Acute promyelocytic leukemia (APL) is associated with chromosomal translocations resulting in fusion proteins of the retinoic acid receptor (RAR). Here, we report a novel murine model system for APL, based on the transduction of purified murine hematopoietic progenitors (lin−) using high-titer retroviral vectors encoding promyelocytic leukemia–RAR (PML-RAR), and the green fluorescent protein (GFP) as a marker. PML-RAR–expressing lin− cells were impaired in their ability to undergo terminal myeloid differentiation and showed increased proliferative potential in vitro. Inoculation of transduced lin− cells into syngeneic, irradiated mice resulted in the development of retinoic acid-sensitive promyelocytic leukemias at high frequency (> 80%) and short latency (approximately 4 months). Morphologic and immunophenotypic analysis revealed no gross abnormalities of the preleukemic bone marrows. However, hematopoietic progenitors from PML-RAR preleukemic mice showed a severe impairment in their ability to undergo myeloid differentiation in vitro. This result, together with the monoclonality or oligoclonality of the leukemic blasts, supports a “multiple-hit” model, where the fusion protein causes a “preleukemic” phase, and leukemia occurs after additional genetic lesions. This model system faithfully reproduces the main characteristics of human APL and represents a versatile tool for the in vitro and in vivo study of mechanisms of leukemogenesis and the design of protocols for differentiation treatment.
Introduction
Expression of the promyelocytic leukemia–retinoic acid receptor (PML-RAR) fusion protein—originated by the t(15;17) chromosomal translocation—is associated with more than 95% of the cases of acute promyelocytic leukemia (APL).1 APL blasts are sensitive to the differentiation action of pharmacologic concentrations of retinoic acid (RA), that, in association with standard chemotherapy, has become the elective treatment for these patients, leading to long-term remissions.2 3
Studies conducted in cell lines showed that PML-RAR is able to block differentiation on several differentiating stimuli and that this effect is reverted by RA treatment.4 These results suggested that PML-RAR is responsible for both the pathogenesis of the disease and for its RA sensitivity. In vivo studies using transgenic chicken and mice confirmed this hypothesis.5-13 Expression of PML-RAR in mice under different myeloid-specific promoters gave 2 kinds of results: (1) in the case of promoters for genes expressed during early myeloid-promyelocytic stages of differentiation, such as cathepsin-G and MRP8, PML-RAR induced slight alterations in myelopoiesis (“preleukemic syndrome”) in 100% of mice, and an APL-like leukemia at low penetrance (15%-20%) and long latency (6-18 months)6,8-10; (2) in the case of promoters for late stages of myeloid differentiation, such as CD11b, expression of PML-RAR provoked a modest impairment in hematopoiesis, without induction of an overt leukemic syndrome.7 These results underscore the relevance of the proper targeting of PML-RAR expression and suggest a “2-hit” model, where expression of the fusion protein is not sufficient for leukemogenesis, but creates a cellular environment favorable for the accumulation of additional genetic lesions.12 13
We have developed an alternative strategy to express PML-RAR in the hematopoietic compartment, by retroviral-based transduction of murine hematopoietic progenitors and subsequent reinoculation of syngeneic mice. Our results confirm and extend the observations obtained by the conventional transgenic models, and offer a new, more versatile model system to study APL pathogenesis.
Materials and methods
Plasmids
Purification of lin− cells
Lin− cells were purified from the bone marrow of 8- to 10-week-old 129SvEv mice. After centrifugation through a Ficoll gradient, mononucleate cells were enriched for progenitors by depletion of cells presenting myeloid, erythroid, and lymphoid differentiation markers using commercially available reagents (Stem Cell Technologies, Vancouver, BC, Canada).
Transduction and sorting of GFP+ cells
Lin− cells were grown for 36 hours in medium containing interleukin 3 (IL-3), IL-6, and stem cell factor (SCF). The cells were then plated onto retronectin-coated (Takara-Shuzo, Shiga, Japan), non–tissue culture–treated plates and exposed to the supernatant of packaging, ecotropic Phoenix cells transiently transfected with the indicated retroviral vectors. Transduced cells were sorted using a Becton Dickinson (Franklin Lakes, NJ) FACS Vantage instrument. Purity of sorted cells was more than 98% in all experiments.
Differentiation and survival assays
To analyze differentiation in vitro, sorted cells or uninfected control lin− cells were plated (5000 cells/plate) in methylcellulose medium containing fetal bovine serum and the following cytokines: IL-3 (20 ng/mL), IL-6 (20 ng/mL), SCF (100 ng/mL), granulocyte colony-stimulating factor (G-CSF; 60 ng/mL), and granulocyte-macrophage colony-stimulating factor (GM-CSF; 20 ng/mL). Seven to 10 days after plating, cells were harvested and characterized by analysis of surface myeloid markers (MAC1 and GR1). For the in vivo studies, cells from peripheral blood, bone marrow, or spleen of healthy and leukemic mice were analyzed for the presence of surface progenitor markers (C-KIT, CD34, and SCA1) and myeloid markers (GR1 and MAC1). Murine antibodies for C-KIT, CD34, SCA1, GR1, MAC1, and their matched isotype controls were purchased from Pharmingen (San Diego, CA) and used according to the manufacturer's instruction. Cells were analyzed by a Becton Dickinson FACScan, using Cell Quest software.
In survival assays in vitro, cells were plated in methylcellulose medium as described for the differentiation assays. After 10 days, pooled colonies were reseeded (10 000 cells/plate) in semisolid medium. The assay was repeated until no colonies developed in control plates (usually, 2-3 platings), due to terminal differentiation of the cells.16
Repopulation of lethally irradiated recipients
129SvEv wild-type mice were lethally irradiated (9 Gy) and reinjected intravenously with 300 000 sorted cells plus 500 000 spleen cells (obtained from an untreated mouse); after 7 days, mice were reinjected intravenously with 500 000 spleen cells. Mice reinjected with spleen cells only survived slightly longer than untreated, irradiated mice (approximately 4 weeks versus 1-2 weeks), but eventually they all died due to impaired hematopoiesis.
The animals were checked periodically for clinical signs of disease and for presence of blast cells by May-Grünwald-Giemsa staining of blood smears.
Morphologic analysis
Mice were humanely killed by CO2 inhalation and underwent necropsy. Main organs were fixed in 10% buffered formalin and processed for histopathologic examination. Samples were embedded in paraffin, sectioned at 4 μm and stained with hematoxylin and eosin. Blood smears, bone marrow smears, and spleen cell imprints were stained with May-Grünwald-Giemsa and Sudan black B.
DNA, RNA, and protein analysis
DNA polymerase chain reaction (PCR) and reverse transcription–PCR (RT-PCR) from colonies derived from methylcellulose plating, or from leukemic animals, were performed according to standard techniques, using primers specific for the human PML-RAR cDNA.17 As a routine control for DNA quality of all of our genomic samples, we have used primers to amplify a genomic subregion of the murine p21 gene. In all of the samples shown in the figures, that test result was positive. For Southern blot analysis, genomic DNAs from spleen, liver, or bone marrow of leukemic mice (primary or secondary recipients) were digested withHindIII, run on an agarose gel, and then blotted to nylon membranes (Amersham, Piscataway, NJ). The filters were hybridized using an HindIII-EcoRI 3-kb fragment containing the entire PML-RAR cDNA. Eight primary leukemic samples and 12 secondary/tertiary leukemic samples were analyzed in this study; 4 representative primary cases/2 secondary cases are shown in Figure 6. For protein analysis, immunoblotting and immunofluorescence experiments were performed using antibodies specific for the human and mouse PML.18 Immunoprecipitations were performed using antibodies against human PML, followed by analysis of the immunoprecipitates by immunoblotting using anti-RAR antibodies.
FISH analysis
RA treatment of secondary recipients
Mice with overt leukemias were humanely killed, and leukemic cells were harvested from the spleen. Leukemic cells were reinjected intravenously (1 × 107 cells/mouse) in nonirradiated, syngeneic recipient mice. When the secondary recipient mice developed overt leukemia (in about 2-3 weeks), a 21-day release pellet containing 5 mg RA or placebo (Innovative Research of America [IRA], Sarasota, FL) was implanted subcutaneously. Absence/presence of blast cells, differentiation, and recovery were evaluated on peripheral blood smears stained with May-Grünwald-Giemsa or Sudan black B.
All procedures involving animals were done in accordance with national and international laws and policies.
Results
Ectopic expression of PML-RAR blocks myeloid differentiation and increases proliferative potential of lin− cells
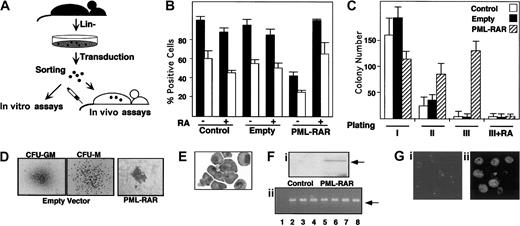
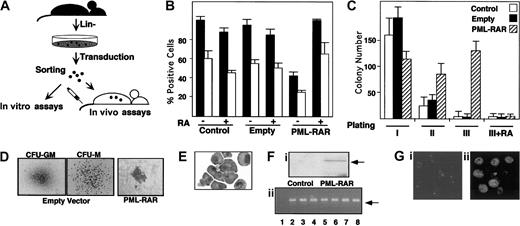
Our experimental strategy is based on the following steps: (1) enrichment of murine bone marrow hematopoietic progenitors by depletion of cells expressing myeloid, lymphoid, and erythroid differentiation markers (lin− cells); (2) infection of lin−cells with high-titer retroviruses expressing PML-RAR, or wild-type RAR, from the 5′ viral long terminal repeat (LTR), and GFP from an internal promoter (cytomegalovirus [CMV]); (3) separation of GFP+ cells by fluorescence-activated cell sorter (FACS); and (4) use of GFP+ cells for in vitro differentiation assays (colony formation in methylcellulose) or in vivo reconstitution of lethally irradiated, syngeneic mice (Figure1A). Western blot analysis showed expression of PML-RAR in the sorted lin− cell population (Figure 1F). Anti-PML immunofluorescence analysis of GFP+cells showed the typical punctuated or microspeckled patterns in cells infected with the empty or PML-RAR retroviruses, respectively (Figure 1G).
PML-RAR expression causes RA-sensitive differentiation block and enhanced survival in vitro.
(A) Scheme of the experimental strategy. (B) Uninfected (Control) or sorted cells infected with the empty vector (Empty), or PML-RAR–expressing cells were plated in methylcellulose medium containing IL-3, IL-6, SCF, G-CSF, and GM-CSF, in the presence or in the absence of RA (1 μM). Colonies were pooled and analyzed for the presence of the myeloid differentiation markers MAC1 (▪) and GR1 (■). (C) Control, empty, or PML-RAR cells were plated as described. Colonies were pooled and 10 000 cells were reseeded in methylcellulose plates. This procedure was repeated twice, and in the third plating RA was also included in the medium. (D) Typical morphology of the colonies observed in control (× 50), or PML-RAR expressing cells (× 100). The “clusters” of PML-RAR–expressing cells are particularly evident from the second plating. Control colonies derive from cells infected with the empty vector. Identical results were obtained from uninfected cells. (E) Wright-Giemsa staining of PML-RAR pooled colonies (third plating), after cytospins. (Fi) Western blot analysis using an anti-RAR antibody of sorted cells from control and PML-RAR infections. The arrow indicates the PML-RAR protein. (Fii) DNA-PCR analysis of several individual colonies (lanes 3-8) obtained from growing PML-RAR expressing cells in semisolid medium. Lane 1: negative control, from one control colony; lane 2: positive control, from plasmid DNA. The arrow indicates the specific, amplified PCR product. (Gi) PML expression pattern in lin− cells by immunofluorescence using anti-mPML antibodies (× 125). (Gii) PML-RAR expression pattern on pooled colonies by immunofluorescence using anti-hPML antibodies (× 125). Double immunofluorescence with anti-mPML antibodies showed delocalization of the normal PML signal from “nuclear bodies” to microspeckles colocalizing with hPML-RAR (data not shown).
PML-RAR expression causes RA-sensitive differentiation block and enhanced survival in vitro.
(A) Scheme of the experimental strategy. (B) Uninfected (Control) or sorted cells infected with the empty vector (Empty), or PML-RAR–expressing cells were plated in methylcellulose medium containing IL-3, IL-6, SCF, G-CSF, and GM-CSF, in the presence or in the absence of RA (1 μM). Colonies were pooled and analyzed for the presence of the myeloid differentiation markers MAC1 (▪) and GR1 (■). (C) Control, empty, or PML-RAR cells were plated as described. Colonies were pooled and 10 000 cells were reseeded in methylcellulose plates. This procedure was repeated twice, and in the third plating RA was also included in the medium. (D) Typical morphology of the colonies observed in control (× 50), or PML-RAR expressing cells (× 100). The “clusters” of PML-RAR–expressing cells are particularly evident from the second plating. Control colonies derive from cells infected with the empty vector. Identical results were obtained from uninfected cells. (E) Wright-Giemsa staining of PML-RAR pooled colonies (third plating), after cytospins. (Fi) Western blot analysis using an anti-RAR antibody of sorted cells from control and PML-RAR infections. The arrow indicates the PML-RAR protein. (Fii) DNA-PCR analysis of several individual colonies (lanes 3-8) obtained from growing PML-RAR expressing cells in semisolid medium. Lane 1: negative control, from one control colony; lane 2: positive control, from plasmid DNA. The arrow indicates the specific, amplified PCR product. (Gi) PML expression pattern in lin− cells by immunofluorescence using anti-mPML antibodies (× 125). (Gii) PML-RAR expression pattern on pooled colonies by immunofluorescence using anti-hPML antibodies (× 125). Double immunofluorescence with anti-mPML antibodies showed delocalization of the normal PML signal from “nuclear bodies” to microspeckles colocalizing with hPML-RAR (data not shown).
Uninfected or GFP+ lin− cells were plated in methylcellulose in the presence of cytokines. Colonies were pooled and analyzed for the expression of the myeloid differentiation markers MAC1 and GR1. PML-RAR–expressing cells were impaired in their capacity to differentiate, unless pharmacologic concentrations of RA were added to the medium (Figure 1B). As previously reported, lower doses of RA had no or marginal effect15,16 (data not shown). Evaluation of proliferative potential by serial replating of methylcellulose colonies revealed the ability of PML-RAR–expressing cells to re-form colonies, in contrast to control or RA-treated PML-RAR cells, which lost the capacity to grow in methylcellulose after a few platings, due to terminal differentiation (Figure 1C). Morphologically, PML-RAR colonies consisted of small clusters (Figure 1D) of immature, promyelocytelike cells (Figure 1E). During these experiments, we noticed that GFP expression decreased dramatically in PML-RAR, but not in control, cells. This might be due to silencing of the CMV promoter by the fusion protein, because this promoter contains binding sites for PML-RAR21; we are currently evaluating the molecular mechanisms responsible for this phenomenon. We picked 20 individual colonies after methylcellulose plating and confirmed by DNA and RNA analysis that all of the colonies from PML-RAR–derived plates originated from cells infected with the PML-RAR retroviral construct (Figure 1F shows representative DNA-PCR data from 6 colonies).
The capacity of PML-RAR to block myeloid differentiation (at the promyelocytic stage), and to prolong cell survival in a RA-reversible fashion, indicates that our model system recapitulates the biologic properties of the human disease in vitro, thus prompting us to examine the effect of fusion protein expression in vivo.
PML-RAR–expressing cells cause an APL-like disease in mice
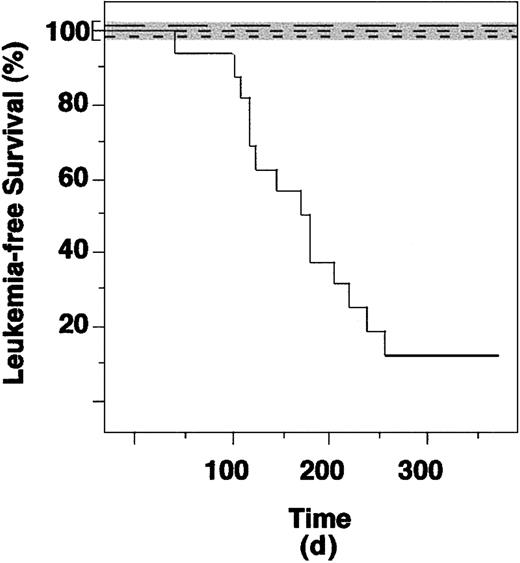
Immediately after sorting, GFP+ cells from control, PML-RAR, or RAR-transduced cells were reinoculated into lethally irradiated recipient mice. More than 95% of the mice reconstituted with control, GFP-expressing cells survived for more than 6 months, whereas the nonreinoculated mice died within 1 to 2 weeks after irradiation (Table 1 and Figure2).
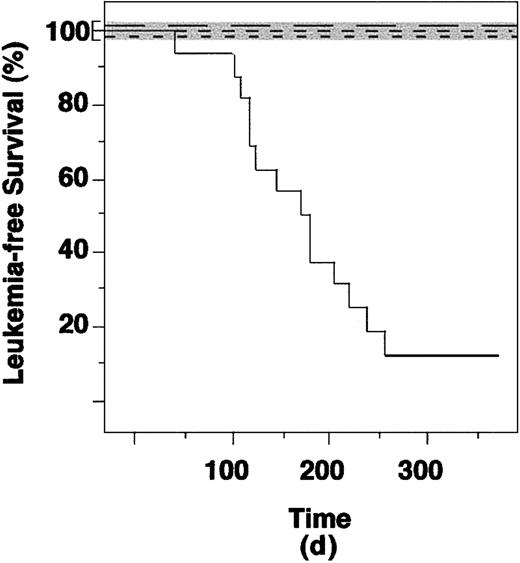
PML-RAR causes leukemias in mice.
Leukemia-free survival curves of mice reinoculated with lin− cells from control (not infected or infected with the empty vector), RAR, and PML-RAR–expressing cells. Continuous line indicates PML-RAR mice; long dashes, uninfected; medium dashes, empty vector; short dashes, RAR. The control curves overlap, because none of the recipient mice developed leukemias. To allow visualization of the 3 curves we have expanded the area in the graph corresponding to 100% leukemia-free survival (shaded area).
PML-RAR causes leukemias in mice.
Leukemia-free survival curves of mice reinoculated with lin− cells from control (not infected or infected with the empty vector), RAR, and PML-RAR–expressing cells. Continuous line indicates PML-RAR mice; long dashes, uninfected; medium dashes, empty vector; short dashes, RAR. The control curves overlap, because none of the recipient mice developed leukemias. To allow visualization of the 3 curves we have expanded the area in the graph corresponding to 100% leukemia-free survival (shaded area).
Mice reconstituted with lin− transduced with the empty vector showed a variable (from 10 to > 80%) degree of GFP+ cells in the peripheral blood or bone marrow (not shown). We did not observe differences in the expression of myeloid, lymphoid, and erythroid differentiation markers in the GFP+and GFP− cells, demonstrating a successful engraftment of GFP-transduced stem cells, and the lack of effects from GFP expression in the target cells (Figure 7A and data not shown). In some cases, mice that received transplantations were killed and their bone marrow used for reinoculation of secondary recipient mice; GFP-expressing cells were detected for up to 18 months from the original transduction experiment (data not shown). We did not observe development of leukemias (or lymphoid tumors) in any of the mice reinoculated with lin− transduced with the empty vector (0 of 25 mice, Table 1). Similar results were obtained with RAR-transduced lin− cells, showing that RAR overexpression per se is not leukemogenic (0 of 7 mice, Table 1). Strikingly, 13 (81.25%) of 16 mice reinoculated with PML-RAR–expressing cells developed an APL-like disease. The minimum and maximum latency times were 42 and 253 days, respectively, with a median of approximately 140 days (Table 1). The calculated, 2-sided P values (Fisher exact test) were in all cases extremely significant (P < .0001 for PML-RAR versus control or empty vector; P < .0005 for PML-RAR versus RAR; refer to Table 1 for further details).
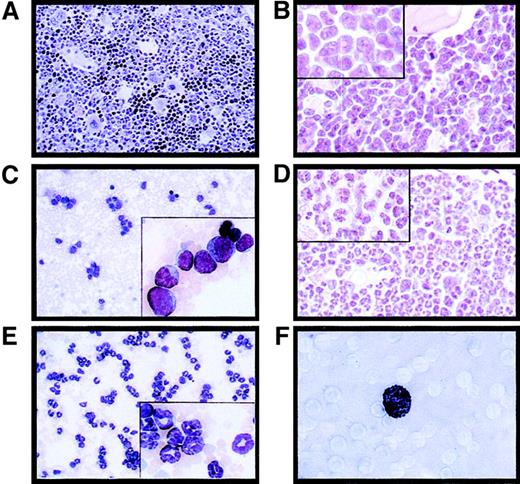
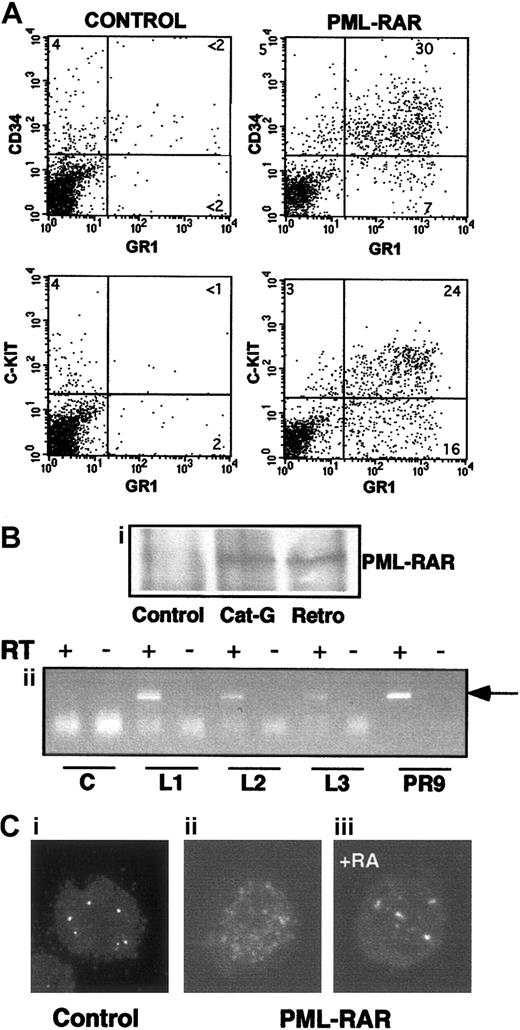
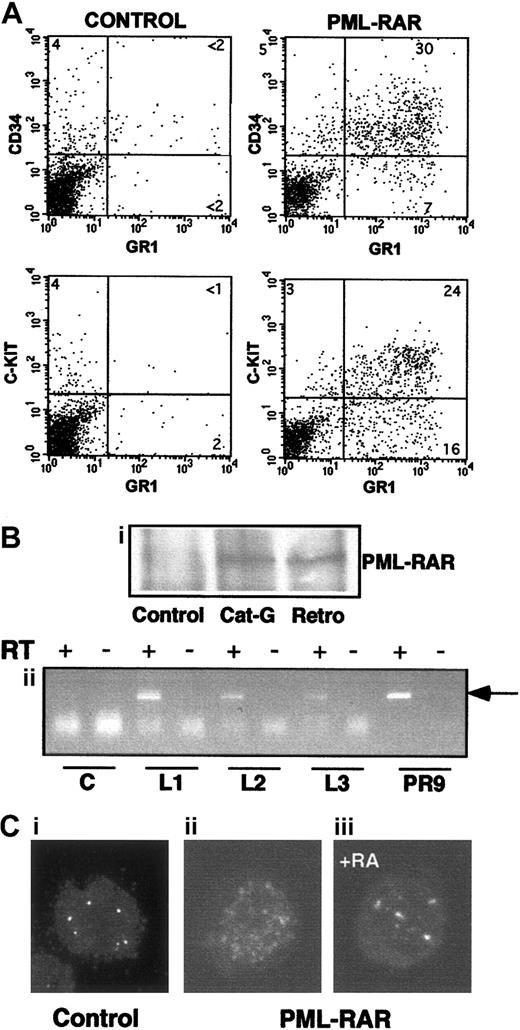
Massive splenomegaly and hepatomegaly were present in all PML-RAR mice, with frequent lymphoadenomegaly (Figure 5A and data not shown). Histopathologic examination showed leukemic cells diffusely invading the bone marrow (Figure 3B), the splenic red pulp, and the liver (not shown and Figure 5C). Moderate to severe infiltration of leukemic cells was also frequently detectable in the lymph nodes, lungs, meninges, and less frequently in the kidneys. Cytologically, the disease was characterized by extensive prevalence of promyelocytes, and, to a lesser degree, metamyelocytes (Figures 3C and5E). Promyelocytes were intensely positive for Sudan black B (Figure3F), had fewer intracytoplasmic granules, and did not show Auer rods, as compared to the human APL cells, thereby resembling the hypogranular variant of human APL.22 Analysis of differentiation surface markers revealed, unlike normal cells, simultaneous expression of the myeloid lineage markers GR1 and MAC1, and of the progenitor markers CD34 and C-KIT, but not of SCA1 (Figure4A, and data not shown). Notably, CD34+ human APL cells are also more frequently associated with the hypogranular variant of disease.23 In all the leukemic mice analyzed, the leukemic cells expressed PML-RAR, as shown by immunoprecipitation, RNA, and immunofluorescence analyses (Figure 4B-C). In contrast, leukemic cells were uniformly negative for GFP, reproducing in vivo the phenotype observed in vitro (see Figure 7).
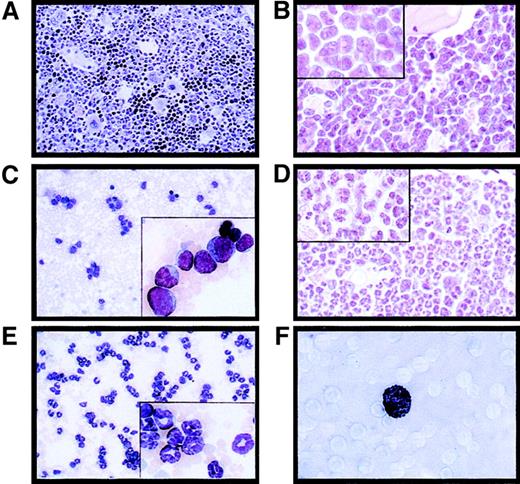
PML-RAR induces an APL-like phenotype, and RA leads to terminal differentiation.
Histologic analysis of the bone marrow from an untreated control mouse (A, original magnification × 400), a leukemic mouse reconstituted with PML-RAR expressing cells (B, original magnification × 600, inset × 1000), and a leukemic mouse after 7 days of RA treatment (D, original magnification × 600, inset × 1000). May-Grünwald-Giemsa staining of peripheral blood smears from a PML-RAR mouse at the leukemic stage (C, original magnification × 400), and after 5 days of RA treatment (E); in the insets, a larger magnification of the same figures (original magnification × 1000). (F) Sudan black staining of APL blasts from a PML-RAR leukemic mouse (original magnification × 1000).
PML-RAR induces an APL-like phenotype, and RA leads to terminal differentiation.
Histologic analysis of the bone marrow from an untreated control mouse (A, original magnification × 400), a leukemic mouse reconstituted with PML-RAR expressing cells (B, original magnification × 600, inset × 1000), and a leukemic mouse after 7 days of RA treatment (D, original magnification × 600, inset × 1000). May-Grünwald-Giemsa staining of peripheral blood smears from a PML-RAR mouse at the leukemic stage (C, original magnification × 400), and after 5 days of RA treatment (E); in the insets, a larger magnification of the same figures (original magnification × 1000). (F) Sudan black staining of APL blasts from a PML-RAR leukemic mouse (original magnification × 1000).
Characteristics of APL blasts.
(A) Mice showing overt signs of disease were killed, and spleen cells were analyzed for expression of several surface markers. Spleen cells from untreated mice are shown for comparison in the left panels. PML-RAR blasts are CD34+/C-KIT+/MAC1+/GR1+. (B,C) In panel B, expression of PML-RAR in APL blasts was confirmed (Bi) by analysis of PML-RAR expression by immunoprecipitation with an anti-PML antibody, followed by Western blot using anti-RAR antibodies. Spleen cell extracts were prepared from control and leukemic animals obtained as outlined in Figure 1, or leukemic animals derived from cathepsin G-PML-RAR transgenic mice.8 Panel Bii shows RT-PCR analysis of RNA obtained from the bone marrow of 3 independently derived leukemic mice (L1-L3). Reverse transcriptase was omitted in the samples marked with the minus sign as control. Negative control (lanes “C”) is from a control mouse, positive control (lanes “PR9”) derives from U937 cells stably transfected with PML-RAR; the arrow indicates the specific, amplified PCR product. (C) Immunofluorescence of bone marrow cells from a leukemic mouse prior to (Cii), or after 5 days of RA treatment (Ciii), using antibodies against mouse PML (original magnification × 630). Control, normal bone marrow cells (Ci).
Characteristics of APL blasts.
(A) Mice showing overt signs of disease were killed, and spleen cells were analyzed for expression of several surface markers. Spleen cells from untreated mice are shown for comparison in the left panels. PML-RAR blasts are CD34+/C-KIT+/MAC1+/GR1+. (B,C) In panel B, expression of PML-RAR in APL blasts was confirmed (Bi) by analysis of PML-RAR expression by immunoprecipitation with an anti-PML antibody, followed by Western blot using anti-RAR antibodies. Spleen cell extracts were prepared from control and leukemic animals obtained as outlined in Figure 1, or leukemic animals derived from cathepsin G-PML-RAR transgenic mice.8 Panel Bii shows RT-PCR analysis of RNA obtained from the bone marrow of 3 independently derived leukemic mice (L1-L3). Reverse transcriptase was omitted in the samples marked with the minus sign as control. Negative control (lanes “C”) is from a control mouse, positive control (lanes “PR9”) derives from U937 cells stably transfected with PML-RAR; the arrow indicates the specific, amplified PCR product. (C) Immunofluorescence of bone marrow cells from a leukemic mouse prior to (Cii), or after 5 days of RA treatment (Ciii), using antibodies against mouse PML (original magnification × 630). Control, normal bone marrow cells (Ci).
A hallmark of APL is the dramatic differentiation response observed on treatment with pharmacologic doses of RA.2 3 To examine the RA response of leukemic mice in vivo, we obtained secondary leukemias by reinoculating spleen cell suspensions of leukemic mice (with 30%-50% infiltration of leukemic cells) into syngeneic, nonirradiated recipients.
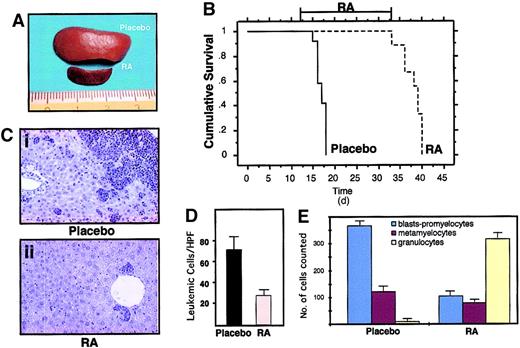
The reinoculated mice developed (100% cases) secondary leukemias with minimal latency (2 weeks), with characteristics identical to those observed in the primary recipient mice (including transplantability to further recipients; data not shown). Because the leukemic phenotype was observed synchronously in the secondary recipients, we could treat a cohort of mice that were at the same stage of disease, and carrying the same leukemic clone(s). RA was administered by subcutaneous implantation of a 21-day release pellet containing 5 mg RA or placebo. Mice were killed at time intervals to monitor the response in the various tissues or maintained until death to monitor survival. Starting from the third day of therapy, cells within the leukemic population revealed clear signs of granulocytic differentiation, which was extensive by the seventh day of treatment (Figures 3D-E and 5E). Immunofluorescence studies revealed that RA, as previously shown, reverted almost completely the delocalized pattern of mouse PML to the normal “nuclear bodies” appearance (Figure 4C: from 30% of cells showing a microspeckled pattern in spleen cells, to < 2% on RA treatment). The leukemic infiltrates in the various organs appeared reduced, as shown by their parallel reduction to normal size (Figure 5A,C,D). All of the mice implanted with a subcutaneous placebo died by progressive multifocal invasion of parenchymal tissues by leukemic blasts, whereas RA treatment significantly prolonged survival (P < .0001), with no apparent signs of disease during treatment, in more than 90% of mice (Figure 5B). Identical results were obtained in 5 independent experiments, using as donors 3 mice that developed APL in 3 independent series of experiments (data not shown).
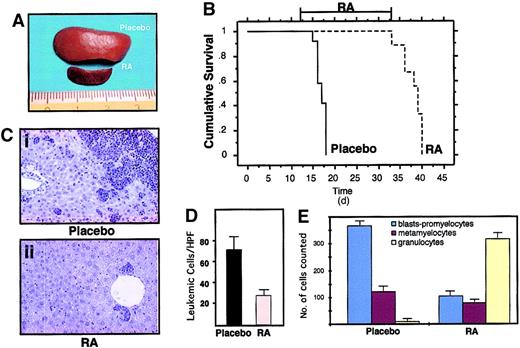
RA treatment induces differentiation of leukemic cells and prolongs survival of leukemic mice.
(A) Macroscopic appearance of spleens from 2 mice treated in the same experimental cohort with placebo (upper) or RA for 7 days (lower). (B) Survival curves of mice inoculated with spleen cells from one leukemic mouse (L3). RA treatment was started at day 12 after inoculation, when secondary leukemias were apparent in recipient mice (from analysis of peripheral blood smears). The RA pellet subcutaneously implanted has a duration of approximately 3 weeks. (C) Histologic analysis of leukemic infiltrates in the liver of one leukemic mouse (Ci), and of one leukemic mouse after 7 days of RA treatment (Cii). Original magnification × 400. (D) Extent of leukemic infiltration within the liver of mice treated with placebo (n = 4) or RA (n = 4). For each mouse examined the leukemic cells infiltrating the liver were counted in 40 high-power fields (HPFs; magnification × 630); for each animal the mean number of leukemic cells per HPF was calculated. The columns represent the mean of the values. (E) Differential cell counts in the bone marrow of mice treated with placebo (n = 4) or RA (n = 4). For each examined mouse, 500 myeloid cells were classified according to morphologic features.
RA treatment induces differentiation of leukemic cells and prolongs survival of leukemic mice.
(A) Macroscopic appearance of spleens from 2 mice treated in the same experimental cohort with placebo (upper) or RA for 7 days (lower). (B) Survival curves of mice inoculated with spleen cells from one leukemic mouse (L3). RA treatment was started at day 12 after inoculation, when secondary leukemias were apparent in recipient mice (from analysis of peripheral blood smears). The RA pellet subcutaneously implanted has a duration of approximately 3 weeks. (C) Histologic analysis of leukemic infiltrates in the liver of one leukemic mouse (Ci), and of one leukemic mouse after 7 days of RA treatment (Cii). Original magnification × 400. (D) Extent of leukemic infiltration within the liver of mice treated with placebo (n = 4) or RA (n = 4). For each mouse examined the leukemic cells infiltrating the liver were counted in 40 high-power fields (HPFs; magnification × 630); for each animal the mean number of leukemic cells per HPF was calculated. The columns represent the mean of the values. (E) Differential cell counts in the bone marrow of mice treated with placebo (n = 4) or RA (n = 4). For each examined mouse, 500 myeloid cells were classified according to morphologic features.
In human APL, treatment with RA alone results in disease remission and subsequent relapse in virtually all patients.2 3 Likewise, suspension of RA treatment in the leukemic mice resulted in relapse in 100% of mice (Figure 5B). Taken together, these results show that our mouse model faithfully reproduced the main biologic and clinical features of human APL.
APL in mice is a monoclonal or oligoclonal disease, which follows a preleukemic state
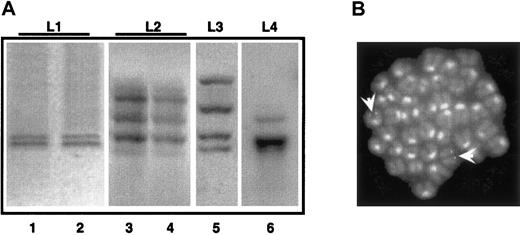
To evaluate the polyclonal or clonal origin of the mouse APL leukemias, we performed Southern blotting analysis of integrated proviruses. A polyclonal origin is consistent with the capacity of PML-RAR to directly transform target cells, whereas a monoclonal or oligoclonal origin suggests a 2-hit model of leukemogenesis, that is, the requirement for additional genetic lesions.6 13 The genomic DNAs from spleen/bone marrow of 4 primary and 2 secondary leukemic mice were digested with the HindIII restriction enzyme (which cuts once in the integrated retroviral sequence, allowing recognition of individual integration events) and hybridized with a probe representative of the human PML-RAR cDNA.
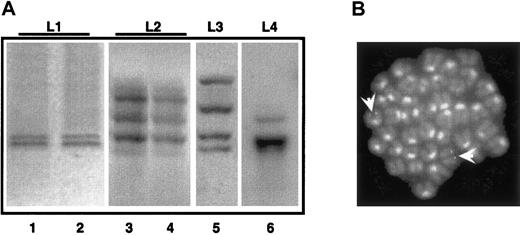
As observed in Figure 6A, all the primary leukemias showed a discrete number of hybridizing DNA fragments (2-4 in the cases shown). Primary and secondary leukemia samples revealed the same patterns of multiple hybridizing fragments (compare lanes 1 and 2, 3 and 4 in Figure 6A). The presence of multiple hybridizing fragments can be the consequence of a single, yet complex, integration site, or of several integration sites in the leukemia initiating cell. To clarify this issue, we performed FISH analysis of leukemic metaphases using a probe encompassing the entire integrated sequence. Strikingly, in the case where 2 hybridizing fragments were observed, we detected 2 hybridizing regions, confirming the monoclonality of the leukemia, and the presence of 2 integration events (Figure 6B). Overall, these results support the monoclonal or oligoclonal origin of the leukemic blasts and the hypothesis that leukemia arose from one PML-RAR expressing cell undergoing additional genetic lesions.
APL is a monoclonal or oligoclonal disease.
Genomic DNA was prepared from leukemic mice (L1-L4, lanes 1, 3, 5, and 6) or from mice with secondary leukemias derived from reinjection of spleen cells from L1-L2 mice (lanes 2, 4). Samples were digested withHindIII, and then analyzed by Southern blot assay using the PML-RAR cDNA as a probe. (B) FISH analysis (using the proviral sequence as a probe) of metaphase-enriched nuclei of cells derived from the case “L1.” Arrowheads highlight the hybridization signals. Original magnification × 630.
APL is a monoclonal or oligoclonal disease.
Genomic DNA was prepared from leukemic mice (L1-L4, lanes 1, 3, 5, and 6) or from mice with secondary leukemias derived from reinjection of spleen cells from L1-L2 mice (lanes 2, 4). Samples were digested withHindIII, and then analyzed by Southern blot assay using the PML-RAR cDNA as a probe. (B) FISH analysis (using the proviral sequence as a probe) of metaphase-enriched nuclei of cells derived from the case “L1.” Arrowheads highlight the hybridization signals. Original magnification × 630.
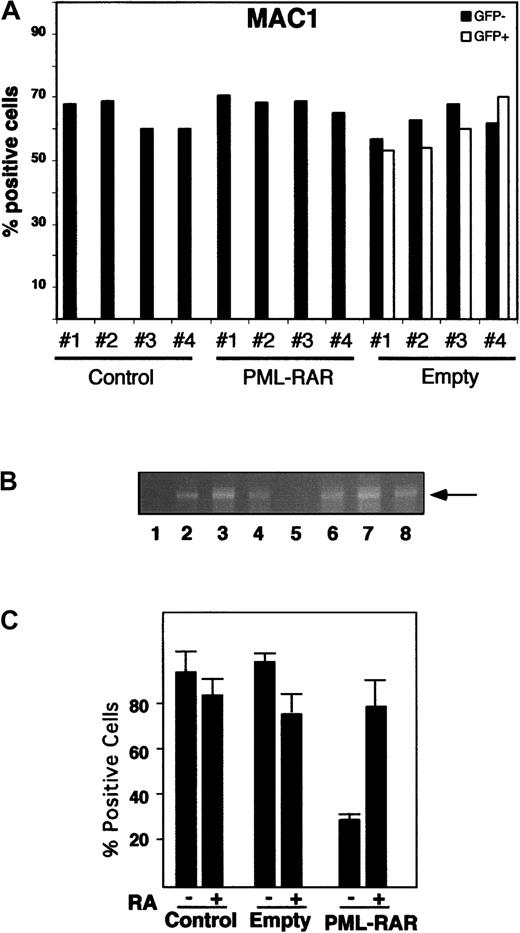
We next investigated whether expression of PML-RAR is sufficient to induce a detectable phenotype, prior to the onset of an overt leukemia. In the transgenic mice carrying PML-RAR under the MRP-8 or cathepsin G promoters, a mild defect in myeloid maturation was consistently seen in 100% of the mice, defined as a “preleukemic” state and characterized by a slight decrease of MAC1 or GR1 expression in the whole bone marrow.6 13 We did not, however, detect a similar phenotype in our PML-RAR mice, as shown by the immunophenotypic analysis of bone marrow cells at 30, 60, and 90 days after reinoculation (Figure 7A and data not shown). Because we could not follow directly the fate of PML-RAR–expressing cells, due to loss of GFP expression, we performed DNA-PCR analysis on several colonies derived from methylcellulose platings of lin− cells from the reinoculated mice to demonstrate that the preleukemic bone marrow cells expressed the fusion protein. Analysis of differentiation markers was then performed in those mice where more than 80% of colonies were positive for PML-RAR cDNA. We conclude, therefore, that the lack of a “preleukemic” condition is not due to the low number of transduced cells repopulating the recipient mouse (Figure 7A-B). Slight variations in the expression of differentiation markers might be, however, masked, in our case, by the complex kinetics of reconstitution of normal hematopoiesis into lethally irradiated mice. We used, therefore, functional assays to evaluate the existence of a preleukemic state. Whole bone marrow, or the lin− population from reconstituted mice (control, GFP only, and PML-RAR–expressing cells), were then analyzed for their capacity to undergo myeloid differentiation in vitro in semisolid medium. PML-RAR–expressing cells showed a strongly reduced capacity to differentiate in vitro, unless RA was added to the medium; similar results were obtained by analyzing cells derived from the MRP-8 and cathepsin G PML-RAR transgenic mice (Figure 7C and data not shown).
PML-RAR expression induces a preleukemic state.
(A) Differentiation marker analysis of bone marrow cells from recipient mice reconstituted with uninfected (Control), GFP-only (PINCO), and PML-RAR–expressing lin− cells (PML-RAR). Four mice per group were analyzed 90 days after reconstitution. In the case of PINCO, the data are presented for both GFP+ and GFP−cells. Peripheral blood and spleen cell analysis gave comparable results (data not shown). In addition to MAC1, we analyzed the following markers: GR1, CD34, SCA1, TER119, and CD3 with comparable results (data not shown). (B) Lin− cells were prepared from recipient mice reconstituted with PML-RAR–expressing cells, 90 days after lethal irradiation. For DNA-PCR analysis, primers specific for human PML-RAR cDNA were used to amplify DNA extracted from colonies derived from methylcellulose plating. Lane 1, negative control; lane 2, positive control. Lanes 3 to 8, 6 colonies from one representative PML-RAR reconstituted mouse, used in the analysis of differentiation markers shown in panel A. (C) In vitro differentiation analysis (MAC1) of pooled colonies derived as in panel B.
PML-RAR expression induces a preleukemic state.
(A) Differentiation marker analysis of bone marrow cells from recipient mice reconstituted with uninfected (Control), GFP-only (PINCO), and PML-RAR–expressing lin− cells (PML-RAR). Four mice per group were analyzed 90 days after reconstitution. In the case of PINCO, the data are presented for both GFP+ and GFP−cells. Peripheral blood and spleen cell analysis gave comparable results (data not shown). In addition to MAC1, we analyzed the following markers: GR1, CD34, SCA1, TER119, and CD3 with comparable results (data not shown). (B) Lin− cells were prepared from recipient mice reconstituted with PML-RAR–expressing cells, 90 days after lethal irradiation. For DNA-PCR analysis, primers specific for human PML-RAR cDNA were used to amplify DNA extracted from colonies derived from methylcellulose plating. Lane 1, negative control; lane 2, positive control. Lanes 3 to 8, 6 colonies from one representative PML-RAR reconstituted mouse, used in the analysis of differentiation markers shown in panel A. (C) In vitro differentiation analysis (MAC1) of pooled colonies derived as in panel B.
These results suggest that, as in the transgenic mice, a “preleukemic” state may be observed in our experimental system, and corresponds to an alteration in the differentiation potential induced by the fusion protein in hematopoietic progenitors.
Discussion
We have presented here a novel model system to study APL in mice, based on transplantation of transduced murine hematopoietic progenitors. This system fully recapitulates the most relevant features of human APLs, that is, a differentiation block at the promyelocyte stage and RA sensitivity. Leukemia is obtained by the expression of PML-RAR (but not RAR) in lin− cells. Previously, similar results have been obtained in chicken and mice model systems. In chicken, retroviral-mediated expression of a gag-PML-RAR fusion protein induced transformation of hematopoietic progenitors and caused leukemias. Point mutations harbored by the provirus, however, modified the intracellular localization of PML-RAR, resulting in a disease not resembling human APL, and that was not sensitive to RA treatment.5 In transgenic mice, PML-RAR was expressed under the control of myeloid-specific promoters (MRP-8 or cathepsin G).6-10 In those systems, RAR carrying a mutation in its ligand binding domain was shown to be nonleukemogenic; the results presented here, using wild-type RAR, further demonstrate that overexpression of RAR does not induce leukemias.10 In the previous studies, PML-RAR leukemias developed after long latency (6-18 months) and at low penetrance (10%-30%). Simultaneous expression of the product of the reciprocal translocation (RAR-PML) was shown to increase frequency (to about 50%), without affecting the latency of the disease.24 In our experiments, instead, induction of a leukemic phenotype was observed in more than 80% of the mice on expression of PML-RAR, with decreased latency (median time of about 4 months) compared to the transgenic models. This discrepancy is not due to different levels of PML-RAR because direct comparison of fusion protein expression revealed comparable levels among the different model systems (cathepsin G, MRP-8 transgenics, and ours; Figure 4B). The long latency and low frequency of leukemias in the transgenic mice suggested a “2-hit” model, which is consistent with the clonal origin of leukemias in our model system. Differences among the various model systems might, instead, be due to different targeting of the PML-RAR cDNA.13 Expression of PML-RAR in transgenic mice strictly depends on the promoter used, as shown by the failure of late myeloid-stage specific promoters (CD11b) to provoke leukemias.7 It appears, therefore, that PML-RAR acquires leukemogenic potential only if expressed at specific stages of hematopoietic development. It is then possible that expression of PML-RAR (by retroviral transduction) in the heterogeneous lin− population increases the frequency of proper PML-RAR targeting, as compared to developmental regulated promoters where the frequency of proper targeting might be lower (MRP-8 and cathepsin G), or close to zero (CD11b). Future experiments based on the transduction and reinoculation of subsets of hematopoietic mouse precursors should allow a better definition of the APL target cell.
Expression of PML-RAR is not sufficient to transform cells; in all model systems, additional genetic/epigenetic events are required, which are favored by PML-RAR expression.25 The fusion protein provokes a so-called “preleukemic” state, characterized, in vivo, by a mild impairment in myeloid differentiation. Lin−cells from preleukemic animals are impaired in their ability to undergo terminal differentiation in vitro, a phenotype that we also detected into freshly transduced, PML-RAR–expressing lin− cells, thereby offering the unique possibility to investigate this phenomenon (and possibly the subsequent steps leading to transformation) in vitro.
The possibility to study the same cell populations in vitro and in vivo is, indeed, one of the greatest advantages of our experimental system. In vitro, we have shown that PML-RAR blocks differentiation and extend survival of lin− cells; by structure-function analysis, it will be possible to dissect these 2 phenotypes, and validate immediately the results in vivo.
Finally, RA sensitivity of primary and secondary leukemias, as well as of the preleukemic state observed in vitro, shows that this system is also available for pharmacologic studies. Aberrant recruitment of histone deacetylases (HDACs) is considered critical for the leukemogenic potential of PML-RAR and other AML fusion proteins, and treatment with HDAC inhibitors is able to induce differentiation in vitro.26 Treatment of the leukemic mice with HDAC inhibitors, or other pharmacologically active compounds, may lead to new proposals for the therapy of APL, and possibly other types of cancers.
We thank Tim Ley, Silvia Soddu, Jeff Medin, Mirco Fanelli, and Francesco Bertolini for discussions and help with the set up of some experimental techniques. S. Monestiroli and S. Giavara contributed equally to this work.
Prepublished online as Blood First Edition Paper, June 14, 2002; DOI 10.1182/blood-2001-11-0089.
Supported by grants from European Community (EEC), Italian Foundation for Cancer Research (FIRC), Italian Association for Cancer Research (AIRC), and Ministero della Sanitá to S.M. and P.G.P.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Pier Giuseppe Pelicci, European Institute of Oncology, Department of Experimental Oncology, Via Ripamonti 435 Milan, 20141 Italy; e-mail: pgpelicci@ieo.it; and Saverio Minucci, European Institute of Oncology, Department of Experimental Oncology, Via Ripamonti 435 Milan, 20141 Italy; e-mail: sminucci@ieo.it.