Anemia is a common clinical problem, and there is much interest in its role in promoting left ventricular hypertrophy through increasing cardiac workload. Normally, red blood cell production is adjusted through the regulation of erythropoietin (Epo) production by the kidney. One important cause of anemia is relative deficiency of Epo, which occurs in most types of renal disease. Clinically, this can be corrected by supplementation with recombinant Epo. Here we describe an oxygen-regulated gene therapy approach to treating homozygous erythropoietin-SV40 T antigen (Epo-TAgh) mice with relative erythropoietin deficiency. We used vectors in which murine Epo expression was directed by an Oxford Biomedica hypoxia response element (OBHRE) or a constitutive cytomegalovirus (CMV) promoter. Both corrected anemia, but CMV-Epo–treated mice acquired fatal polycythemia. In contrast, OBHRE-Epo corrected the hematocrit level in anemic mice to a normal physiologic level that stabilized without resulting in polycythemia. Importantly, the OBHRE-Epo vector had no significant effect on the hematocrit of control mice. Homozygous Epo-TAgh mice display cardiac hypertrophy, a common adaptive response in patients with chronic anemia. In the OBHRE-Epo–treated Epo-TAgh mice, we observed a significant reversal of cardiac hypertrophy. We conclude that the OBHRE promoter gives rise to physiologically regulated Epo secretion such that the hematocrit level is corrected to healthy in anemic Epo-TAghmice. This establishes that a hypoxia regulatory mechanism similar to the natural mechanism can be achieved, and it makes EPOgene therapy more attractive and safer in clinical settings. We envisage that this control system will allow regulated delivery of therapeutic gene products in other ischemic settings.
Introduction
An important cause of anemia is relative deficiency in the production of the glycoprotein hormone erythropoietin (Epo), which regulates the formation of red blood cells (RBCs). Relative Epo deficiency occurs in almost all patients with chronic renal failure.1,2 Decreased RBC production reduces the oxygen-carrying capacity of the blood, resulting in tissue hypoxia. Pathophysiologic consequences correlate with the severity of the anemia and range from fatigue and reduced exercise tolerance to cardiac hypertrophy. Erythropoietin deficiency in renal disease can be treated remarkably effectively by regular administration of recombinant human Epo (rhEpo) several times a week. Erythropoietin can also be used to treat anemia in patients with cancer and chronic inflammatory diseases such as rheumatoid arthritis.3 4 Although it is safe, treatment with erythropoietin is relatively costly and entails some inconvenience in monitoring and administration. Hence, there has been considerable interest in developing a gene therapy strategy for the delivery of Epo whereby single administration of the EPOgene would ensure the long-term delivery of Epo.
Although the EPO gene has been delivered to animals using various plasmid and viral vectors, the relevance of these studies to chronic anemia and its sequelae have been limited. This is because theEPO gene has been delivered to healthy animals5-10 or has been used in models such as β-thalassemic mice11,12 or mice with acute renal failure.13 In these studies, any response in the hematocrit level has been taken as a measure of successful gene therapy. We have now assessed the potential of genetic delivery of theEPO gene in a more relevant model of chronic anemia. The erythropoietin-SV40 T antigen (Epo-TAgh) transgenic mouse has a targeted disruption in the 5′ untranslated region of theEPO gene that dramatically reduces expression such that the homozygous animal is severely anemic and has a hematocrit level of approximately 13.2% ± 3.3% (normal level, 43% ± 5.1%).14 In the current study we examined the long-term consequences in these mice and show that, in addition to a low hematocrit level, pronounced cardiac hypertrophy, a cardinal feature of chronic anemia, results.15 The Epo-TAgh mouse, therefore, provides an excellent model for studying the effects of introducing a functional EPO gene.
We wanted to model a potential human clinical protocol, and it was important that EPO gene therapy could be regulated. Uncontrolled delivery of the EPO gene leads to an expanded red cell mass (polycythemia),5,16 with hemodynamic and rheologic problems including increased risk for vascular thrombosis.17 One approach to control gene expression is to use a regulated promoter that can switch EPO gene expression on and off, such as the tetracycline- or rapamycin-responsive promoters. To date, these promoters have only been shown to regulate the hematocrit level above normal baseline rather than to maintain normal levels.8 18-20 We envisage that the use of these extrinsic regulation systems in a patient could be costly and cumbersome, especially because of the addition of the pharmacologic regulatory agents may interfere with other patient medications. Therefore, we set out to develop a homeostatic system of gene therapy based on sensing and correcting tissue hypoxia given the normal regulation of the erythropoietin gene.
Reduced oxygenation of blood reaching the kidney is the key physiologic signal for increasing erythropoiesis through increased expression of the EPO gene by the fibroblasts of the renal cortex and outer medulla14,21 and, to a lesser degree, in the adult by the hepatocytes and Ito cells of the liver. In hypoxia, a heterodimeric transcription complex (hypoxia-inducible factor-1 [HIF-1]) binds to a hypoxia-response element (HRE) lying 3′ to the EPO gene to stimulate transcription.22 Although erythropoietin gene expression is highly cell-type specific, most (if not all) cell types activate the HIF-1 pathway in response to hypoxia.23,24Consistent with this, many genes active in a broad range of cell types and tissues are now known to contain HREs and to respond to stabilization of the HIF-1 transcription factor in hypoxia.25 Various natural and synthetic HRE-containing promoters have been used to direct heterologous gene expression in response to hypoxia—for example, tumor cells, muscle cells, and macrophages.26-29 We reasoned that the Epo-TAgh mouse would have hypoxia in other tissues besides the kidney and liver as a consequence of reduced oxygen delivery and that this could be sufficient to activate gene expression from a hypoxia-responsive promoter. Ideally, once sufficient Epo is produced to restore the RBC level to normal, the cells in the selected tissue would revert to physiological normoxia and the HRE should cease to drive transcription. This would reduce Epo production so that polycythemia would not develop. We have now tested this concept by using a recombinant adeno-associated virus (AAV) vector to express murine Epo under the control of a constitutive cytomegalovirus (CMV) promoter or the Oxford Biomedica hypoxia response element (OBHRE) promoter. The method of gene delivery was chosen because the vascularity of skeletal muscle allows for the distribution of secreted proteins.30 In addition, we know that the hypoxia signaling pathway is functional in muscle, that AAV vectors give good gene transfer to muscle, and, in a clinical setting, that skeletal muscle is easily targeted by injection. The effect of intramuscular delivery of these vectors on the hematocrit and organ structure of healthy and Epo-TAgh mice has been assessed over a 7-month study. Our data indicate that Epo can be delivered on physiological demand to reverse a chronic state of anemia.
Materials and methods
Healthy and anemic mice
Generation of anemic (Epo-TAgh) transgenic mice in which the simian virus 40 (SV40) large T-antigen marker gene is integrated in the regulatory sequence of the endogenous mouseEPO gene is described elsewhere.14 The official designation of this mouse strain is now TgH(eposvT)1Pjr. The breeding colony of Epo-TAgh and healthy (C57B16/CBA) mice used in this study was maintained at CAMR (Centre for Applied Microbiology and Research; Porton Down, Wiltshire, United Kingdom). The 7- to 16-week-old female Epo-TAgh homozygote mice were generated from F1 breeding pairs of heterozygote females and homozygote males. The genotype was determined by hematocrit level (homozygote, 17.5% ± 4%; heterozygote, 35.5% ±4.1%; normal level, 52%). These values are slightly higher than were reported originally for this transgenic colony.14 Each group of mice, healthy or Epo-TAgh homozygote, contained 6 animals. The mice were injected with a total volume of 100 μL containing 1 × 1010 particles of recombinant AAV virus that was divided for 4 injection sites. Two 30-μL injections were made into the quadriceps, and two 20-μL injections were made into the anterior tibialis muscle of the left hindlimb.
Cell lines
T47D and HT1080 cell lines (ECACC, Wiltshire, United Kingdom) were used to assess hypoxic regulation of the Epo expression vectors because they have previously shown good hypoxic induction in vitro.27 Cells were maintained in RPMI 1640 or Dulbecco modified Eagle medium, respectively, supplemented with 10% (vol/vol) fetal calf serum, 2 mM glutamine, and 2 mM nonessential amino acids (Sigma-Aldrich, Dorset, United Kingdom).
Transient transfections
Typically, cells seeded in a 24-well dish were brought to 70% confluence and were transfected with 0.21 μg plasmid using the Fugene-6 transfection reagent (Boehringer Mannheim, Indianapolis, IN).
Hypoxia in vitro
Twenty-four hours after transduction or transfection, cells were either incubated for another 16 hours under normoxic conditions in a standard incubator (21% O2, 5% CO2, 74% N2) or under hypoxic conditions (0.1% O2, 5% CO2, 95% N2) using a multigas incubator purchased from Heto-Holten (Allerod, Denmark).
Construction of recombinant AAV vectors
The murine erythropoietin cDNA was cloned through nested polymerase chain reaction (PCR) on murine kidney cDNA (Quickclone cDNA; Clontech, United Kingdom) using 2 pairs of nested PCR primers: primer set 1, 5′-GACAGTGACCACTTTCTTCCAG-3′ and 5′-GGACAGACTGGTAAGAAGGTAATG-3′; primer set 2, 5′-CAGCTAGGCGCGGAGATG-3′ and 5′-CAGCAGCATGTCACCTGTC-3′.
The mEpo PCR product was cloned into the pUC18 plasmid (Panvera, Madison, WI) and was subsequently removed as anXbaI-EcoRI fragment and cloned in to the pCI-Neo (Promega, Southampton, United Kingdom) NheI-EcoRI sites to create pCMV-Epo. The CMV/IE promoter in pCMV-Epo was replaced with the OBHRE promoter to create pHRE-Epo.27
An oligonucleotide was cloned into the BamHI andSpeI restriction sites in the multiple cloning site of the pSL1180 plasmid (Amersham Pharmacia Biotech, Buckinghamshire, United Kingdom) to generate the following restriction sites:BamHI-NheI-MluI-XhoI-StuI-NruI-BclI-SpeI-BglII.
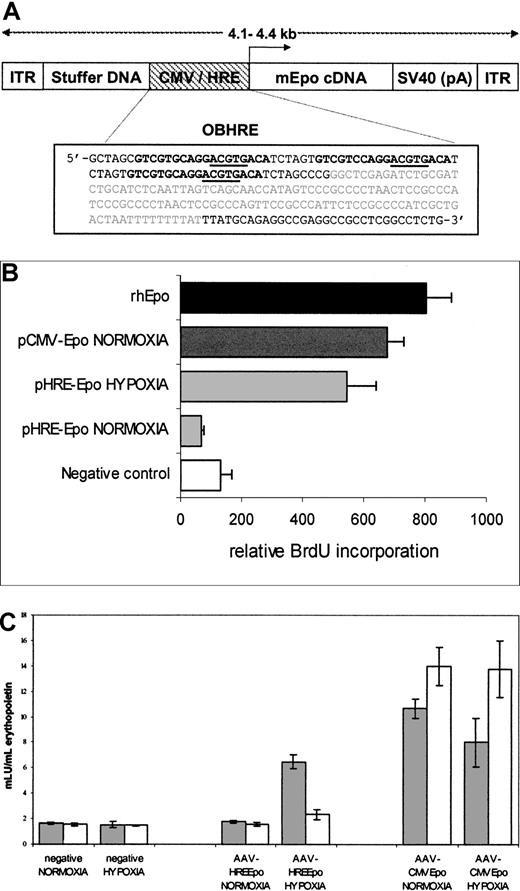
The AAV-CMVEpo vector genome was constructed by creating a 145–base pair (bp) oligonucleotide consisting of the wild-type AAV-2 inverted terminal repeat (ITR) (GenBank accession number: NC_001401) flanked byBamHI and NheI compatible ends. The ITR was cloned sequentially in reverse and forward orientation into theBamHI-NheI, SpeI, and BglII sites of the modified pSL1180 vector. The CMV-EpoBsaBI-BglII fragment from pCMVEpo was cloned into the StuI-BglII sites of the modified pSL1180 vector, together with a 1.7-kb BclI-BglI stuffer fragment from the LacZ gene such that the complete internal cassette measured 4.2 kilobases (kb). The AAV-HREEpo vector genome was created by exchanging the CMV/IE NotI- Eco47III promoter fragment in AAV-CMVEpo for the OBHRENotI-XmnI promoter fragment in pHRE-Epo (Figure1A).
Construction and analysis of recombinant AAV-Epo vectors.
(A) Diagrammatic representation of recombinant AAV vectors used for this study. The AAV-CMVEpo and AAV-HREEpo virus vectors differ only in the nature of the promoter sequence. ITR indicates AAV-2–inverted terminal repeats; CMV, immediate/early promoter enhancer elements from CMV; HRE, hypoxia responsive promoter; mEpo, murine erythropoietin; SV40 (pA), polyadenylation signal from the SV40 virus; stuffer DNA, fragment of the 3′ β-galactosidase gene to ensure genome size is larger than 4 kb. The configuration of the OBHRE promoter is boxed below: the PGK HRE trimer encompassing −307/−290 of murine PGK is shown in bold, the core HIF-1 consensus binding site is underlined, and the minimal SV40 promoter is shown in gray. (B) Proliferation of splenocytes incubated with supernatants from HT1080 cells transfected with pCMV/HRE-Epo plasmids. The assay shows that the increased proliferation of the splenocyte cells when exposed to the supernatant from the pHRE-Epo–transfected cells exposed to hypoxia. Negative control represents untreated cells. The rhEpo is the recombinant human Epo positive control used in this assay at a concentration of 0.5 U/mL. Data are the mean relative light units per second ± SD of 3 samples. (C) Hypoxia-regulated Epo expression is maintained in a rAAV vector. T47D cells were transduced with rAAV vectors, AAV-CMVEpo, and AAV-HREEpo at a multiplicity of infection (MOI) of 10. Supernatants were harvested 1 day (gray bars) and 4 days (white bars) after hypoxia treatment and were analyzed in an Epo ELISA assay to show the hypoxia-mediated expression of Epo was transient. Data are the mean mIU/mL Epo values ± SD of 3 samples.
Construction and analysis of recombinant AAV-Epo vectors.
(A) Diagrammatic representation of recombinant AAV vectors used for this study. The AAV-CMVEpo and AAV-HREEpo virus vectors differ only in the nature of the promoter sequence. ITR indicates AAV-2–inverted terminal repeats; CMV, immediate/early promoter enhancer elements from CMV; HRE, hypoxia responsive promoter; mEpo, murine erythropoietin; SV40 (pA), polyadenylation signal from the SV40 virus; stuffer DNA, fragment of the 3′ β-galactosidase gene to ensure genome size is larger than 4 kb. The configuration of the OBHRE promoter is boxed below: the PGK HRE trimer encompassing −307/−290 of murine PGK is shown in bold, the core HIF-1 consensus binding site is underlined, and the minimal SV40 promoter is shown in gray. (B) Proliferation of splenocytes incubated with supernatants from HT1080 cells transfected with pCMV/HRE-Epo plasmids. The assay shows that the increased proliferation of the splenocyte cells when exposed to the supernatant from the pHRE-Epo–transfected cells exposed to hypoxia. Negative control represents untreated cells. The rhEpo is the recombinant human Epo positive control used in this assay at a concentration of 0.5 U/mL. Data are the mean relative light units per second ± SD of 3 samples. (C) Hypoxia-regulated Epo expression is maintained in a rAAV vector. T47D cells were transduced with rAAV vectors, AAV-CMVEpo, and AAV-HREEpo at a multiplicity of infection (MOI) of 10. Supernatants were harvested 1 day (gray bars) and 4 days (white bars) after hypoxia treatment and were analyzed in an Epo ELISA assay to show the hypoxia-mediated expression of Epo was transient. Data are the mean mIU/mL Epo values ± SD of 3 samples.
Recombinant AAV-2 vectors were produced according to the published method.31 AAV particles were determined by dot blot quantification of genome copy and direct comparison with a recombinant AAV vector expressing CMV-GFP of known biologic titer.
Spleen cell proliferation assay
The functionality and regulation of the cloned Epo cDNA was verified using a biologic spleen cell proliferation assay based on a published method.32 Briefly, 2- to 3-month-old mice (C57BL/6J × C3H/HeB F1 hybrid), each weighing 25 to 35 g, were given 2 consecutive daily intraperitoneal injections of 60 mg/kg phenylhydrazine hydrochloride. Spleens were isolated 3 days after the second injection. Single-cell suspensions from the spleen were prepared 3 days after the second injection and were seeded into black-walled, 96-well plates (Canberra Packard, Mississauga, ON, Canada) at a density of 4 × 105 cells per well. Supernatants were collected from HT1080 cells 5 days after transfection with either pCMV-EPO or pHRE-EPO plasmids, and 1 μL of each supernatant was added to the splenocyte cell cultures. As a positive control, rhEpo was used at 500 U/mL. Splenocyte cell cultures were incubated for 22 hours and then were assayed for proliferation using a chemiluminescent 5-bromodeoxyuridine (BrdU) assay (Roche, Mannheim, Germany).
Detection of erythropoietin in vitro
Erythropoietin was detected in cell supernatants using the Quantikine IVD Epo enzyme-linked immunosorbent assay (ELISA) kit at a detectable threshold of 2 mU/mL (R&D Systems, Abingdon, Oxon, United Kingdom).
Histologic analyses
Standard hematoxylin and eosin staining was carried out for assessment of tissue morphology. To determine the distribution of iron in the liver, sections were stained using Perl Prussian blue. For immunologic analysis, tissue sections were fixed in acetone. The TER-119 antibody (BD PharMingen, Oxford, United Kingdom) was used to recognize cells committed to the erythroid lineage, from proerythroblasts to mature erythrocytes. It was used at a dilution of 1:50 overnight followed by the horseradish peroxide (HRP)–conjugated secondary antibody (HRP rabbit anti-rat; DAKO, Glostrup, Denmark) at a dilution of 1:50 in 10% mouse serum. DAB (3,3′-diaminobenzidine) was added for 10 minutes, and the slides were counterstained with hematoxylin.
For electron microscopy, the heart was isolated from one mouse from each of the untreated and the AAV-HREEpo–treated healthy and Epo-TAgh groups on day 189 of the study. The hearts were dissected into 1-mm cubes and were immersion-fixed in 1% gluteraldehyde/2.5% paraformaldehyde. Samples were washed in phosphate-buffered saline (PBS) and were postfixed in 1% OsO4 in 0.1 M phosphate buffer, washed in distilled water overnight at 4°C, dehydrated in alcohol, and embedded in Durcupan epoxy resin. Ultrathin cross-sections of the myocardium were stained with 2% uranyl acetate, followed by 1% lead citrate (Reynold stain) and were examined under the Philips 401 transmission electron microscope (Wilmington, MA). Sarcomere measurements were made from randomly taken photographs.
Statistical analysis
The unpaired Student t test was used to determine whether there was a significant difference between groups of data. Groups were considered significantly different whenP < .05.
Results
Hypoxia-mediated regulation of functional murine Epo expression in vitro
We have observed that a synthetic HRE multimer, referred to as OBHRE, can combine a good induction ratio with a high level of expression comparable to that achieved by strong constitutive promoters such as the CMV promoter, but only when the oxygen concentration is low.27 The OBHRE promoter was inserted into plasmid and AAV vectors to produce pHRE and AAV-HRE, respectively (Figure 1A). Similar vectors containing the human CMV promoter were pCMV and AAV-CMV. A cDNA for murine EPO was inserted into these vectors, and green fluorescence protein (GFP)–expressing vectors were used as negative controls. We wanted to use the murineEPO rather than the human EPO to avoid immune responses that would compromise the efficacy of the gene therapy. We first confirmed that the murine EPO gene functioned in vitro. The production of mEpo in the culture supernatant of HT1080 cells, transfected with pHRE-Epo or pCMV-Epo and maintained in normoxia or hypoxia, was determined using a spleen cell proliferation assay (Figure 1B). Both plasmids directed the expression of functional mEpo, but with pHRE-Epo the expression was at least 8-fold higher from cells maintained in hypoxia compared with normoxia. Similarly, the recombinant AAV vectors were transduced into T47D cells, placed in normoxia, or exposed to hypoxia for 16 hours and then returned to normoxia (Figure 1C). Secretion of mEpo into the supernatant was assessed in an Epo ELISA 1 day and 4 days after hypoxic induction. AAV-CMV–directed mEpo expression increased during the 4 days in normoxia and hypoxia, whereas AAV-HRE–directed mEpo expression increased from basal levels only in the hypoxia-exposed cultures as measured at day 1. By day 4, levels of mEpo had returned to baseline. Collectively, these data from the 2 assays indicated that the mEPO gene was functional and that the expression could be activated by hypoxia and switched off in normoxia. This reversible expression was the profile that would be required for a gene therapy vector that could deliver Epo under anemic conditions but that would be shut down once normal oxygenation was restored.
Regulated delivery of Epo in vivo
In preliminary studies we examined the Epo-TAghhindlimb skeletal muscle for evidence of hypoxia. As expected, immunologic analysis for CD31 showed a modest increase in muscle capillarity. Although variable, immunolabeling for vascular endothelial growth factor (VEGF) showed more signal in specimens from the Epo-TAgh mice than control mice (data not shown). This suggested that the skeletal muscle was likely to be sufficiently hypoxic to activate the OBHRE promoter because VEGF gene expression is activated by hypoxia, predominantly through the HIF-1–mediated transcriptional pathway.
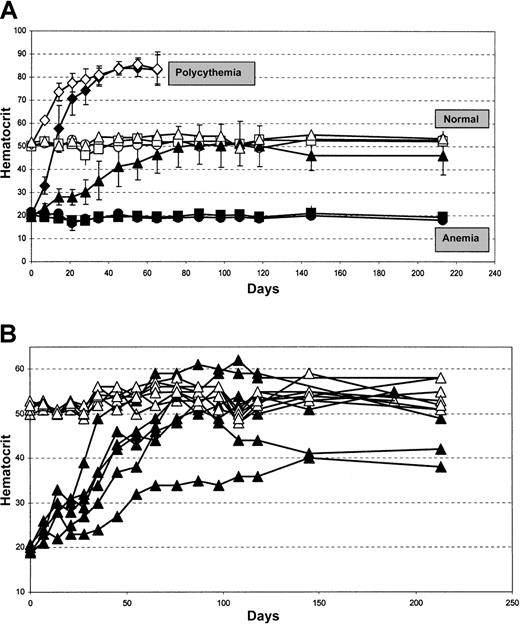
Healthy or Epo-TAgh mice were injected in the left hindlimb with AAV-CMVGFP–, AAV-CMVEpo–, or AAV-HREEpo–recombinant AAV viruses. Hematocrit measurements were made regularly over a period of 7 months (Figure 2). The control vector was AAV-CMVGFP, and this produced no change in the hematocrit level of healthy mice, which was maintained at approximately 52% (Figure 2A, open squares), or of Epo-TAgh mice, which was maintained at approximately 18% (Figure 2A, closed squares) throughout the duration of the study. These levels were identical to those of untreated controls (Figure 2A, healthy mice, open circles; Epo-TAghmice, closed circles). In marked contrast, when the healthy and Epo-TAgh mice were injected with the constitutive Epo vector, AAV-CMVEpo, there was a dramatic increase in the hematocrit level in each group that was significant after only 7 days and that increased to 85% after 45 days (Figure 2A, diamonds). Two mice in this group died suddenly at day 60, by which time the blood in the remaining animals became too viscous to obtain samples for hematocrit analysis; therefore, the animals in these groups were killed. However, a different result was obtained when the hypoxia-regulated vector, AAV-HREEpo, was used. In healthy mice (Figure 2, open triangles) there was no statistically significant effect on the hematocrit level after AAV-HREEpo treatment. In the Epo-TAgh mice, the AAV-HREEpo vector led to a steady increase in the hematocrit of 2% to 2.5% per week for 11 weeks, until a plateau was reached at the normal level. This plateau was an average hematocrit level between 46% and 50%—that is, it was in the normal range (Figure 2, closed triangles). This normal hematocrit was maintained for the remaining 5 months of the study. Individual data for each mouse in the AAV-HREEpo–treated groups are shown in Figure 2B. Hematocrit levels of the AAV-HREEpo–treated healthy mice are all within the normal range of 48% to 59% for the duration of the experiment. Hematocrit levels of the AAV-HREEpo–treated Epo-TAgh mice showed some variation in terms of the rate of increase and the plateau level indicated by maximum and minimum levels at day 108 of 62% and 36%, respectively. This could be because of variations in the efficiency of transduction in the skeletal muscle between different mice or the genetic heterogeneity in the Epo-TAgh mice colony that was not inbred. However, in no case did the hematocrit level reach that obtained by the constitutive AAV-CMVEpo vector. Treatment with the hypoxia-regulated AAV-HREEpo vector restored and maintained normal hematocrit levels in the Epo-TAgh mice for the duration of the 7-month study.
AAV-HREEPO–treated EPO-TAgh transgenic mice display physiological correction of the hematocrit.
(A) Filled symbols represent Epo-TAgh mice groups; open symbols, healthy mice groups. Circles represent untreated groups showing baseline hematocrit levels of the healthy and Epo-TAgh mice; squares, AAV-CMVGFP–treated groups showing no change in hematocrit level compared with that of untreated counterparts; diamonds, AAV-CMVEpo–treated groups show a rapid, unregulated rise in the hematocrit leading to fatal polycythemia in 2 mice; triangles, AAV-HREEpo–treated groups. Epo-TAgh mice responded to AAV-HREEpo treatment with correction of the hematocrit to the normal physiological level, whereas there was no change in hematocrit level in the healthy mice treated with the same AAV-HREEpo vector. Unpaired student t test was used to compare day 7 and day 213 hematocrit measurements. P = .81,P = .36, P = .98, and P = .0001, respectively, for untreated healthy mice, healthy mice + AAVHREEpo, untreated Epo-TAgh mice, and Epo-TAgh mice + AAVHREEpo. Hematocrit levels are plotted as a mean value for 6 animals in each treatment group ± SD. (B) Hematocrit data from each individual animal treated with the AAV-HREEpo vector is plotted. ▴ represents Epo-TAgh mice; ▵, healthy mice.
AAV-HREEPO–treated EPO-TAgh transgenic mice display physiological correction of the hematocrit.
(A) Filled symbols represent Epo-TAgh mice groups; open symbols, healthy mice groups. Circles represent untreated groups showing baseline hematocrit levels of the healthy and Epo-TAgh mice; squares, AAV-CMVGFP–treated groups showing no change in hematocrit level compared with that of untreated counterparts; diamonds, AAV-CMVEpo–treated groups show a rapid, unregulated rise in the hematocrit leading to fatal polycythemia in 2 mice; triangles, AAV-HREEpo–treated groups. Epo-TAgh mice responded to AAV-HREEpo treatment with correction of the hematocrit to the normal physiological level, whereas there was no change in hematocrit level in the healthy mice treated with the same AAV-HREEpo vector. Unpaired student t test was used to compare day 7 and day 213 hematocrit measurements. P = .81,P = .36, P = .98, and P = .0001, respectively, for untreated healthy mice, healthy mice + AAVHREEpo, untreated Epo-TAgh mice, and Epo-TAgh mice + AAVHREEpo. Hematocrit levels are plotted as a mean value for 6 animals in each treatment group ± SD. (B) Hematocrit data from each individual animal treated with the AAV-HREEpo vector is plotted. ▴ represents Epo-TAgh mice; ▵, healthy mice.
Stimulation of extramedullary hematopoiesis in AAV-HREEpo–treated Epo-TAgh mice
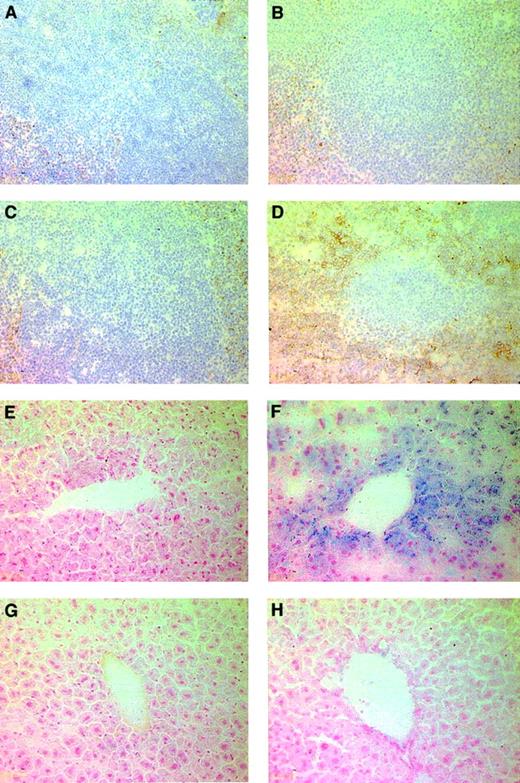
To further characterize the response to regulated erythropoietin in Epo-TAgh mice, we analyzed the liver, spleen, and bone marrow for evidence of hematopoiesis. For this we used TER-119, an antibody that recognizes cells in the late stages of the erythroid lineage.33 The liver and bone marrow showed no discernible difference in the TER-119 staining in all groups examined. However, the spleen taken from the Epo-TAgh mice treated with AAV-HREEpo (Figure 3D) contained elevated levels of erythroid (TER-119+) cells in the red pulp region compared to the spleens taken from untreated Epo-TAgh (Figure 3B) mice. Spleens taken from untreated and AAV-HREEpo–treated healthy mice did not show any difference in the level of erythroid cells (Figure3A,C). This indicates that the production of Epo from the AAV-HREEpo vector stimulated extramedullary hematopoiesis in the spleens of the Epo-TAgh mice but not in the healthy mice.
Effect of regulated Epo gene therapy on the spleen and liver in Epo-TAgh mice.
(A-D) TER-119 staining to reveal cells of the erythropoietic lineage in the spleen. TER-119 recognizes a molecule associated with cell-surface glycophorin A. Therefore, erythroid cells were identified by membrane-associated staining. (A) Healthy spleen. (B) Epo-TAgh spleen. (C) Healthy spleen treated with AAV-HREEpo. (D) Epo-TAgh spleen treated with AAV-HREEpo. Treatment of the Epo-TAgh mice with AAV-HREEpo stimulates erythropoiesis in the spleen. Compare level of TER-119 staining in panels B and D. (E-H) Perl Prussian blue staining to reveal nonheme iron in (E) healthy liver, (F) Epo-TAgh liver, (G) healthy liver treated with AAV-HREEpo, and (H) Epo-TAghliver treated with AAV-HREEpo. Treatment of the Epo-TAghmice with AAV-HREEpo causes a reduction in the hepatic iron loading. Compare Perl staining in panels F and H. Original magnification, × 200.
Effect of regulated Epo gene therapy on the spleen and liver in Epo-TAgh mice.
(A-D) TER-119 staining to reveal cells of the erythropoietic lineage in the spleen. TER-119 recognizes a molecule associated with cell-surface glycophorin A. Therefore, erythroid cells were identified by membrane-associated staining. (A) Healthy spleen. (B) Epo-TAgh spleen. (C) Healthy spleen treated with AAV-HREEpo. (D) Epo-TAgh spleen treated with AAV-HREEpo. Treatment of the Epo-TAgh mice with AAV-HREEpo stimulates erythropoiesis in the spleen. Compare level of TER-119 staining in panels B and D. (E-H) Perl Prussian blue staining to reveal nonheme iron in (E) healthy liver, (F) Epo-TAgh liver, (G) healthy liver treated with AAV-HREEpo, and (H) Epo-TAghliver treated with AAV-HREEpo. Treatment of the Epo-TAghmice with AAV-HREEpo causes a reduction in the hepatic iron loading. Compare Perl staining in panels F and H. Original magnification, × 200.
Impact of AAV-HREEpo on liver iron loading in Epo-TAgh mice
It has previously been shown that the Epo-TAgh mice display hepatic iron loading because of enhanced mucosal iron uptake in the absence of erythropoiesis.34 Hepatic iron loading can lead to functional impairment in the liver. We were interested to see the distribution of nonheme iron in the livers of untreated and AAV-HREEpo–treated Epo-TAgh mice using Perl Prussian blue. Livers from untreated Epo-TAgh mice (Figure 3F) showed an increase in the amount of nonheme iron in the cytoplasm of the liver Kupffer and parenchymal cells compared with the livers from healthy mice (Figure 3E). Interestingly, the livers from AAV-HREEpo–treated Epo-TAgh mice showed a decrease in the amount of nonheme iron. This suggests the stored iron has been mobilized from the liver and used for erythropoiesis.
Organ analysis of study animals
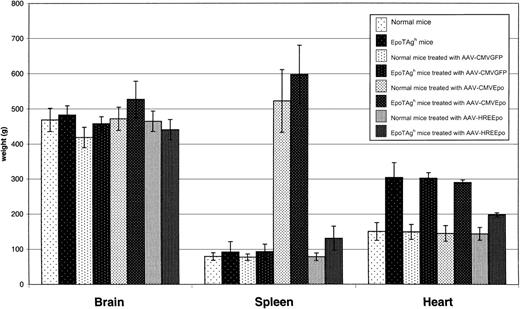
We wanted to determine whether Epo gene therapy caused any further structural changes to internal organs. Changes in red blood cell composition affect the volume and the pressure of blood. In chronic anemia, this hemodynamic alteration leads to gradual development of cardiac enlargement (hypertrophy) as the cardiac output increases to compensate for the decreased oxygen-carrying capacity of the blood. We compared the weights of some of the organs in the untreated and treated Epo-TAgh and healthy mice at the end of the experiment (Figure 4). There was no significant difference between any of the groups in brain size. However, a marked difference was noted in the spleen. The AAV-CMVEpo–treated healthy and Epo-TAgh mice had significant splenomegaly. This is most likely a result of extramedullary hematopoiesis and vascular congestion caused by the increase in RBC load. Consistent with this, AAV-HREEpo–treated Epo-TAgh mice had only slightly larger spleens than their healthy counterparts.
Analysis of organ weights in the Epo-TAghand healthy mice at the end of the experiment.
Healthy mice, Epo-TAgh mice, healthy mice treated with AAV-CMVGFP, Epo-TAgh mice treated with AAV-CMVGFP, healthy mice treated with AAV-CMVEpo, Epo-TAgh mice treated AAV-CMVEpo, healthy mice treated with AAV-HREEpo, Epo-TAgh mice treated AAV-HREEpo. Average weights of organs are plotted ± SD (n = 4-6). Spleens in the AAV-CMVEpo–treated groups are significantly enlarged. Hearts of Epo-TAgh mice are enlarged compared with those of healthy mice. This enlargement was partially reversed after treatment with the AAV-HREEpo vector that physiologically corrected the hematocrit. In this experiment, AAV-CMVEpo–treated mice were culled on day 76, and all other mice were culled on day 218.
Analysis of organ weights in the Epo-TAghand healthy mice at the end of the experiment.
Healthy mice, Epo-TAgh mice, healthy mice treated with AAV-CMVGFP, Epo-TAgh mice treated with AAV-CMVGFP, healthy mice treated with AAV-CMVEpo, Epo-TAgh mice treated AAV-CMVEpo, healthy mice treated with AAV-HREEpo, Epo-TAgh mice treated AAV-HREEpo. Average weights of organs are plotted ± SD (n = 4-6). Spleens in the AAV-CMVEpo–treated groups are significantly enlarged. Hearts of Epo-TAgh mice are enlarged compared with those of healthy mice. This enlargement was partially reversed after treatment with the AAV-HREEpo vector that physiologically corrected the hematocrit. In this experiment, AAV-CMVEpo–treated mice were culled on day 76, and all other mice were culled on day 218.
Hearts taken from the untreated Epo-TAgh mice weighed 200% more than those taken from healthy mice, consistent with anemia-associated hypertrophy. Overproduction of Epo from the AAV-CMVEpo vectors had no significant effect on heart weight in either the Epo-TAgh or the healthy mice. Most interesting was the reduction in heart weight in the AAV-HREEpo–treated Epo-TAgh mice such that it was now only 33% greater than in healthy mice, showing that the hypertrophy had partially been reversed. AAV-HREEpo treatment had no effect on heart weight in the healthy mice.
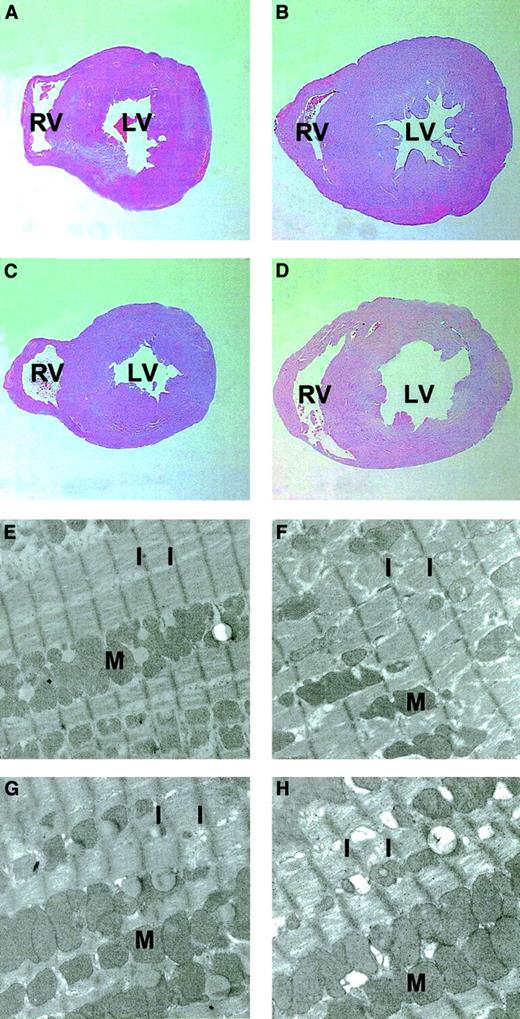
Histologic and ultrastructural analysis of the hearts confirmed gross hypertrophy in the Epo-TAgh mice (Figure5). Hypertrophy occurs as a result of an increase in the size of individual cells, and this can be seen in the Epo-TAgh heart as an increase in the average length of the sarcomere (the width between the muscle striations) from 0.97 ± 0.12 μm in healthy mice to 1.20 ± 0.20 μm in Epo-TAghmice. AAV-HREEpo treatment had no effect on sarcomere length in the healthy mice; however, in the Epo-TAgh mice, it was reduced to 1.05 ± 0.08 μm, further confirming partial correction of the cardiac hypertrophy in the AAV-HREEpo–treated Epo-TAghmice.
Histologic and ultrastructural analyses of the cross-sections of hearts from AAV-CMVGFP– and AAV-HREEpo–treated Epo-TAgh and healthy mice.
(A-D) Histologic analyses. (E-H) Ultrastructural analyses. (A,E) Healthy heart. (B,F) Epo-TAgh heart showing enlargement/hypertrophy illustrated by the increased size of the heart and the increase in sarcomere length. (C,G) Healthy heart treated with AAV-HREEpo. (D,H) Epo-TAgh heart showing partial correction of the hypertrophy such that there is a decrease in the sarcomere length compared with the untreated Epo-TAghhearts. Note the reduction in the width of the left ventricle wall in panel D (AAV-HREEpo–treated Epo-TAgh mouse) compared with panel B (untreated mouse). LV indicates left ventricle; RV, right ventricle; M, mitochondria; I, I band of the striated cardiac muscle cell (the distance between these I bands is the sarcomere). Sarcomere length in anemic mice was 1.20 ± 0.20 μm, and in anemic mice treated with AAV-HREEpo it was 1.05 ± 0.08 μm (P = .0001), showing that the decrease in sarcomere length between these hearts was statistically significant. Original magnifications: × 12.5 for histologic analysis, × 2100 for ultrastructural analysis.
Histologic and ultrastructural analyses of the cross-sections of hearts from AAV-CMVGFP– and AAV-HREEpo–treated Epo-TAgh and healthy mice.
(A-D) Histologic analyses. (E-H) Ultrastructural analyses. (A,E) Healthy heart. (B,F) Epo-TAgh heart showing enlargement/hypertrophy illustrated by the increased size of the heart and the increase in sarcomere length. (C,G) Healthy heart treated with AAV-HREEpo. (D,H) Epo-TAgh heart showing partial correction of the hypertrophy such that there is a decrease in the sarcomere length compared with the untreated Epo-TAghhearts. Note the reduction in the width of the left ventricle wall in panel D (AAV-HREEpo–treated Epo-TAgh mouse) compared with panel B (untreated mouse). LV indicates left ventricle; RV, right ventricle; M, mitochondria; I, I band of the striated cardiac muscle cell (the distance between these I bands is the sarcomere). Sarcomere length in anemic mice was 1.20 ± 0.20 μm, and in anemic mice treated with AAV-HREEpo it was 1.05 ± 0.08 μm (P = .0001), showing that the decrease in sarcomere length between these hearts was statistically significant. Original magnifications: × 12.5 for histologic analysis, × 2100 for ultrastructural analysis.
Discussion
This study demonstrates physiologically controlled regulation of Epo gene expression in genetically anemic Epo-TAgh mice. Each mouse received a single intramuscular dose of recombinant AAV vector, where EPO expression is regulated by the OBHRE promoter, AAV-HREEpo. The Epo-TAghmice treated with AAV-HREEpo showed correction of the hematocrit to aphysiologically normal level that was stabilized for the duration of the 7-month experiment. In other studies, sequences from the native EPO enhancer have been used to boost expression of EPO in an adenovirus or in encapsulated skeletal muscle cells.35 36 However, this study is the first example of the physiological regulation of EPO expression using an optimized hypoxia-responsive promoter in a clinically relevant anemic animal model. These data illustrate that the OBHRE promoter can sense the level of tissue hypoxia in the skeletal muscle and switch the expression of EPO on and off accordingly. The physiological regulation of the AAV-HREEpo vector in the skeletal muscle was further reinforced by the finding that it had no effect on the hematocrit level in healthy mice.
It is perhaps surprising that such exquisite physiological regulation was achieved in the skeletal muscle because it has been argued that the kidney is uniquely suited to sensing the hematocrit and regulating the production of Epo.37 These data suggest that the ability to sense and respond effectively to changes in the hematocrit may not be limited to the kidney and liver.
The correction of chronic anemia is a clinically important challenge. Anemia alone can lead to cardiac hypertrophy in the absence of any other cardiovascular disorder, and in renal patients anemia is an important contributor to left ventricular hypertrophy. Importantly, in patients with renal disease, the degree of left ventricular hypertrophy is a strong independent predictor of death.38In the presence of heart disease, especially coronary artery disease, anemia intensifies angina and contributes to a high incidence of cardiovascular complications. Clinically, it is essential to tailor the dose of rhEpo carefully to obtain an optimal response and to avoid complications related to polycythemia. The appropriate dose not only varies from patient to patient, it requires frequent adjustment based on hematocrit level. The approach we have taken here has 3 main advantages. First, the results described in this study suggest that anEPO therapy regulated by the OBHRE promoter might require less tailoring to individual patient requirements. Second,EPO gene therapy might achieve more precise correction of anemia, allowing more normal functioning of important organs such as the heart. The potential of this is illustrated by the substantial correction of left ventricular hypertrophy in the Epo-TAghmice in this study. Third, EPO gene therapy might be used in conditions such as myeloma, cancer, and thalassemia, which are often relatively resistant to Epo and would require higher doses than those administered to renal patients. Illustrating the problem of unregulated erythropoietin in this setting, when Epo-secreting hematopoietic cells were engrafted into a mouse model of β-thalassemia, the β-thalassemic phenotype was corrected but lethal polycythemia developed.39 It would clearly be of interest to determine whether AAV-HREEpo treatment could be used effectively and safely in these mice. In addition, the results described in this study show that the kinetics of EPO expression from the OBHRE promoter gave rise to a gradual increase in the hematocrit to the relevant normal level. This feature is important because excessively rapid increases in the red cell mass, as seen with the CMV promoter, can be detrimental to the cardiovascular system as a consequence of hyperviscosity.
We have clearly shown in this study that the OBHRE promoter functions to gives rise to physiologically controlled regulation ofEPO gene expression in a relevant anemic mouse model. Before we evaluate this gene therapy approach clinically, we would like to test the activity of the OBHRE promoter in larger animal models of Epo-responsive anemia—cats, dogs,40 and primates. The lack of toxicity seen in primate studies involving recombinant AAV-expressing Epo bodes well for the safety of these vectors for human gene therapy application.5 16 The lack of toxicity may be partly attributed to the intramuscular route of delivery because this allows little vector to reach the liver or lungs. Therefore, it is especially significant that the OBHRE promoter works to a physiologically relevant level in the skeletal muscle.
Although recombinant Epo has good safety and clinical records, the exquisite regulation observed in this model may lead to the consideration of genetic delivery strategies. In addition, the system described here shows the potential of combining efficient gene transfer to the skeletal muscle, with the individual's own cells supplying the regulatory component of the transcriptional machinery, HIF-1. We envisage that this approach could be useful in controlled local delivery of other polypeptides in diverse ischemic diseases.
In summary, this study describes the development of a physiologically regulated gene therapy modality that can deliver long-term, physiologically regulated expression of EPO, leading to correction of the hematocrit level and associated cardiac hypertrophy in chronically anemic mice.
We thank Peter Ratcliffe (The Henry Wellcome Building of Genomic Medicine, Oxford, United Kingdom) for helpful contributions to the work and Michael Dennis (CAMR, Salisbury, Wiltshire, United Kingdom) for maintenance and management of the animals. We also thank Sarah Busby (Department of Pharmacology, University of Oxford, United Kingdom) for the electron microscopy work.
Prepublished online as Blood First Edition Paper, June 14, 2002; DOI 10.1182/blood-2002-02-0605.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Katie Binley, Oxford BioMedica (UK) Ltd, Medawar Centre, Oxford Science Park, Oxford OX4 4GA, United Kingdom; e-mail: k.binley@oxfordbiomedica.co.uk.