMSCs and IL-6 secretion.
(A) Patient MM-derived BMSCs (patients A-C) were treated with 2ME2 (3-9 μM) for 72 hours, and viability was assessed by MTT assay. Results are the mean ± SD from triplicate samples; P = .002. (B) 2ME2 induced proteolytic cleavage of PARP in patient MM-BMSCs cells. BMSCs from 2MM patients were treated with 2ME2 (3 μM) for 72 hours and harvested; total protein lysates were subjected to SDS-PAGE analysis. Immunoblot analysis of the lysates was performed with anti-PARP antibody. FL indicates full length; CF, cleaved fragment. (C) Effect of 2ME2 on MM cell adhesion–induced IL-6 secretion in BMSCs. IL-6 levels were measured using IL-6–specific ELISA in supernatants of 24-hour cultures of BMSCs, MM.1S cells, and BMSCs + MM.1S cells, in the presence or absence of 2ME2 (3 μM).
Effect of 2ME2 on BMSCs and IL-6 secretion.
(A) Patient MM-derived BMSCs (patients A-C) were treated with 2ME2 (3-9 μM) for 72 hours, and viability was assessed by MTT assay. Results are the mean ± SD from triplicate samples; P = .002. (B) 2ME2 induced proteolytic cleavage of PARP in patient MM-BMSCs cells. BMSCs from 2MM patients were treated with 2ME2 (3 μM) for 72 hours and harvested; total protein lysates were subjected to SDS-PAGE analysis. Immunoblot analysis of the lysates was performed with anti-PARP antibody. FL indicates full length; CF, cleaved fragment. (C) Effect of 2ME2 on MM cell adhesion–induced IL-6 secretion in BMSCs. IL-6 levels were measured using IL-6–specific ELISA in supernatants of 24-hour cultures of BMSCs, MM.1S cells, and BMSCs + MM.1S cells, in the presence or absence of 2ME2 (3 μM).
Effect of 2ME2 on IL-6 secretion in BMSCs
Adhesion of MM cells to BMSCs triggered the secretion of factors such as IL-6 from BMSCs, which regulate the growth of MM cells in an autocrine or a paracrine manner.34 To determine whether 2ME2 also affected the secretion of IL-6, we next examined the effect of 2ME2 on IL-6 production in the BM microenvironment. As seen in Figure 2C, 2ME2 significantly (P < .005) inhibited IL-6 secretion in MM patient BMSCs triggered by MM cell adhesion. Reports that high serum levels of IL-6 contribute to clinical chemoresistance and treatment failure,35 coupled with our demonstration that 2ME2 decreases MM adhesion-induced IL-6 secretion from BMSCs, suggest that 2ME2 may overcome drug resistance in patients with advanced MM.
Effect of Dex and IL-6 on response of MM cells to 2ME2
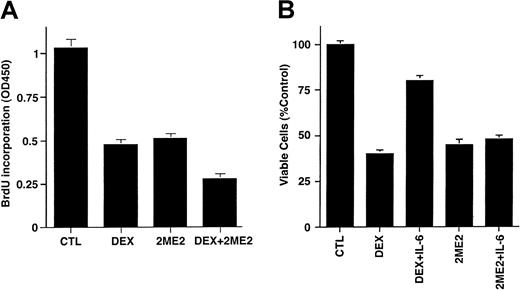
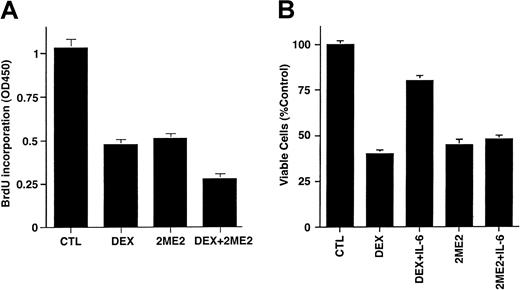
To determine whether the effects of 2ME2 are additive with conventional therapies, we next examined whether Dex (0.0005-0.5 μM) enhanced 2ME2 (3 μM) effects on proliferation of Dex-sensitive (MM.1S) MM cells. As seen in Figure 3A, the combination of 2ME2 and Dex induces more growth inhibition than the treatment of MM.1S cells with 2ME2 alone. Dex (0.05 μM) and 2ME2 (3 μM) increased growth inhibition by 30% relative to cultures with 2ME2 alone (P = .05, one-sided Wilcoxon rank-sum test). PI staining after Dex (0.05 μM) and 2ME2 (3 μM) revealed 93% ± 6.1% apoptosis versus 66% ± 4.3% cell death induced by Dex alone.
Effects of Dex and IL-6 on 2ME2-induced cytotoxicity in MM cells.
(A) MM.1S cells were cultured in control media alone and with Dex (0.05 μM), 2ME2 (3 μM), or Dex + 2ME2. At 48 hours, cells were harvested and analyzed by BrdU assay. Results are mean ± SD of 3 independent experiments (P < .005). (B) MM.1S cells were treated with 2ME2 (3 μM) or Dex (0.05 μM) in the presence or absence of IL-6 (10 ng/mL). At 48 hours, cells were harvested, and viability was analyzed by MTT assay. Median viability was 41% for Dex and 78% for Dex + IL-6 (P = .05, as determined by one-sided Wilcoxon rank-sum test), whereas for 2ME2 the median viability was 45% with 2ME2 alone and 48% with 2ME2 + IL-6 (P = .20, Wilcoxon test, as above). Results are mean ± SD of 3 independent experiments.
Effects of Dex and IL-6 on 2ME2-induced cytotoxicity in MM cells.
(A) MM.1S cells were cultured in control media alone and with Dex (0.05 μM), 2ME2 (3 μM), or Dex + 2ME2. At 48 hours, cells were harvested and analyzed by BrdU assay. Results are mean ± SD of 3 independent experiments (P < .005). (B) MM.1S cells were treated with 2ME2 (3 μM) or Dex (0.05 μM) in the presence or absence of IL-6 (10 ng/mL). At 48 hours, cells were harvested, and viability was analyzed by MTT assay. Median viability was 41% for Dex and 78% for Dex + IL-6 (P = .05, as determined by one-sided Wilcoxon rank-sum test), whereas for 2ME2 the median viability was 45% with 2ME2 alone and 48% with 2ME2 + IL-6 (P = .20, Wilcoxon test, as above). Results are mean ± SD of 3 independent experiments.
We and others36-39 have shown that IL-6 protects against Dex-induced apoptosis; hence we next examined the effect of IL-6 on 2ME2-induced apoptosis. MM.1S cells were treated with 2ME2 (3 μM) or Dex (0.05 μM), in the presence and absence of IL-6 (10 ng/mL). As seen in Figure 3B, the median viability was 41% for Dex and 78% for Dex + IL-6 (P = .05, as determined by one-sided Wilcoxon rank-sum test), whereas for 2ME2 the median viability was 45% with 2ME2 alone and 48% with 2ME2 + IL-6 (P = .20, Wilcoxon test, as above). These findings suggest that IL-6 failed to abrogate the effects of 2ME2 on MM.1S cells. In contrast, and as in our prior studies,38 IL-6 blocked Dex-induced decreases in MM.1S cell viability. Taken together, our data suggest that 2ME2 overcomes the growth and protective effects of IL-6 on MM cells, and they indicate distinct mechanisms of action for 2ME2 and Dex against MM cells.
2ME2 induces mitochondrial release of cytochrome-c and Smac
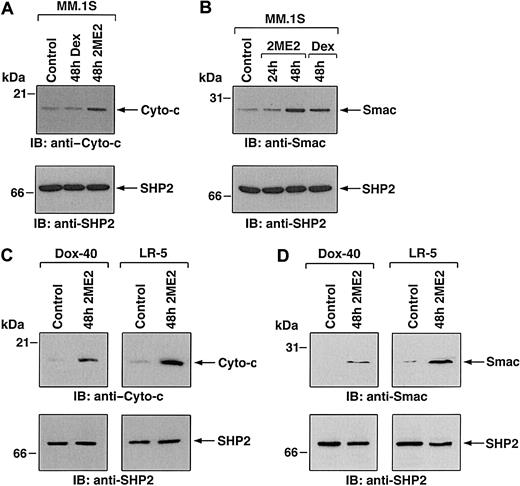
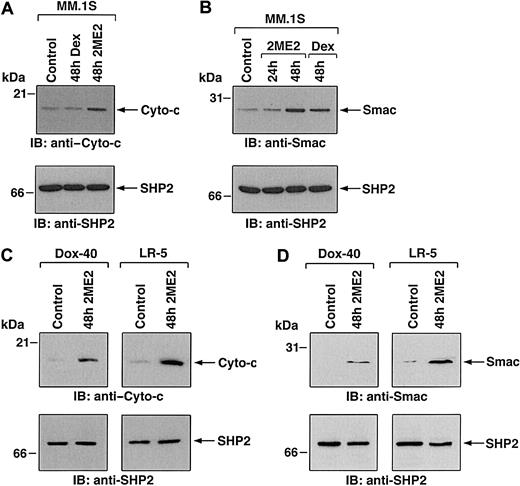
Having shown the biologic sequelae of 2ME2 in MM cells, we next examined the signaling mechanisms mediating apoptosis triggered by 2ME2. Our prior studies have characterized Dex-induced apoptotic mechanisms in MM.1S MM cells, and we therefore studied 2ME2 effects using these cells to compare differential signaling cascades triggered by Dex versus 2ME2. Previous studies have shown that 2ME2-induced apoptosis involves mitochondrial damage.32 We first determined whether treatment of MM.1S cells is associated with the release of mitochondrial apoptogenic proteins, cyto-c and Smac. Our recent studies have demonstrated that Smac, but not cyto-c, is released from mitochondria into the cytosol during Dex-induced apoptosis in MM.1S cells.24,40 To determine whether 2ME2-induced apoptosis in MM.1S cells is associated with the mitochondrial release of cyto-c, Smac, or both, we treated MM.1S cells with 2ME2 for various times and analyzed cytosolic and mitochondrial extracts for levels of cyto-c and Smac. As seen in Figure 4A-B (upper panels), 2ME2 increased cyto-c and Smac levels in cytosol at 48 hours. Densitometric analysis of the immunoblot demonstrated that 2ME2 induces a 4- to 5-fold increase in cytosolic cyto-c and Smac levels compared with untreated cells (Figure 4A-B). The increase in cyto-c and Smac levels was associated with a concomitant decrease in mitochondrial Smac or cyto-c levels (data not shown). Moreover, the 2ME2-induced increases in cytosolic cyto-c or Smac levels were specific because no changes were observed in levels of cytosolic SHP2 (Figure 4A-B, lower panels). These results suggest that 2ME2-induced apoptosis is accompanied by the accumulation of Smac and cyto-c in the cytosol. In contrast, and as in our prior studies,24 Dex-induced apoptosis was associated with the mitochondrial release of Smac, but not of cyto-c (Figure 4A-B).
2ME2 induces mitochondrial release of cytochrome-c and Smac.
(A,B) MM.1S cells were treated with 2ME2 (3 μM) or Dex (0.05 μM) and were harvested at 48 hours. Cytosolic proteins were separated by 12.5% SDS-PAGE and analyzed by immunoblotting with anti–cyto-c (A, upper panel) or anti-Smac (B, upper panel) antibodies. As a control for equal loading of proteins, filters were also reprobed with anti-SHP2 antibody (A and B, lower panels). Blots are representative of 3 independent experiments. (C,D) Dox-40 and LR-5 MM cells were treated with 2ME2 (3 μM) and harvested at 48 hours. Cytosolic proteins were separated by 12.5% SDS-PAGE and analyzed by immunoblotting with anti–cyto-c (C, upper panels) or anti-Smac (D, upper panel) antibodies. As a control for equal loading of proteins, filters were reprobed with anti-SHP2 antibody (C and D, lower panels). Blots are representative of 3 independent experiments. Densitometric analysis of the immunoblot demonstrated that 2ME2 induced a 4- to 5-fold increase in the cytosolic cyto-c and Smac levels compared with untreated cells.
2ME2 induces mitochondrial release of cytochrome-c and Smac.
(A,B) MM.1S cells were treated with 2ME2 (3 μM) or Dex (0.05 μM) and were harvested at 48 hours. Cytosolic proteins were separated by 12.5% SDS-PAGE and analyzed by immunoblotting with anti–cyto-c (A, upper panel) or anti-Smac (B, upper panel) antibodies. As a control for equal loading of proteins, filters were also reprobed with anti-SHP2 antibody (A and B, lower panels). Blots are representative of 3 independent experiments. (C,D) Dox-40 and LR-5 MM cells were treated with 2ME2 (3 μM) and harvested at 48 hours. Cytosolic proteins were separated by 12.5% SDS-PAGE and analyzed by immunoblotting with anti–cyto-c (C, upper panels) or anti-Smac (D, upper panel) antibodies. As a control for equal loading of proteins, filters were reprobed with anti-SHP2 antibody (C and D, lower panels). Blots are representative of 3 independent experiments. Densitometric analysis of the immunoblot demonstrated that 2ME2 induced a 4- to 5-fold increase in the cytosolic cyto-c and Smac levels compared with untreated cells.
To determine whether 2ME2 also induces cyto-c or Smac release in other MM cell lines, we treated Dox-40, MM.1R and LR-5 MM cells with 2ME2 (3 μM) for 48 hours, followed by analysis of cytosolic extracts for levels of cyto-c and Smac. As seen in Figure 4C and 4D (upper panels), 2ME2 triggers accumulation of cyto-c and Smac levels in the cytosol of Dox-40 and LR-5 MM cells. Similar results were obtained using the MM.1R MM cell line (data not shown). 2ME2-induced increases in cytosolic cyto-c or Smac levels were specific because no changes were observed in levels of cytosolic SHP2 (Figure 4C-D, lower panels). Taken together, these results suggest that 2ME2-induced apoptosis is accompanied by the accumulation of Smac and cyto-c in the cytosol.
2ME2-induced apoptosis is mediated by activation of the caspase cascade
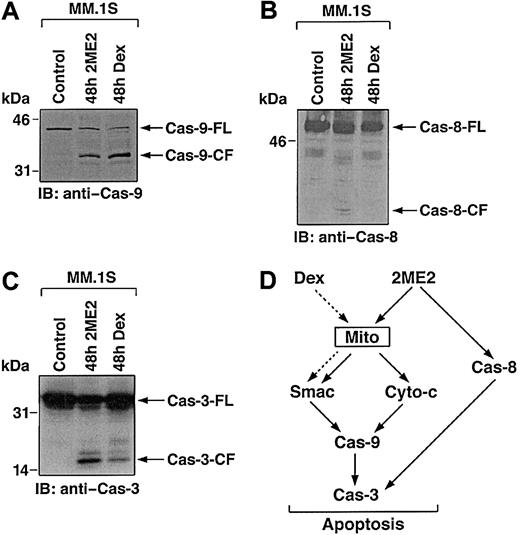
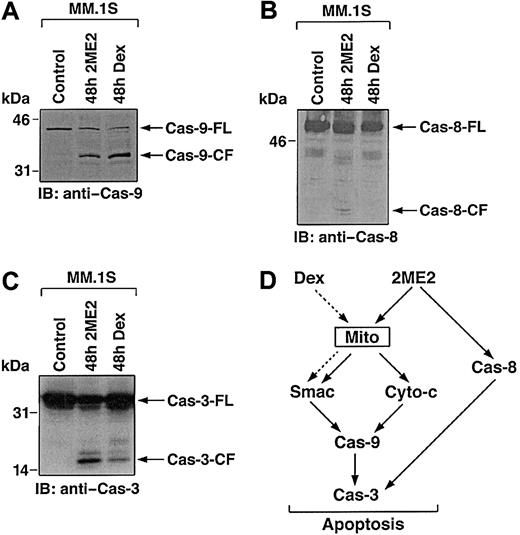
Smac promotes caspase activation through the cyto-c/Apaf-1/caspase-9 pathway,41 and our prior study in MM cells demonstrated that Dex triggers the release of Smac from mitochondria into cytosol, with sequential activation of caspase-9 and caspase-3.40 To examine whether 2ME2-induced Smac release and apoptosis in MM cells is associated with activation of caspase-9, cytosolic extracts from 2ME2-treated MM.1S cells were subjected to immunoblot analysis using anti–caspase-9 antibody. As seen in Figure5A, treatment of MM.1S cells with 2ME2 (3 μM) induces proteolytic cleavage of procaspase-9 into 37- and 35-kDa fragments. We also assayed for catalytic activity of caspase-9 using LEHD-pNA conjugated substrate in a colorimetric protease assay.40 Incubation of cytosolic extracts from 2ME2-treated MM.1S cells with LEHD-pNA was associated with cleavage of LEHD-pNA (data not shown). Taken together, these findings demonstrate that the treatment of MM.1S cells with 2ME2 is associated with the activation of caspase-9.
Delineation of 2ME2 and Dex-induced caspase cascade.
2ME2 induces the activation of caspase-9 (A) and -8 (B). MM.1S cells were treated with 2ME2 (3 μM) and Dex (0.05 μM) and harvested at 48 hours. Cytosolic proteins were separated by 12.5% SDS-PAGE and analyzed by immunoblotting with anti–caspase-8 (cas-8) and -9 (Cas-9) antibodies. Blots are representative of 3 independent experiments. (C) Cleavage of caspase-3 induced by 2ME2. MM.1S cells were treated with 2ME2 (3 μM) and Dex (0.05 μM) and were harvested at 48 hours. Total cell lysates were analyzed by immunoblotting with anti–caspase-3 antibody. FL indicates full length; CF, cleaved fragment. (D) 2ME2 and Dex-induced differential signaling cascades in MM.
Delineation of 2ME2 and Dex-induced caspase cascade.
2ME2 induces the activation of caspase-9 (A) and -8 (B). MM.1S cells were treated with 2ME2 (3 μM) and Dex (0.05 μM) and harvested at 48 hours. Cytosolic proteins were separated by 12.5% SDS-PAGE and analyzed by immunoblotting with anti–caspase-8 (cas-8) and -9 (Cas-9) antibodies. Blots are representative of 3 independent experiments. (C) Cleavage of caspase-3 induced by 2ME2. MM.1S cells were treated with 2ME2 (3 μM) and Dex (0.05 μM) and were harvested at 48 hours. Total cell lysates were analyzed by immunoblotting with anti–caspase-3 antibody. FL indicates full length; CF, cleaved fragment. (D) 2ME2 and Dex-induced differential signaling cascades in MM.
Multiple studies have shown that upstream events mediating mitochondrial cyto-c release induce sequential activation of caspase-8, cleavage of Bid, translocation of cleaved Bid from the cytoplasm into mitochondria, and release of cyto-c.42,43 Because 2ME2 induces the release of cyto-c, we next assayed for activation of caspase-8 by 2ME2. As seen in Figure 5B, 2ME2 (3 μM) also triggers proteolytic cleavage of caspase-8. In contrast, and as in our prior study,40 Dex-induced apoptosis is not associated with caspase-8 activation (Figure 5B).
Previous studies have demonstrated that caspase-8 and -9 activate downstream apoptotic caspase-3 (CPP32).44 Furthermore, in a cell-free system, the addition of purified cyto-c to cyto-c–depleted extracts activates caspase-3 and DNA fragmentation.45Given that treatment of MM.1S cells with 2ME2 activates caspase-8 and -9, we next determined whether caspase-3 is cleaved and activated in response to 2ME2. MM.1S cells were treated with 2ME2 (3 μM), and cell lysates were subjected to immunoblotting with either caspase-3 or its known substrate, PKC δ.24 2ME2 induces caspase-3 cleavage and activation, as evidenced by the cleavage of caspase-3 (Figure 5C and data not shown). Dex treatment served as a positive control.
Collectively, comparative analyses of 2ME2 and Dex-induced apoptotic signaling cascades suggest that 2ME2, in contrast to Dex, triggers multiple signaling pathways (Figure 5D). For example, 2ME2 induces the release of cyto-c and Smac, whereas Dex triggers the release of only Smac; 2ME2 activates caspase-8 and -9, whereas Dex triggers activation of only caspase-9; and finally, IL-6 protects against Dex-, but not 2ME2-induced apoptosis. Because 2ME2 triggers apoptosis in Dex-resistant MM cells, elucidating the 2ME2-induced apoptotic signaling pathways may identify additional apoptotic targets and delineate abnormalities in signaling pathways that confer Dex resistance.
2ME2 suppresses in vivo tumor growth in SCID mouse model
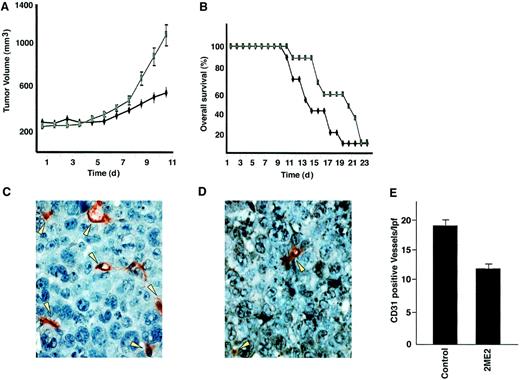
Having shown the signaling mechanisms mediating the anti-MM effects of 2ME2 in vitro, we next determined whether these in vitro effects correlate with the in vivo activity of 2ME2 using our SCID mouse model. Immune-deficient beige-nude-xid (BNX) mice were inoculated subcutaneously in the flank with 3 × 107 RPMI 8226 MM cells in 100 μL RPMI 1640 medium, together with 100 μL Matrigel. Daily oral administration of 2ME2 (100 mg/kg) starting after the development of measurable tumor significantly reduced MM tumor growth (35%-40%; P < .05) (Figure6A) and increased survival (Figure 6B) compared with the control group treated with vehicle (Matrigel) only. No significant toxicity, as evidenced by lack of weight changes, was observed in any treatment groups. These findings suggest that 2ME2 at molar concentrations is effective in controlling the growth of human MM in mice. Other in vivo preclinical studies have shown that 2ME2 is also effective in reducing tumor burden in Meth-A sarcoma or B16 melanoma, in non–estrogen-dependent human breast carcinoma (MDA-MB-435), and in human pancreatic (MIA PaCa-2) xenograft mouse models.2 14
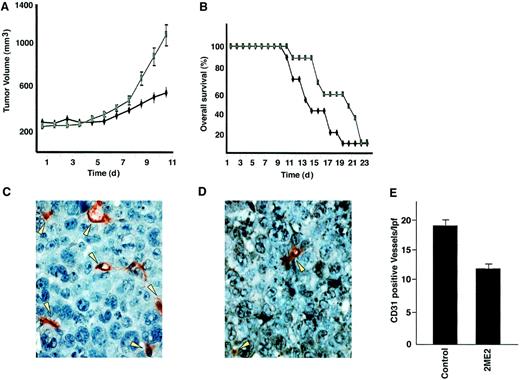
2ME2 suppresses plasmacytoma growth and increases survival in immune deficient beige-nude-xid (BNX) mice.
Mice were inoculated subcutaneously in the flank with 3 × 107 RPMI 8226 MM cells in 100 μL RPMI 1640 medium, together with 100 μL Matrigel. 2ME2 (100 mg/kg) was started after the development of measurable tumor and was given daily using an orogastric feeding tube; carboxymethylcellulose 0.5% served as a control. Serial caliper measurements of perpendicular diameters were taken every other day to calculate tumor volume (A). Each time point represents the mean ± SD of 10 mice. Survival of 2ME2 versus control animals (B). Frozen tumor samples were stained by anti–CD-31 antibody, and the vessels were counted and averaged in control (C) versus 2ME2-treated (D) animals. Arrows denote CD31+ blood vessels. (E) Significantly fewer CD31+ blood vessels were observed in 2ME2-treated than in control animals (P < .05).
2ME2 suppresses plasmacytoma growth and increases survival in immune deficient beige-nude-xid (BNX) mice.
Mice were inoculated subcutaneously in the flank with 3 × 107 RPMI 8226 MM cells in 100 μL RPMI 1640 medium, together with 100 μL Matrigel. 2ME2 (100 mg/kg) was started after the development of measurable tumor and was given daily using an orogastric feeding tube; carboxymethylcellulose 0.5% served as a control. Serial caliper measurements of perpendicular diameters were taken every other day to calculate tumor volume (A). Each time point represents the mean ± SD of 10 mice. Survival of 2ME2 versus control animals (B). Frozen tumor samples were stained by anti–CD-31 antibody, and the vessels were counted and averaged in control (C) versus 2ME2-treated (D) animals. Arrows denote CD31+ blood vessels. (E) Significantly fewer CD31+ blood vessels were observed in 2ME2-treated than in control animals (P < .05).
Because 2ME2 is a known antiangiogenic agent and recent reports show increased BM angiogenesis in MM,15-17 we examined the effect of 2ME2 on intratumoral microvessel density (MVD), evidenced by CD-31 immunostaining of human MM tumors in mice. A significant decrease in MVD was noted in 2ME2-treated animals (Figure 6D) versus the cohort treated with control vehicle alone (Figure 6C). The number of CD-31+ blood vessels per low-power (400 ×) field was 10.30 ± 1.0 in 2ME2-treated versus 19.10 ± 1.9 in control groups (P < .05) (Figure 6E). Taken together, these studies demonstrate that 2ME2 is effective in inhibiting tumor cell growth and angiogenesis in MM.
Having shown the in vivo effects of 2ME2 on angiogenesis, we determined whether 2ME2 altered VEGF secretion in the BM microenvironment. 2ME2 induces a significant (P < .05) decrease in VEGF secretion in MM.1S cells (34% ± 2.1% decrease, n = 3) and in BMSCs (41% ± 3.2% decrease, n = 3). Our results are consistent with other studies suggesting that VEGF expression, as evaluated by immunohistochemical analysis, is down-regulated concomitantly with tumor angiogenic suppression.46 Because we and others16 17 have recently demonstrated that VEGF is an angiogenic factor and a trigger of growth and migration in MM cells, our present findings suggest an additional mechanism whereby 2ME2 mediates anti-MM activity.
Our study, therefore, demonstrates that 2ME2 can directly induce apoptosis in MM cells and act in the BM microenvironment to decrease cytokines mediating tumor cell growth survival and angiogenesis. These studies provide the framework for clinical trials of 2ME2 to overcome drug resistance and improve patient outcome in MM.
We thank Dr Xiaodong Wang for providing Smac-related reagents. We also thank Kamal Chauhan for technical assistance.
Prepublished online as Blood First Edition Paper, May 24, 2002; DOI 10.1182/blood-2002-02-0376.
Supported by National Institutes of Health grants R0-1 50947 and PO-1 78378, Multiple Myeloma Research Foundation Senior Research Awards (D.C., T.H.), International Myeloma Foundation Fellow Award (K.P.), and Doris Duke Distinguished Clinical Research Scientist Award (K.C.A.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Kenneth C. Anderson, Dana-Farber Cancer Institute, 44 Binney St, Boston, MA 02215; e-mail:kenneth_anderson@dfci.harvard.edu.


 ) with the indicated doses of 2ME2 for 72 hours. Results are mean ± SD from 5 independent experiments; P < .0001 for all cell lines. (C) Effect of treatment with 2ME2 (0-20 μM) for 72 hours on normal lymphocyte viability, assessed by MTT assay. Results are the mean ± SD of 5 independent experiments; P = 0.23 from J-T test for trend. (D) MM cells (CD138+) from 5 patients (patients 1-5) were treated with 2ME2 (9 μM) for 72 hours, followed by BrdU assay. Values are the mean ± SD of triplicate samples (P = .06); experiments were repeated 3 times with similar results. (E) 2ME2 induces proteolytic cleavage of PARP in patient MM cells. CD138+ cell from 2 MM patients and normal lymphocytes from 2 healthy donors were treated with 2ME2 (9 μM) for 72 hours and harvested, and total protein lysates were subjected to SDS-PAGE analysis. Immunoblot analysis of the lysates was performed with anti-PARP antibody. FL indicates full length; CF, cleaved fragment.
) with the indicated doses of 2ME2 for 72 hours. Results are mean ± SD from 5 independent experiments; P < .0001 for all cell lines. (C) Effect of treatment with 2ME2 (0-20 μM) for 72 hours on normal lymphocyte viability, assessed by MTT assay. Results are the mean ± SD of 5 independent experiments; P = 0.23 from J-T test for trend. (D) MM cells (CD138+) from 5 patients (patients 1-5) were treated with 2ME2 (9 μM) for 72 hours, followed by BrdU assay. Values are the mean ± SD of triplicate samples (P = .06); experiments were repeated 3 times with similar results. (E) 2ME2 induces proteolytic cleavage of PARP in patient MM cells. CD138+ cell from 2 MM patients and normal lymphocytes from 2 healthy donors were treated with 2ME2 (9 μM) for 72 hours and harvested, and total protein lysates were subjected to SDS-PAGE analysis. Immunoblot analysis of the lysates was performed with anti-PARP antibody. FL indicates full length; CF, cleaved fragment.