Homing and repopulation of nonobese diabetic/severe combined immunodeficient (NOD/SCID) mice by enriched human CD34+stem cells from cord blood, bone marrow, or mobilized peripheral blood are dependent on stromal cell-derived factor 1 (SDF-1)/CXCR4 interactions. Recently, human cord and fetal blood CD34+CD38−CXCR4− and CXCR4+ cells, sorted with neutralizing anti-CXCR4 monoclonal antibody (mAb), were shown to have similar NOD/SCID repopulation potential. Herein we report that human cord blood CD34+CXCR4+ (R4+) and CD34+CXCR4− (R4−) subsets, sorted with neutralizing anti-CXCR4 mAb, engrafted NOD/SCID mice with significantly lower levels of human cells compared with nonsorted and SDF-1–migrated CD34+ cells. Coinjection of purified cells with 10 μg anti-CXCR4 mAb significantly reduced engraftment of all CD34+ subsets, and 50 μg completely abrogated engraftment by R4− and CD34+ cells. Importantly, R4− cells harbor intracellular CXCR4, which can be rapidly induced to cell surface expression within a few hours. Moreover, 48 hours of cytokine stimulation resulted in up-regulation of both cell surface and intracellular CXCR4, restoring migration capacities toward a gradient of SDF-1 and high-level NOD/SCID repopulation potential. In addition, homing of sorted R4− cells into the murine bone marrow and spleen was significantly slower and reduced compared to CD34+ cells but yet CXCR4 dependent. In conclusion, R4− cells express intracellular CXCR4, which can be functionally expressed on the cell membrane to mediate SDF-1–dependent homing and repopulation. Our results suggest dynamic CXCR4 expression on CD34+ stem and progenitor cells, regulating their motility and repopulation capacities.
Introduction
Hematopoietic stem cells migrate during embryonic development from the fetal liver through the blood circulation, home to the bone marrow (BM) microenvironment, and repopulate it with immature and maturing blood cells of all lineages. Similarly, in clinical and experimental stem cell transplantation protocols, hematopoietic stem cells, which are infused into the blood circulation of patients and experimental animals, home and repopulate the BM.1 The molecular mechanisms that regulate the homing and repopulation processes are crucial for stem cell function and development.2-5
The CXC chemokine stromal cell-derived factor 1 (SDF-1) plays a major role in migration, proliferation, differentiation, and survival of many cell types including human and murine hematopoietic stem/progenitor cells.6,7 SDF-1 is produced by multiple BM stromal cell types and by epithelial cells in many organs8,9 and is highly expressed by human and murine BM endothelium.10-12CXCR4, the 7-transmembrane receptor of SDF-1, is widely expressed by a variety of hematopoietic cell types, neuronal cells, and different stromal cells.13 SDF-1 is a chemotactic agent for human lymphoid, myeloid, and immature CD34+ progenitor cells.6,7,14,15 This chemokine induces integrin-dependent adhesion of CXCR4+ human T lymphocytes16 and immature CD34+CXCR4+ cells17 under shear flow and also mediates transendothelial migration of human progenitors.18 In vivo cell migration and localization are also mediated by SDF-1/CXCR4 interactions. Murine T cells overexpressing human CXCR4 and CD4 accumulated in the BM of transgenic mice.19 Prevention of CXCR4 expression by introducing SDF-1 intrakine blocked in vitro migration and in vivo dissemination of a T-cell hybridoma.20 More important, mice reconstituted with progenitor cells expressing SDF-1–intrakine suffered impaired lymphoid and myeloid hematopoiesis, whereas transplantation of progenitors overexpressing SDF-1 led to increased myeloid and B-lymphoid hematopoiesis.21 The key role of SDF-1 and CXCR4 in embryonic development was demonstrated by knockout studies in mice. The lack of either SDF-1 or its receptor in murine fetuses results in multiple lethal defects including impaired BM hematopoiesis.22-25
Recently, Wright and colleagues have demonstrated that SDF-1 is the sole chemokine mediating in vitro migration of purified adult murine BM stem cells.26 This important study suggests a major role for SDF-1/CXCR4 interactions also in adult murine stem cell migration and development.
We demonstrated the essential role of SDF-1/CXCR4 interactions in both homing and high-level multilineage repopulation of nonobese diabetic/severe combined immunodeficient (NOD/SCID) and NOD/SCID/B2mnull mice that have received primary and secondary serial transplants of enriched human CD34+ stem and progenitor cells derived from cord blood (CB), BM, and mobilized peripheral blood.10,27,28 The antihuman CXCR4-neutralizing monoclonal antibody (mAb; clone 12G5) binds CXCR4 on the first and second extracellular domain as its ligand SDF-1, interfering with SDF-1 binding and signaling.29,30Coinjecting enriched human CD34+ cells with neutralizing anti-CXCR4 mAb blocked homing and repopulation of human SCID-repopulating stem cells. Similar inhibition was achieved by using neutralizing anti–SDF-1 antibody or desensitizing CD34+cells with high doses of SDF-1.27,28 Increasing SDF-1 levels within the recipient BM by either preconditioning the murine hosts with DNA-damaging agents or by direct injection of human SDF-1 led to increased homing and repopulation.10,28 Short-term (24-48 hours) stimulation with stem cell factor (SCF) and interleukin 6 (IL-6) up-regulated surface CXCR4 expression by immature human CD34+ cells and increased in vitro migration toward a gradient of SDF-1 and in vivo homing and repopulation,27,28 demonstrating functional dynamic expression of CXCR4. We therefore recharacterized human SCID repopulating stem cells as CD38−/lowCXCR4+cells.27 Rosu-Myles et al recently reported that human CB and fetal blood CD34+CD38−CXCR4−and CD34+CD38−CXCR4+ sorted cells both have similar repopulating capacity in NOD/SCID mice.31 Their study rules out both the need for functional CXCR4 expression and the crucial role of SDF-1 signaling in human SCID-repopulating cell (SRC) function, that is, homing, retention, and high-level multilineage repopulation.
The aim of the present study was to elucidate the regulation of CXCR4 expression in sorted human CB CD34+CXCR4− and CXCR4+ cells and to assess the role of this receptor in their homing and repopulation.
Materials and methods
Human cells
Human CB cells from full-term deliveries, leftover BM, or granulocyte colony-stimulating factor (G-CSF)–mobilized peripheral blood cells from healthy donors for clinical transplantation were obtained after informed consent was obtained and were used in accordance with procedures approved by the human ethics committee of the Weizmann Institute. The samples were separated on Ficoll-Paque (Pharmacia Biotech, Uppsala, Sweden). CD34+ cells were enriched using the MACS cell isolation kit and the auto MACS magnetic cell sorter (Miltenyi Biotec, Bergisch Gladbach, Germany) according to the manufacturer's instructions, obtaining purity of about 80%. Purified cells were used freshly or frozen in 10% dimethyl sulfoxide (DMSO) for later usage. For cell sorting, enriched CB CD34+cells from multiple donors were pooled and labeled with human-specific mAb anti-CD34 fluorescein isothiocyanate (FITC; Becton Dickinson, San Jose, CA) and neutralizing anti-CXCR4 phycoerythrin (PE; clone 12G5, Pharmingen, San Diego, CA) according to the manufacturer's instructions. The 12G5 mAb is nontoxic to human CD34+ cells as indicated by colony-forming assays.27 Cells were washed twice and sorted for CD34+CXCR4− or CD34+CXCR4+ purified subpopulations by FACSVantage (Becton Dickinson), obtaining purity of more than 97%. Where indicated, sorted cells (2 × 105 cells/mL) were cultured for 24 or 48 hours in RPMI supplemented with 10% fetal calf serum (FCS) or in serum-free media32 supplemented with the following recombinant human cytokines31: 5-cytokine combination—SCF, FLT-3 ligand (FLT3-L 300 ng/mL each), G-CSF (50 ng/mL), IL-3 (10 ng/mL), all from R & D Systems (Minneapolis, MN) and IL-6 (10 ng/mL; Interpharm Laboratories, Ares-Serono Group, Ness Ziona, Israel); 2-cytokine combination—SCF plus IL-6 (50 ng/mL each). SDF-1 desensitization was performed in the presence of the 5-cytokine combination and 1 μg/mL SDF-1 for 24 hours. Cultures without cytokines served as controls. Serum-free media or media supplemented with 10% FCS gave similar results. The cultures were incubated at 37°C in a humidified atmosphere containing 5% CO2.
Mice
NOD/LtSz-Prkdcscid (NOD/SCID) mice were bred and maintained under defined flora conditions at the Weizmann Institute in sterile microisolator cages. All the experiments were approved by the animal care committee of the Weizmann Institute. Eight- to 10-week-old mice were sublethally irradiated (375 cGy, from a60Co source) and received transplants of human cells as indicated (1-2 × 105 cells/mouse) about 6 hours after irradiation. Mice were killed at different time points after transplantation as indicated; BM and spleen cells were harvested and resuspended into single-cell suspension.
Human cell engraftment
Where indicated, human cells (1-2 × 105cells/mouse) were preincubated with nonconjugated neutralizing antihuman CXCR4 mAb (10 μg or 50 μg/mouse, clone 12G5, R&D Systems) before transplantation. Incubated cells were not washed and the entire dose of anti-CXCR4 mAb was coinjected with the cells. In other experiments, 10 μg anti-CXCR4 mAb was injected intraperitoneally at different time points after transplantation as indicated. Two or 6 weeks later, a single-cell suspension was prepared from the BM and spleen of mice that underwent transplantation. Human cell engraftment was assayed by flow cytometry (FACSCalibur, Becton Dickinson), using specific antihuman CD45-FITC mAb (Immuno Quality Products, Groningen, The Netherlands), anti–CD19-PE (Coulter, Miami, FL), or anti–CXCR4-PE (12G5, Pharmingen). Human plasma and mouse IgG were used to block Fc receptors. Isotype control antibodies and cells obtained from mice that did not undergo transplantation were used as negative controls and human cells were used as a positive control.
Intracellular CXCR4 staining
CXCR4 expressed on the cell surface was blocked with nonconjugated antihuman CXCR4 mAb (clone 12G5, 10 μg/mL, 1 hour, 4°C). Cells were fixed with paraformaldehyde (4%, 20 minutes at room temperature; BDH, Poole, England) and then permeabilized with Triton X-100 (0.5%-1%,10 minutes at room temperature; Sigma, St Louis, MO). Antihuman CXCR4-PE mAb was used to label the cells for flow cytometry for 30 minutes, 4°C. The cells were washed with phosphate-buffered-saline without Mg++/Ca++after each step.
Homing assay
Human CD34+CXCR4− sorted cells (≥ 7 × 105 cells/mouse) and human CD34+-enriched cells (5 × 105 cells/mouse) from the same donors were injected into sublethally (375 cGy) irradiated mice 24 hours after irradiation. Where indicated, cells were incubated with antihuman CXCR4 mAb (10 μg/mouse) and coinjected without washing. Cells were recovered from the BM and spleen of mice that underwent transplantation 16 or 29 hours after transplantation and analyzed for the presence of human cells by using human-specific anti–CD34-FITC (Becton Dickinson), antihuman CD38-PE (Coulter), and anti–CXCR4-PE antibodies acquiring at least 106cells/sample. Mouse IgG and human plasma were used to block Fc receptors. Cells obtained from mice that did not undergo transplantation or labeled with mouse isotype control antibodies were used as negative controls. Human cells were used as a positive control and propidium iodide (PI) staining was used to exclude dead cells.
Migration assay
Human CB-enriched CD34+ cells were allowed to migrate toward a gradient of SDF-1 as previously described.27 Briefly, 125 ng/mL SDF-1 was added to the lower chamber of a Costar 24-well transwell (Corning, NY). CD34+ cells (1-2 × 105) or R4−cells (1 × 105 cells, 2, 24, and 48 hours after sorting and incubation with the indicated cytokines) were loaded to the upper chamber and were allowed to migrate for 4 hours at 37°C. CD34+ migrating cells (which are about 25% of the total CD34+ population) were collected from the lower chamber, washed, and transplanted (1-2 × 105 cells/mouse) into NOD/SCID mice as indicated.
Results
Sorted CB CD34+CXCR4+ and CD34+CXCR4− cells have reduced repopulating potential
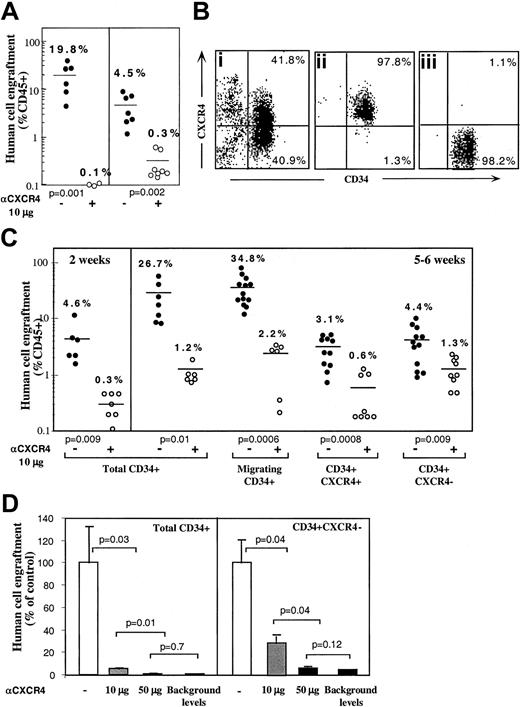
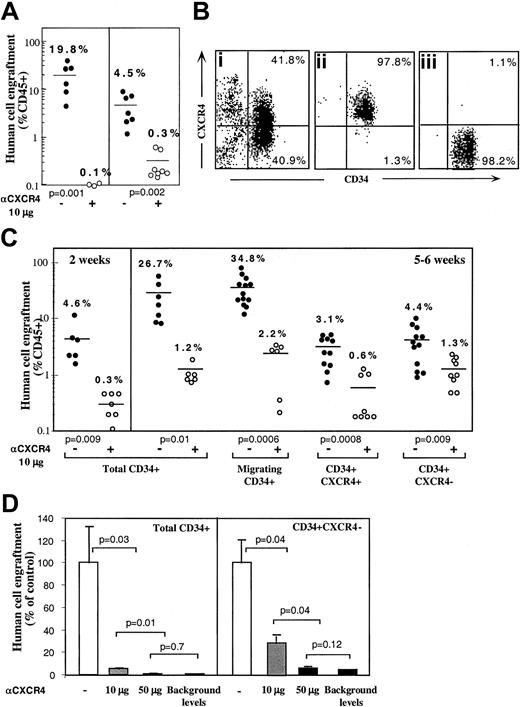
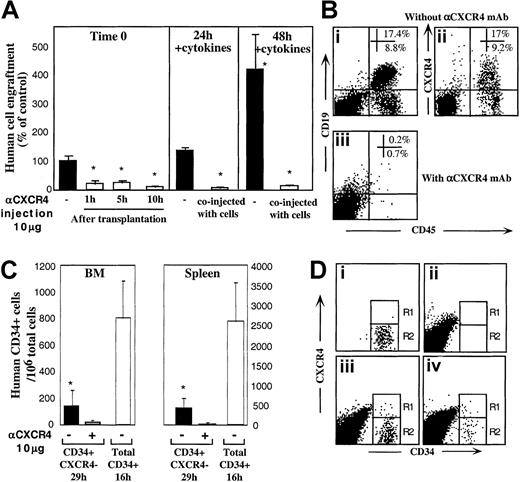
Enriched human CD34+ cells derived from mobilized peripheral blood, BM, or CB engraft NOD/SCID mice in a CXCR4-dependent manner as demonstrated by the levels of human progenitors in the BM of mice that underwent transplantation.27 This result was confirmed when enriched human BM and mobilized peripheral blood CD34+ cells were preincubated and coinjected with 10 μg neutralizing antihuman CXCR4 mAb leading to more than 90% reduction in total human cell engraftment (Figure 1A). To further investigate the potential role of CXCR4 in homing and repopulation of CD34+ cells that do not express cell surface CXCR4, enriched human CB CD34+ cells were further sorted into CD34+CXCR4+ (R4+) and CD34+CXCR4− (R4−) purified subsets, yielding a purity of more than 97% (Figure 1Bi,ii,iii, respectively). Because R4+ cells required staining with neutralizing anti-CXCR4 mAb for their sorting, a process that also blocks SDF-1 signaling, enriched CD34+ cells from the same donors were kept untreated as a positive control. In addition, enriched CD34+ cells from the same donors were allowed to migrate toward a low gradient of SDF-1 in transwells, to functionally select CXCR4+ cells based on their responsiveness to SDF-1, without blocking SDF-1 binding and signaling, and with only minimal CXCR4 internalization. Equal numbers of all CD34+ subsets were then transplanted into sublethally irradiated NOD/SCID mice (1-2 × 105 cells/mouse) that were assayed for the level of human/mouse chimerism 2 weeks (only for control, total CD34+ cells) or 5 or 6 weeks later, as indicated. Enriched CD34+ cells and CD34+ cells migrating to a gradient of SDF-1 demonstrated significantly higher levels of human cell engraftment (26.7% and 34.8%, respectively) compared to R4+ and R4− sorted cells (3.1% and 4.4%, respectively; Figure 1C). These data demonstrate that the use of neutralizing antibodies to sort R4+ cells significantly impairs their repopulating potential by preventing SDF-1 binding and signaling. Repopulation levels by R4+ sorted cells are thus similarly reduced to the low levels obtained with R4−cells. However, the low but significant repopulation ability of R4− and neutralized R4+ cells required further investigation.
CXCR4-dependent engraftment of human CD34+ cells.
(A) BM engraftment of NOD/SCID mice, by enriched human CD34+ cells derived from peripheral blood of G-CSF–treated donors (MPB) or BM aspiration of healthy donors. BM of mice that underwent transplantation was harvested 1 month later; human/mouse chimerism was determined by flow cytometry using antihuman-specific CD45-FITC mAb. Human CD34+ cells (2 × 105cells/mouse) were transplanted without (●) or with (○) anti-CXCR4 mAb (10 μg/mouse). Each dot represents one mouse. Data summarize results of 3 independent experiments. P values are indicated. (B) CB enriched CD34+ cells (Bi) were further sorted with neutralizing antihuman CXCR4 mAb to R4+ (Bii), and R4− (Biii) purified subsets. (C) Murine BM engraftment of CB CD34+ subsets. Enriched CD34+ subset cells were pretreated and coinjected as described for panel A. Data summarize results of 4 independent experiments. P values are indicated. (D) Dose-dependent inhibition of BM repopulation by anti-CXCR4 mAb. Dose-dependent activity of 12G5 mAb is demonstrated by coinjecting 10 μg/mouse versus 50 μg/mouse. Human cell engraftment in the murine BM is presented. ■ indicates untreated CD34+ cells. ░ indicates coinjection of 10 μg/mouse. ▪ indicates coinjection of 50 μg/mouse or background levels determined by staining with isotype control mAb, as indicated.P values are indicated. Data present values of 3 experiments.
CXCR4-dependent engraftment of human CD34+ cells.
(A) BM engraftment of NOD/SCID mice, by enriched human CD34+ cells derived from peripheral blood of G-CSF–treated donors (MPB) or BM aspiration of healthy donors. BM of mice that underwent transplantation was harvested 1 month later; human/mouse chimerism was determined by flow cytometry using antihuman-specific CD45-FITC mAb. Human CD34+ cells (2 × 105cells/mouse) were transplanted without (●) or with (○) anti-CXCR4 mAb (10 μg/mouse). Each dot represents one mouse. Data summarize results of 3 independent experiments. P values are indicated. (B) CB enriched CD34+ cells (Bi) were further sorted with neutralizing antihuman CXCR4 mAb to R4+ (Bii), and R4− (Biii) purified subsets. (C) Murine BM engraftment of CB CD34+ subsets. Enriched CD34+ subset cells were pretreated and coinjected as described for panel A. Data summarize results of 4 independent experiments. P values are indicated. (D) Dose-dependent inhibition of BM repopulation by anti-CXCR4 mAb. Dose-dependent activity of 12G5 mAb is demonstrated by coinjecting 10 μg/mouse versus 50 μg/mouse. Human cell engraftment in the murine BM is presented. ■ indicates untreated CD34+ cells. ░ indicates coinjection of 10 μg/mouse. ▪ indicates coinjection of 50 μg/mouse or background levels determined by staining with isotype control mAb, as indicated.P values are indicated. Data present values of 3 experiments.
Sorted CB CD34+CXCR4− and CD34+CXCR4+ cells engraft NOD/SCID mice in a CXCR4-dependent manner
The involvement of CXCR4 in the engraftment of all CD34+ subsets was demonstrated by preincubation with the neutralizing antihuman CXCR4 mAb (clone 12G5, 10 μg/mouse) that was also coinjected with transplanted cells without washing.27As expected, engraftment levels of the positive controls, total CD34+ cells and SDF-1–migrating CD34+ cells, were significantly reduced (1.2% and 2.2% respectively, Figure 1C). Interestingly, the same treatment also further reduced the low engraftment levels obtained with R4+ sorted cells (from 3.1% to 0.6%), despite the fact that these cells were already binding lower levels of conjugated neutralizing mAb used for the sorting process. These findings suggest that during cell sorting and the homing process additional CXCR4 receptors free of neutralizing mAb are functionally expressed on the cell surface. These newly expressed receptors, which contributed to the limited engraftment levels by untreated R4+ cells, were further blocked by coinjection of 10 μg neutralizing mAb. Most important, the low engraftment levels obtained by sorted R4− cells (4.4%) were also significantly reduced by coinjection with 10 μg neutralizing anti-CXCR4 (1.3%), providing evidence for CXCR4-dependent engraftment also by the R4− cells (Figure 1C). Moreover, the in vivo inhibition capacity of the neutralizing anti-CXCR4 mAb is dose-dependent. Coinjection of 50 μg/mouse neutralizing anti-CXCR4 mAb significantly reduced further BM repopulation by both control, total CD34+ cells (0.1% ± 0.03%) and by sorted CD34+CXCR4− cells (0.2% ± 0.05%) similar to the minimal background levels detected by isotype control mAb staining (0.1%; Figure 1D). This high dose was used to demonstrate that BM repopulation could be totally blocked with a single treatment of anti-CXCR4 mAb (Figure 1D).
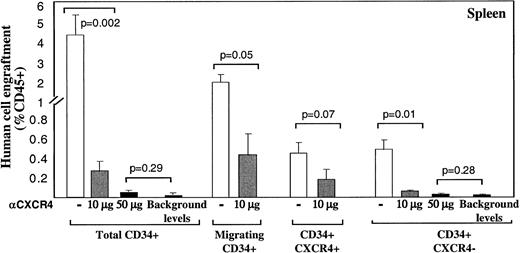
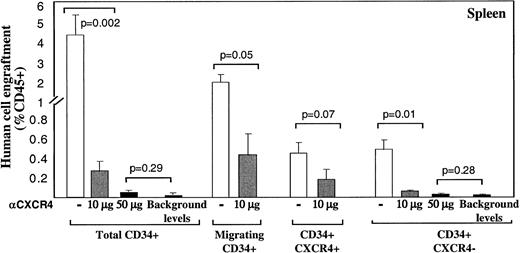
A similar pattern of CXCR4-dependent repopulation by the different human CD34+ subsets was also observed in the spleen of mice that received transplantations, which is a hematopoietic organ as well (Figure 2). Repopulation by CD34+ subset coinjected with neutralizing anti-CXCR4 mAb, was also significantly reduced (to 0.26% by using 10 μg/mouse and 0.05% with 50 μg/mouse) compared to their untreated counterparts (3.5%; Figure 2). Similarly, repopulation by CD34+CXCR4− sorted cells cotransplanted with anti-CXCR4 mAb, was also significantly reduced (to 0.04% by using 10 μg/mouse and 0.02% with 50 μg/mouse) compared to their untreated counterparts (0.42%; Figure 2). In both fractions, using a high dose of 50 μg/mouse abrogated repopulation capacity to background levels determined by staining with isotype control mAb.
Murine spleen engraftment by human CD34+subsets is mediated by CXCR4.
Dose dependent inhibition of spleen repopulation by anti-CXCR4 mAb. The levels of human cell engraftment in the spleen of mice presented in Figure 1, panels C and D, were summarized. ■ represents transplantation of cells without additional mAb; ░ indicates coinjection with anti-CXCR4 mAb (10 μg/mouse) and ▪ represents 50 μg/mouse or background levels determined by staining with isotype control mAb, as indicated. Transplanted subsets and P values are indicated.
Murine spleen engraftment by human CD34+subsets is mediated by CXCR4.
Dose dependent inhibition of spleen repopulation by anti-CXCR4 mAb. The levels of human cell engraftment in the spleen of mice presented in Figure 1, panels C and D, were summarized. ■ represents transplantation of cells without additional mAb; ░ indicates coinjection with anti-CXCR4 mAb (10 μg/mouse) and ▪ represents 50 μg/mouse or background levels determined by staining with isotype control mAb, as indicated. Transplanted subsets and P values are indicated.
Sorted CD34+CXCR4− cells express intracellular CXCR4, which can be up-regulated and expressed on the cell surface in response to cytokine stimulation
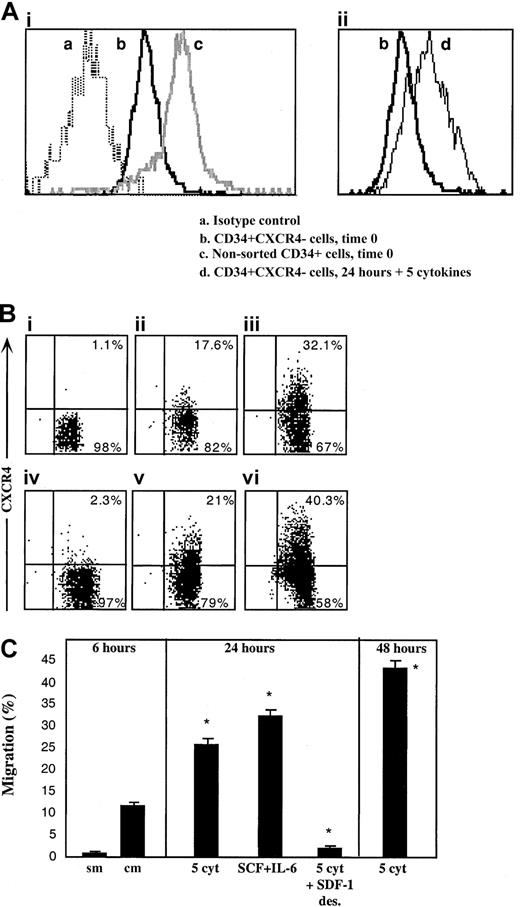
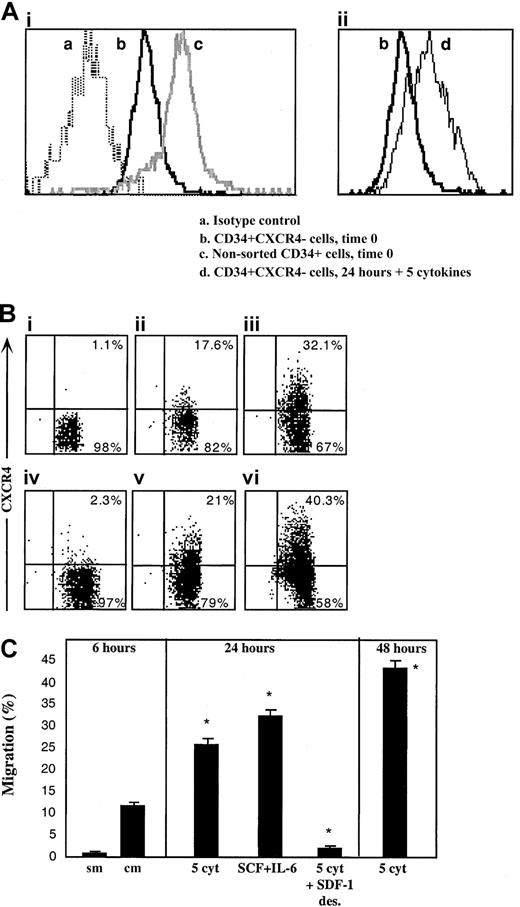
Next we postulated that like R4+ sorted cells, sorted R4− cells as well can rapidly express functional cell surface CXCR4 that, in turn, mediates their limited SDF-1–dependent repopulation capacity. To investigate this hypothesis, the levels of cell surface and intracellular CXCR4 expressed by the cells were determined immediately after sorting and following 24 hours of in vitro incubation with cytokines that are known to support SCID repopulating cells.31,33 We documented low intracellular CXCR4 levels in sorted R4− cells (Figure3Aib), whereas almost no detectable CXCR4 was expressed on the cell surface (Figure 3Bi). R4− sorted cells that were cultured with a 5-cytokine combination (SCF, FLT3-L, IL-6, IL-3, and G-CSF)31 33 demonstrated limited cell surface CXCR4 expression after 24 and even more so after 48 hours, similar to normal levels, (Figure 3Biii and vi, respectively), which was associated with increased expression of intracellular CXCR4 (Figure 3Aiid). Similarly, spontaneous up-regulation of surface CXCR4 expression within 24 hours was also observed when R4−cells were cultured in serum-free media without additional cytokines, suggesting autocrine secretion of cytokines capable of CXCR4 up-regulation, although cell viability was significantly reduced (56% viability, Figure 3Bv). Most notably is the fact that short-term cytokine-stimulated R4− sorted cells express similar levels of intracellular CXCR4 compared to freshly isolated CD34+ cells (Figure 3Aic and iid, respectively), indicating the potential of both subsets to rapidly express surface CXCR4 within 24 hours.
Up-regulation of intracellular and cell surface CXCR4, functionally expressed by R4− sorted cells.
CXCR4 expression by R4− cells was determined following cell sorting and after in vitro cultures. (A) Intracellular CXCR4 expression. (Ai) Time 0. (a) Isotype control antibody, (b) R4− sorted cells time 0, (c) nonsorted total CD34+ cells. (Aii) Up-regulation following 24 hours with 5 cytokines. (b) R4− sorted cells time 0, (d) R4− cells cultured for 24 hours with 5 cytokines. (B) Cell surface staining. (Bi) Time 0. (Bii) Migrating cells following 2 hours of sorting plus 4 hours in transwells. (Biii) 24 hours with 5 cytokines. (Biv) 24 hours with 5 cytokines plus a high concentration of SDF-1, 1μg/mL. (Bv) 24 hours without cytokines. Cell viability was 56%. (Bvi) 48 hours with 5 cytokines. (C) In vitro migration. sm indicates spontaneous migration—without SDF-1 at the lower chamber; cm, migration of sorted cells toward SDF-1. Culture conditions: 5 cyt indicates 5-cytokine combination. SDF-1 des indicates 1μg/mL SDF-1. A-B, representative FACS analyses. Data summarize 3 experiments.
Up-regulation of intracellular and cell surface CXCR4, functionally expressed by R4− sorted cells.
CXCR4 expression by R4− cells was determined following cell sorting and after in vitro cultures. (A) Intracellular CXCR4 expression. (Ai) Time 0. (a) Isotype control antibody, (b) R4− sorted cells time 0, (c) nonsorted total CD34+ cells. (Aii) Up-regulation following 24 hours with 5 cytokines. (b) R4− sorted cells time 0, (d) R4− cells cultured for 24 hours with 5 cytokines. (B) Cell surface staining. (Bi) Time 0. (Bii) Migrating cells following 2 hours of sorting plus 4 hours in transwells. (Biii) 24 hours with 5 cytokines. (Biv) 24 hours with 5 cytokines plus a high concentration of SDF-1, 1μg/mL. (Bv) 24 hours without cytokines. Cell viability was 56%. (Bvi) 48 hours with 5 cytokines. (C) In vitro migration. sm indicates spontaneous migration—without SDF-1 at the lower chamber; cm, migration of sorted cells toward SDF-1. Culture conditions: 5 cyt indicates 5-cytokine combination. SDF-1 des indicates 1μg/mL SDF-1. A-B, representative FACS analyses. Data summarize 3 experiments.
The potential of newly expressed receptors to function in vitro was assessed by SDF-1 transwell migration assay, comparing R4−cells 2 hours after sorting, to cytokine-stimulated cells. Within 6 hours after cell sorting (including 4 hours of migration toward a low gradient of SDF-1), low surface CXCR4 expression is already initiated on sorted R4− cells (Figure 3Bii) and a basal level of migration toward a low SDF-1 gradient (Figure 3 C, sm—spontaneous migration without the chemokine, and cm—with 125 ng/mL SDF-1 in the lower well) is documented. The 5-cytokine combination or SCF plus IL-6 stimulation for 24 hours induced 2.5- to 3-fold increases in SDF-1–mediated cell motility (middle panel) and a 4-fold increase following 48 hours (right panel). Additionally, functional evidence for the physiologic activity of newly expressed CXCR4 receptors is provided by culturing sorted R4− cells in the presence of a high SDF-1 concentration (1 μg/mL), which is known to cause CXCR4 internalization and desensitization for 24 hours together with the 5-cytokine cocktail. CXCR4 is internalized and its surface expression is prevented despite the presence of cytokines in the culture (Figure 3Biv). As expected, washed SDF-1–desensitized cells did not migrate toward a gradient of SDF-1 (Figure 3C, middle panel).
Slower and reduced CXCR4-dependent homing of sorted CD34+CXCR4− cells
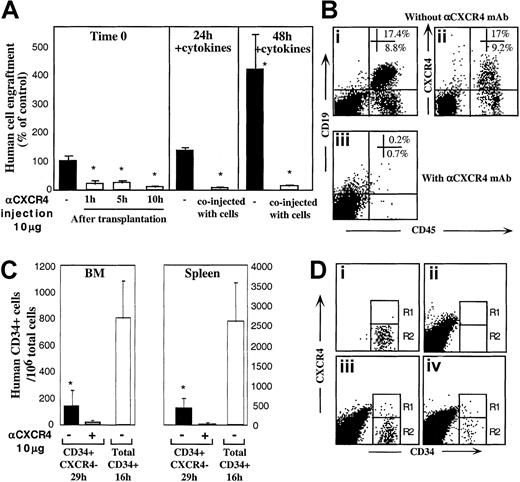
To evaluate the ability of R4− sorted cells to express functional CXCR4 during transplantation, in vivo experiments were performed. In one set of experiments we introduced anti-CXCR4 mAb into the recipients at multiple time points up to 10 hours after transplantation to provide R4− cells with time to regulate cell surface CXCR4 expression in vivo. Engraftment of R4−cells was significantly reduced regardless of whether mAb was injected together with the cells (Figure 1C) or separately 1, 5, or 10 hours later by intraperitoneal injection (Figure4A, left panel; P < .005). Moreover, the partial up-regulation of both intracellular and cell surface CXCR4 induced by cytokine stimulation within 24 and 48 hours (Figure 3Aii,Biii,vi) correlated well with improved CXCR4-dependent engraftment capacities (1.4- and 4.2-fold increase, Figure 4A, middle and right panels). Fluorescence-activated cell-sorter scanner (FACS) analyses of highly engrafted mice that received transplants of R4− cells that were cultured with 5 cytokines for 48 hours reveals that once the cells engraft the murine BM, they give rise to normally distributed multilineage differentiation as indicated by the presence of myeloid and B-lymphoid human cells (Figure 4Bi). Interestingly, these cells expressed variable levels of surface CXCR4 (Figure 4Bii) demonstrating the ability of R4−transplanted cells to give rise to a heterogeneous, normal cell profile in the murine BM. No human cell engraftment could be determined when the cultured cells were coinjected with neutralizing anti-CXCR4 mAb (Figure 4Biii).
CXCR4 mediates homing and repopulation of NOD/SCID mice by R4− cells.
(A) Transplantation of R4− cells at day 0 (▪, left panel), followed by antihuman CXCR4 mAb intraperitoneal injection at the indicated time points (■, left panel). R4− cells cultured with 5-cytokine combination for 24 hours (middle panel) or 48 hours (right panel) were coinjected without (▪) or with (■) anti-CXCR4 mAb (10 μg/mouse). Engraftment levels were determined 5 to 6 weeks later. Data present mean ± SE values of 3 independent experiments, 4 mice per group; P < .05. (B) Representative FACS analysis of BM samples of highly engrafted mice that received transplants of R4− cells cultured for 48 hours with 5 cytokines. (Bi) Cells injected without (Bi-Bii) or with (Biii) anti-CXCR4 mAb (10 μg/mouse). Samples were stained with antihuman CD45 and CD19 (Bi, Biii) or CXCR4 (Bii). (C) Homing of R4− cells and enriched CD34+ cells into the BM and spleen of NOD/SCID mice that underwent transplantation at indicated time points; P < .05. Three experiments are summarized. (D) Representative FACS analysis of homed human cells to the murine BM. (Di) R4− cells, time 0, before transplantation. (Dii) Nontransplanted mouse BM. (Diii) A mouse that received a transplant of total CD34+ cells (16 hours following transplantation). (Div) A mouse that received a transplant of R4− sorted cells, time 0 sorted cells and cells in the murine BM 29 hours following transplantation.
CXCR4 mediates homing and repopulation of NOD/SCID mice by R4− cells.
(A) Transplantation of R4− cells at day 0 (▪, left panel), followed by antihuman CXCR4 mAb intraperitoneal injection at the indicated time points (■, left panel). R4− cells cultured with 5-cytokine combination for 24 hours (middle panel) or 48 hours (right panel) were coinjected without (▪) or with (■) anti-CXCR4 mAb (10 μg/mouse). Engraftment levels were determined 5 to 6 weeks later. Data present mean ± SE values of 3 independent experiments, 4 mice per group; P < .05. (B) Representative FACS analysis of BM samples of highly engrafted mice that received transplants of R4− cells cultured for 48 hours with 5 cytokines. (Bi) Cells injected without (Bi-Bii) or with (Biii) anti-CXCR4 mAb (10 μg/mouse). Samples were stained with antihuman CD45 and CD19 (Bi, Biii) or CXCR4 (Bii). (C) Homing of R4− cells and enriched CD34+ cells into the BM and spleen of NOD/SCID mice that underwent transplantation at indicated time points; P < .05. Three experiments are summarized. (D) Representative FACS analysis of homed human cells to the murine BM. (Di) R4− cells, time 0, before transplantation. (Dii) Nontransplanted mouse BM. (Diii) A mouse that received a transplant of total CD34+ cells (16 hours following transplantation). (Div) A mouse that received a transplant of R4− sorted cells, time 0 sorted cells and cells in the murine BM 29 hours following transplantation.
We previously showed that freshly isolated human CB CD34+-enriched cells home rapidly in a CXCR4-dependent manner and can be detected in the BM and spleen of NOD/SCID recipient mice as early as 2 to 4 hours after transplantation.28 The potential function of cell surface CXCR4, expressed by transplanted R4− sorted cells during their migration in the murine blood circulation, was further evaluated by using in vivo homing assays. R4− sorted cells (≥ 7 × 105cells/mouse) and control CD34+ cells (5 × 105 cells/mouse) from the same donors were transplanted into NOD/SCID mice 24 hours following sublethal irradiation. In all experiments, R4− sorted cells homed to significantly lower extent and at a much slower pace compared to control CD34+ enriched cells. More important, coinjection of sorted R4− cells with 10 μg neutralizing anti-CXCR4 mAb further reduced their homing to the BM and spleen of NOD/SCID mice (Figure 4C; P ≤ .005). Comparing in vivo surface CXCR4 expression by total human CD34+ BM-homed cells 16 hours after transplantation (Figure 4Diii), with R4−-homed cells 29 hours after transplantation (Figure 4Div), reveals a similar profile for both populations: whereas CXCR4 is down-regulated by the majority of cells (Figure 4Diii-iv, R2), most probably by murine BM SDF-1, a minority of the homed R4− cells (R1) maintain up-regulated surface CXCR4 compared to the sorted cells before transplantation (Figure 4Di, R1). Low CXCR4 expression by BM-homed human CD34+ cells may be a result of high murine BM SDF-1 concentrations induced by conditioning the mice with total body irradiation before transplantation.10 These experiments provide additional evidence for the central role of murine SDF-1 signaling via human CXCR4 in the homing of all human CD34+subset cells in NOD/SCID mice that underwent transplantation.
Taken together, these results corroborate the notion that following in vitro cytokine incubation and subsequent in vivo stimulation of R4− sorted cells, intracellular CXCR4 is functionally expressed on the cell surface and accounts for their low SDF-1–dependent homing and engraftment potential.
In vitro and in vivo dynamic CXCR4 expression on CXCR4+ sorted cells
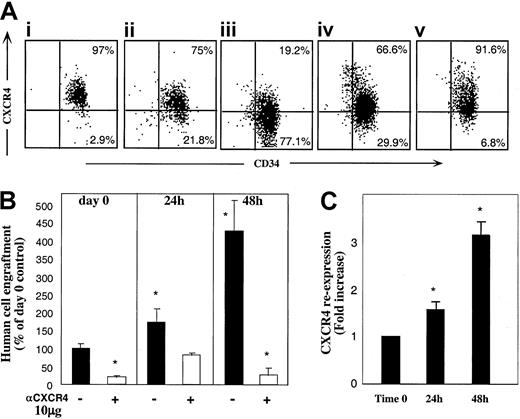
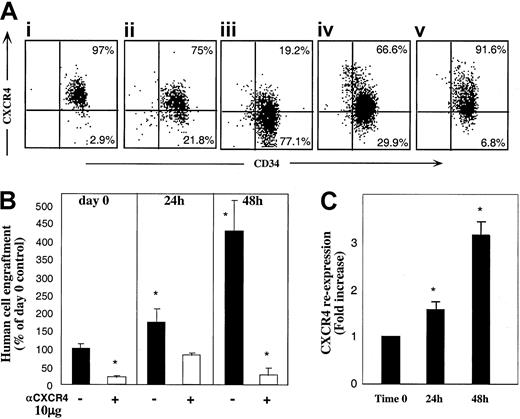
As shown in Figure 1C, binding of anti-CXCR4 mAb used for sorting (in which the excess of conjugated mAb was washed) did not fully abolish the SDF-1–dependent repopulating potential of sorted R4+ cells. We considered the possibility that CXCR4 expression by R4+ sorted cells is not constant and is dynamically regulated during the sorting process and moreover while circulating in vivo, enabling partial recovery of receptor function and homing/repopulation activities. Thus, R4+ cells were incubated in the same cytokine combination as R4− cells. Unexpectedly, the expression of cell surface CXCR4 was reduced following 24 and furthermore 48 hours of cytokine stimulation with SCF, FLT3-L, IL-6, IL-3, and G-CSF (Figure5Ai-iii). Interestingly, cells incubated with SCF and IL-6 alone maintained higher levels of CXCR4 on the cell surface compared to the 5-cytokine combination (Figure 5Aiv). Moreover, viable sorted R4+ cells cultured in serum-free media without cytokines preserved the highest level of CXCR4 surface expression (Figure 5Av), suggesting involvement of an autocrine loop of cytokine signaling. However, human cytokine deprivation led to reduced cell viability (40% viable cells compared to 97% with cytokines). Similarly, CXCR4 down-regulation was previously documented by primitive CD34+CD38−CXCR4+ sorted cells within 24 hours of cytokine stimulation in an SDF-1–responsive manner.31 Intracellular CXCR4 levels expressed by R4+ cells could not be documented due to the high background contributed by the cell surface receptors stained with the conjugated antibody used for sorting.
R4+ cells functionally modulate CXCR4 expression.
(A) Cell surface CXCR4 staining. R4+ sorted cells (Ai) were cultured for 24 hours (Aii), and 48 hours (Aiii), with 5 cytokines, or for 48 hours with SCF plus IL-6 (Aiv) or in serum-free medium alone (Av). At each time point, cells were restained with antihuman CXCR4-PE for flow cytometry analysis. A representative FACS analysis of 3 independent experiments is shown. (B) Enhancement of CXCR4-dependent repopulation by cytokine stimulation. CXCR4 surface expression of cells from cultures in panels Ai-Aiii was analyzed before and after restaining with anti–CXCR4-PE. CXCR4 re-expression was calculated by dividing values obtained in restained samples by those of cells without restaining. (C) Surface CXCR4 re-expression. Cells cultured with 5 cytokines were also transplanted to determine their engraftment potential. R4+ cells were pretreated and coinjected with antihuman CXCR4 (10 μg/mouse) as indicated. Data present mean ± SE values of 3 independent experiments. *P < .03 compared to cells transplanted in day 0 without anti-CXCR4 mAb.
R4+ cells functionally modulate CXCR4 expression.
(A) Cell surface CXCR4 staining. R4+ sorted cells (Ai) were cultured for 24 hours (Aii), and 48 hours (Aiii), with 5 cytokines, or for 48 hours with SCF plus IL-6 (Aiv) or in serum-free medium alone (Av). At each time point, cells were restained with antihuman CXCR4-PE for flow cytometry analysis. A representative FACS analysis of 3 independent experiments is shown. (B) Enhancement of CXCR4-dependent repopulation by cytokine stimulation. CXCR4 surface expression of cells from cultures in panels Ai-Aiii was analyzed before and after restaining with anti–CXCR4-PE. CXCR4 re-expression was calculated by dividing values obtained in restained samples by those of cells without restaining. (C) Surface CXCR4 re-expression. Cells cultured with 5 cytokines were also transplanted to determine their engraftment potential. R4+ cells were pretreated and coinjected with antihuman CXCR4 (10 μg/mouse) as indicated. Data present mean ± SE values of 3 independent experiments. *P < .03 compared to cells transplanted in day 0 without anti-CXCR4 mAb.
To evaluate whether the cells, which express the highest CXCR4 levels also gain the highest stem cell activity, we next examined the repopulating potential of cytokine-stimulated R4+ cells. Despite reduction in the total level of surface CXCR4 expression detected during the culture period (Figure 5Ai-iii), 5-cytokine–treated R4+ cells had significantly increased engraftment capacities compared to nonstimulated R4+ sorted cells (Figure 5B, P < .03). This contradiction between decreasing CXCR4 expression and increasing NOD/SCID repopulation led us to re-examine the cytokine-treated cells for newly expressed CXCR4 receptors. Still binding the labeled, neutralizing anti-CXCR4 antibodies used for sorting, the cultured cells were analyzed with and without restaining for CXCR4. Figure 5C shows the fold increase of CXCR4 re-expression calculated by dividing nonrestained by restained values. A strong correlation between new surface CXCR4 re-expression (C) and the potential to repopulate NOD/SCID (B) can be clearly observed.
These results imply a dynamic expression of CXCR4: highly expressed receptors, which bind neutralizing anti-CXCR4 mAb, are down-regulated with time most probably mimicking interactions mediated by the ligand. On the other hand, new receptor molecules free of inhibitory mAb are expressed in vitro and in vivo and mediate SDF-1–dependent repopulation. These newly expressed functional receptors can also be efficiently blocked by coinjecting 10 μg neutralizing anti-CXCR4 mAb leading to significantly reduced engraftment levels (Figure 5B,P < .03).
Discussion
The study reported here shows that the mechanism whereby SDF-1/CXCR4 interactions regulate the homing and repopulation of human stem cells is a dynamic process. We demonstrate that despite the absence of CXCR4 on the cell surface, CD34+CXCR4− sorted cells express intracellular CXCR4 that can be induced to cell surface expression by stimulation with cytokines. Furthermore, we show that this cell surface CXCR4 expression mediates both homing and repopulation of NOD/SCID recipients by CD34+CXCR4− sorted cells. Our study depicts the important potential of CXCR4−/low cell transplants and the ability to manipulate them in vitro prior to transplantation, to improve their SDF-1–mediated migration and repopulation capacity.
The low repopulation capacity of CD34+CXCR4+sorted cells results from the neutralizing activity of anti-CXCR4 mAb that was used for sorting. This mAb (12G5) binds conformation-dependent epitopes34 comprised of the first and second extracellular loops of CXCR4, a site that also serves for SDF-1 binding and signaling.29,30 Thus, conjugated 12G5 mAb used to sort CD34+CXCR4+ cells also neutralizes receptors expressed on the cell surface, leading to reduced potential of positively labeled cells to respond to SDF-1. Homing of enriched human CB CD34+ cells was not impaired by either antihuman CD34 antibody or by in vivo infusion of isotype control antibody (data not shown). Nevertheless, an indirect effect of infused neutralizing anti-CXCR4 mAb has to be considered as well. Recently, Tanaka et al produced a new antihuman CXCR4 mAb, A80, which binds the third extracellular loop of CXCR4. A80 did not interfere with SDF-1 signaling, but triggered agglutination of T cells.35Therefore, the possibility of using nonneutralizing anti-CXCR4 mAb such as A80 to sort CXCR4 subsets of CD34+ cells without interfering with their SDF-1 signaling will be evaluated in future studies. As long as neutralizing mAbs are used, the repopulating potential of CD34+ subsets sorted on the basis of CXCR4 expression have to be compared with unmanipulated CD34+cells. In the present study 2 crucial control cell subsets were added: unmanipulated enriched CD34+ cells and CD34+cells migrating toward a relatively low concentration of SDF-1, that is, CXCR4+ cells that retain the potential to respond to murine SDF-1 in vivo. Both control populations demonstrated significantly high levels of engraftment as opposed to the low levels obtained with sorted R4+ and R4− cells. The impaired repopulation capacity of CD34+CD38−CXCR4+ and CD34+CD38−CXCR4− sorted cells was previously demonstrated indirectly, by transplanting extremely high cell doses needed to achieve adequate engraftment levels31; in contrast, 60-fold fewer CD34+CD38− sorted cells that were not blocked by 12G5 and expressed normal levels of CXCR4 with full potential to respond to SDF-1–mediated stimulation, were sufficient for achievement of similar engraftment levels.36
We demonstrate that freshly isolated R4− sorted cells already express low levels of intracellular CXCR4 and can express surface CXCR4 within a few hours in vitro. Importantly, following 24 to 48 hours of cytokine stimulation, both intracellular and cell surface CXCR4 expressed by R4− cells were functionally up-regulated correlating with both increased in vitro SDF-1 chemotaxis and in vivo repopulating potential. Interestingly, Rosu-Myles et al also documented up-regulation of CXCR4 on the cell surface of primitive Lin−CD34+CD38−CXCR4−sorted cells following 24 hours of cytokine stimulation. Moreover, these newly expressed receptors are functional because they could be partially down-regulated by adding high levels of SDF-1 (1 μg/mL) to the cytokine cocktail.31 A similar up-regulation process can also occur in vivo; while these cells are circulating in the murine blood, they may be exposed to cross-reactive murine and human autocrine secreted cytokines, and up-regulation of CXCR4 is apparently induced. This process facilitates low levels of homing and engraftment in an SDF-1–dependent manner. Alternative mechanisms involved in CXCR4 potentiation in vivo, such as the effect of accessory cells, could play an additional role.37 Of interest, platelet-derived microparticles that express CXCR4 and respond to SDF-1 signaling were reported to increase homing and repopulation by murine stem cells and to increase adhesion of immature human CD34+-enriched cells to SDF-1. This activity was mediated by binding progenitor cells via P-selectin and Mac-1 integrin antigens, and moreover by increasing their adhesion to SDF-1 expressed by the BM endothelium.38Platelet-derived microparticles induced also proliferation, chemotaxis, and SDF-1 signaling by human CD34+ cells.39Despite their ability to up-regulate intracellular and partially cell surface CXCR4 in vitro within 24 hours, the homing potential of unstimulated R4− sorted cells to the murine BM and spleen was inferior and slower compared to control CD34+-enriched cells. These results imply that there was only partial CXCR4 up-regulation and function in vivo. A careful examination of cell surface CXCR4 expression by BM-homed cells revealed a similar profile demonstrated by either total CD34+ or R4−-homed cells 16 and 29 hours after transplantation, respectively. Most of the cells do not express cell surface CXCR4 within the recipient BM, whereas low or medium levels of the receptor are detected on a minority of the cells. CXCR4 down-regulation and internalization by BM-homed cells are apparently induced by high levels of SDF-1 within the murine BM,27 which are most probably induced by total body irradiation of the host 24 hours before transplantation.10 Engraftment studies, including in vivo administration of 12G5 mAb at different time points after transplantation, also suggest that in vivo up-regulation of CXCR4 by circulating R4− sorted cells, is not significant within 10 hours after transplantation. Of interest, the clearance of human IgG1 antibodies from the blood circulation of NOD/SCID mice was shown to be relatively slow and their half-life is about 63 hours.40Although an IgG2a mAb was used here, a similar clearance rate can be assumed. In vitro manipulation by cytokine stimulation of R4− cells for 24 to 48 hours aimed to enhance surface CXCR4 expression, yields improved SDF-1–mediated migration and repopulation of the murine BM, 6 weeks after transplantation. Because SDF-1 levels within the BM of irradiated recipients are back to normal, repopulating human cells demonstrate normal distribution of CXCR4 expression. This chemokine is also a pre–B-cell growth factor, therefore in many immunodeficient mice that received transplants of human stem cells, elevated levels of CD19+ human B-lymphoid cells are documented in the murine BM.27 41
In contrast to R4− cells, we found that R4+sorted cells down-regulate cell surface receptor following 24 and moreover 48 hours in vitro in the presence of the 5-cytokine stimulation. An inverse correlation between total cell surface CXCR4 expression and the functionality of expressed receptors was found; whereas receptor expression was reduced following cytokine stimulation, SDF-1–dependent engraftment obtained by cultured cells was significantly increased. Inconsistencies relating CXCR4 expression and function were also reported by others. Shen et al found inverse correlation relating CXCR4 expression to SDF-1 response measured by calcium flux, transendothelial migration, and desensitization induced by SDF-1, therefore concluding no physiologic relevance of receptor expression levels.42 Voermans et al found a correlation between hematopoietic recovery after clinical autologous CD34+ cell transplantation and SDF-1 chemotaxis, but not with CXCR4 expression.43 A significantly shorter time frame needed for platelet production was associated with higher CXCR4 expression in allogeneic CD34+-enriched cell transplantation.44 CXCR4 undergoes slow constitutive internalization45 as well as down-regulation and internalization on binding to its ligand SDF-1. Furthermore internalized molecules can recycle and functionally be re-expressed on the cell surface.45-47
Our results show that in parallel to down-regulation of antibody bound surface CXCR4 by highly expressing R4+ cells in a process that mimics receptor-ligand interactions, newly expressed receptors were documented, which could compensate for antibody-bound internalized receptors by facilitating an increased repopulation potential. Different cytokine combinations might differently regulate the turnover of cell surface CXCR4. We found that SCF plus IL-6 better induce surface CXCR4 up-regulation.27 These studies support the notion of dynamic regulation of both CXCR4 expression and function, which are rapid, and moreover stress the important role of using biologic assays to assess cell function based on specific activities rather than surface markers that reveal only a frozen snapshot and can rapidly be changed with time. Other surface markers expressed on hematopoietic stem cells, such as CD34, also oscillate on both human and murine stem cells.48 49
Oscillated CXCR4 expression is also observed when stem and progenitor cells egress from the BM into the blood circulation. Recently others and we demonstrated that SDF-1/CXCR4 interactions are also implicated in both human and murine G-CSF–induced mobilization.50-53Interestingly, G-CSF administration, associated with SDF-1 decrease, induced a pattern of CXCR4 oscillation; a rapid reduction was followed by increased expression within 0.5 to 1 hour after each G-CSF injection,50 demonstrating that dynamic regulation of CXCR4 expression is involved in cell migration and localization in vivo. Proteolytic enzymes such as neutrophil elastase are actively involved in SDF-1 degradation and surface CXCR4 inactivation by cleavage of the signaling N-terminus of the receptor.50,54Matrix metalloproteinase–9 (MMP-9) secretion, induced by SDF-1 within the BM, regulates the shedding of c-kit ligand (SCF) from the BM during stem cell mobilization.55 Interestingly, mobilized peripheral blood CD34+ cells express reduced levels of surface c-kit, demonstrating dynamic regulation of this receptor on migrating stem and progenitor cells.56
The key role of CXCR4 signaling in stem cell activity was demonstrated by documenting in vitro and in vivo effects of SDF-1 on repopulating cells. SDF-1 is a survival factor for both human and mouse stem cells.57,58 It was previously shown that the majority of human CD34+-enriched cells, the more mature CD34+CD38+ cells, which include CXCR4+ cells, also secrete low levels of SDF-159,60 and therefore are less sensitive or dependent on exogenous SDF-1 compared with the more primitive CD34+CD38− cells that demonstrate higher CXCR4 expression and SDF-1 responsiveness.27,61 Cashman and colleagues62,63 showed that 2 daily injections of a high dose (10 μg) of SDF-1 blocked the cycling of long-term culture initiating cells (LTC-ICs) and primitive human repopulating cells in engrafted NOD/SCID mice, leading to higher levels of human engraftment in immune-deficient mice that underwent secondary transplantations. In addition, 2 days of in vitro stimulation with a low dose of this chemokine (100 ng/mL), significantly increased engraftment of NOD/SCID mice by human CB Lin−CD34+ cultured cells, demonstrating the crucial role of SDF-1 signaling via functional CXCR4 for efficient repopulation and that both primary and secondary human NOD/SCID repopulating cells have active CXCR4.62,64 We documented CXCR4 up-regulation by R4− cells cultured in serum-free media without cytokine supplement, suggesting that immature human CD34+ cells and progenitor colony-forming cells, which secrete many cytokines, chemokines, and growth factors,65 can also regulate CXCR4 expression and their responsiveness to SDF-1 by an autocrine manner. However, human cytokine deprivation also caused enhanced cell death.
In summary, our data provide further evidence for the key role of CXCR4 regulation and cell surface expression in motility and tissue localization of human stem and progenitor cells in a preclinical, small animal model. CXCR4 expression is a dynamic process, which is regulated by environmental factors such as cytokines, chemokines, stromal cells, and adhesion molecules. Our findings that CXCR4 oscillation has biologic roles in regulating human stem and progenitor cell migration, homing, and repopulation, suggest in vitro stimulation of human progenitors prior to clinical stem cell transplantation to improve human SDF-1–dependent stem cell homing and repopulation.
Special thanks to Drs John Dick and Dov Zipori for fruitful discussions and for critically reviewing this manuscript.
Prepublished online as Blood First Edition Paper, June 21, 2002; DOI 10.1182/blood-2002-02-0564.
Supported in part by grants from the Israel Academy of Science, The Ares Serono group, and MINERVA Foundation. T.L. is Incumbent of the Pauline Recanati Career Development Chair of Immunology.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Tsvee Lapidot, Department of Immunology, The Weizmann Institute of Science, Rehovot 76100, Israel; e-mail:tsvee.lapidot@weizmann.ac.il.