Abstract
To establish a more appropriate animal recipient for xenotransplantation, NOD/SCID/γ mice double homozygous for the severe combined immunodeficiency (SCID) mutation and interleukin-2Rγ (IL-2Rγ) allelic mutation (γ) were generated by 8 backcross matings of C57BL/6J-γ mice and NOD/Shi-scidmice. When human CD34+ cells from umbilical cord blood were transplanted into this strain, the engraftment rate in the peripheral circulation, spleen, and bone marrow were significantly higher than that in NOD/Shi-scid mice treated with anti-asialo GM1 antibody or in the β2-microglobulin–deficient NOD/LtSz-scid (NOD/SCID/β2mnull) mice, which were as completely defective in NK cell activity as NOD/SCID/γ mice. The same high engraftment rate of human mature cells was observed in ascites when peripheral blood mononuclear cells were intraperitoneally transferred. In addition to the high engraftment rate, multilineage cell differentiation was also observed. Further, even 1 × 102 CD34+ cells could grow and differentiate in this strain. These results suggest that NOD/SCID/γ mice were superior animal recipients for xenotransplantation and were especially valuable for human stem cell assay. To elucidate the mechanisms involved in the superior engraftment rate in NOD/SCID/γ mice, cytokine production of spleen cells stimulated with Listeria monocytogenesantigens was compared among these 3 strains of mice. The interferon-γ production from dendritic cells from the NOD/SCID/γ mouse spleen was significantly suppressed in comparison with findings in 2 other strains of mice. It is suggested that multiple immunological dysfunctions, including cytokine production capability, in addition to functional incompetence of T, B, and NK cells, may lead to the high engraftment levels of xenograft in NOD/SCID/γ mice.
Introduction
Efforts to develop animal recipients for xenotransplantation, especially human cells, to establish an animal model for human diseases or to investigate mechanisms during the growth and differentiation of human stem cells have long been pursued. The epoch-making discovery of nude mice by Isaason and Cattanach in 19621—mice defective in the thymus with T-cell deficiency—has contributed to progress in this research. Consequently, C.B-17-Prkdcscid (scid) mice, which are defective in rearrangements of T-cell receptor (TCR) and B-cell receptor (BCR) resulting in defects of functional T and B cells, were discovered in 1983.2 After the successful engraftment of human peripheral hematopoietic cells, especially lymphoid cells,3,4 and the establishment of HIV-1 infection to T cells developed in these strains,5,6 many attempts have been made to develop modified severe combined immunodeficiency (SCID) mice by genetic crossings with inbred or other mutant strains of mice to obtain a more efficient model.7-10 As a result, NOD/LtSz-scid mice established by Grenier et al11 were found to be superior recipients for human cells. The high engraftment rates of human cells in NOD/LtSz-scid mice have been attributed to immunological multidysfunction, including reductions in macrophage function, complement-dependent hemolytic activity, and NK cell activity.12 We also developed NOD/Shi-scid mice independently from NOD/LtSz-scid mice and described the high engraftment rate of human hematopoietic cells in them.13,14 At present, the NOD/SCID mice have been considered an appropriate model for analysis of human stem cell development and function. However, in NOD/LtSz-scid and NOD/Shi-scid mice, the injection of anti-NK cell antibody before transplantation ameliorates engraftment efficiency for transplanted human cells, thus indicating that residual NK cell activity might interfere with engraftment efficiency.14,15To genetically eliminate NK cell activity, additional impairment of the gene responsible for NK cell development was introduced into NOD/LtSz-scid mice. The NOD/SCID/β2mnull mouse was developed, and it was lacking NK cell activity and higher engraftment of human cells.16,17 In the present study, we examined the newly developed NOD/SCID/γ mouse by backcrossing the γ mouse to the NOD/Shi-scid mouse and the superior engraftment capacity of transferred human cells. The γ mouse was found to lack NK cell activity.18 When comparing the efficiencies of engraftment levels in NOD/SCID/γmice, NOD/SCID/β2mnull mice, and NOD/Shi-scidmice injected with anti-NK cell antibody, NOD/SCID/γ mice were confirmed to be superior in engraftment of human hematopoietic cells. The immunologically detected severe reduction of interferon-γ (IFN-γ) production from dendritic cells in the NOD/SCID/γ mouse spleen and the comprehensive impairment of immunological functions may lead to efficient engraftment of human hematopoietic cells.
Materials and methods
Mice
Mice used in this study were C.B-17/Jic-scid, NOD/ShiJic-scid, NOD/LtSz-scid, NOD/SCID/β2mnull mice (NOD/LtSz-scid with deficient β2-microglobulin), CB57B/6J-γ mice, and newly developed NOD/SCID/γ(NOD/ShiJic-scid with γ) mice. C.B-17/Jic-scid mice were donated by Dr M. Bosma (Institute for Cancer Research, Philadelphia, PA) in 1985 and were maintained in our institute. NOD/ShiJic-scid mouse was established by 8 or more backcross matings of the C.B-17-scid mouse to the NOD/ShiJic mouse that were donated by Dr Makino (Shionogi Pharmaceutical) and were maintained in our institute. NOD/LtSz-scid mice were donated by Dr L. D. Shultz (Jackson Laboratory, Bar Harbor, ME). NOD/SCID/β2mnullmice were purchased from Jackson Laboratory. C57B/6J-γ mice were produced by 8 backcross matings of γ mice to C57B/6JJic and were maintained in our institute. NOD/ShiJic-scid with the γ mouse was developed as follows: Female NOD/Shi-scid mice were crossed with male C57BL/6J-γ mice in which the interleukin-2Rγ (IL-2Rγ) gene was X-chromosome–linked. F1 females were mated with NOD/Shi-scid males. Males obtained were backcrossed 7 times with NOD/Shi-scid mice. Mice obtained by 8 backcrossings were intercrossed to obtain mice homologous for the scid and γ genes. Mice were genetically typed for polymerase chain reaction (PCR) to detect wild-type and γ genes and immunodiffusion to detect serum immunoglobulin M (IgM) for confirming homozygosity for thescid gene. The mice obtained, homologous for both genes, were then maintained by sibling mating. All mice were maintained or mated in vinyl isolators under specific pathogen-free conditions. All materials including bedding, food (CL-2; Clea Japan, Tokyo), and tap water were autoclaved at 128°C for 30 minutes in a laminar firm-capped container and were moved to the vinyl isolators in a sterile manner. For cell transplantation experiments, mice were moved and maintained in micro-isolator boxes with laminar flow hoods in conventional animal SPF facilities of CIEA, Tohoku University School of Medicine and Kyoto University School of Medicine.
Genotyping of γ gene
Mice were PCR genotyped using 2 sets of primers to distinguish between the wild-type and mutant alleles, as described previously.18 DNA was extracted from mouse blood from the orbital vein, using nucleic acid purification kits (Genome, MagExtractor, MFX-2000; Toyobo, Osaka, Japan). To identify the wild-type allele, CTGCTCAGAATGCCTCCAATTCC was used for the 5′ primer, and GATCCAGATTGCCAAGGTGAGTAG was used for the 3′ primer. To identify the mutant allele, CTGCTCAGAATGCCTCCAATTCC was used for the 5′ primer, and CCTGCGTGCAATCCATCTTGTTCAAT was used for the 3′ primer. Both PCR reactions were carried out for 35 cycles (94°C, 1 minute; 55°C, 1 minute; 72°C, 1 minute) in a reaction buffer containing 1 mM MgCl2, 0.25 mM dNTP, and Taq polymerase (Life Technologies, Grand Island, NY). Multiplied fragments to detect the mutant and wild-type alleles were approximately 350 bp and 660 bp, respectively.
Genotyping of microsatellite loci
Forty-six microsatellite markers, one marker for each chromosome, were also analyzed to determine genetic backgrounds. PCR amplification of microsatellite loci was performed using reported methods.19 Amplified products were electrophoresed on a 3% to 4% agarose gel, and ethidium bromide was used to facilitate visualization.
NK cell activity
NK cell activity was determined according to methods described by Shultz et al,12 Mice were intraperitoneally inoculated with 100 μg polyinosinic-polycytidylic acid (poly I:C; Sigma Chemical, St Louis, MO) to stimulate NK cell activity for 48 hours before assay. Spleen cells were separated from 4 mice of each strain of mice, pooled and cocultured with chromium 51Cr- labeled YAC-1 cells as target cells for 4 hours at 37°C in 5% CO2 in 96 semi–V-bottom plates (BioTec, Tokyo, Japan) with various effector-target (E/T) cell ratios. Each sample was made in triplicate, and the supernatants harvested from each well were assayed on a gamma counter (ARC300; Aloka, Tokyo, Japan). The present specific51Cr release was calculated using the following formula, where X is the mean experimental release from triplicate wells. Total release (T) was determined from wells with51Cr-labeled YAC-1 cells and 1 H HCl, and spontaneous release (S) was determined from wells with51Cr-labeled YAC-1 cells and medium: Percent Specific Release = [(X − S)/(T − S)] × 100.
Complement-dependent hemolytic activity
Complement-dependent hemolytic activity in sera from mice was also assayed, as described previously.12 With the mice anesthetized, blood was collected from a right axillary vein. While blood was kept at room temperature for 1 hour, sera were collected by centrifugation at 2000 rpm for 10 minutes. Pooled sera from 4 to 5 mice of each strain were stored at −80°C until assay. Defibrinated sheep red blood cells (SRBCs) (Nippon Bio-Test Laboratories, Tokyo, Japan) were washed 3 times with RPMI 1640 by centrifugation at 1500 rpm for 5 minutes at 4°C. Five milliliters packed SRBCs was resuspended in RPMI 1640 and labeled with 200 μCi (7.4 MBq) 51Cr (ICN Biochemicals, Irvine, CA) by shaking for 2 hours at 37°C in 5% CO2. Labeled SRBCs were again washed with RPMI 1640, resuspended to 3% (vol/vol), and incubated with rabbit anti-SRBC polyclonal antiserum (1/30 dilution; ICN Pharmaceuticals) for 30 minutes on ice. The SRBC-antibody conjugates were washed twice in RPMI 1640, resuspended in RPMI 1640 at 2% vol/vol, and kept on ice until use. Pooled sera were thawed on ice and serially diluted 2-fold in RPMI 1640. One hundred microliters diluted sera was placed on a 96-well V-bottom plate. Immediately, 100 μL 51Cr SRBC-antibody conjugate suspension was added to the well, and the plate was incubated for 2 hours at 37°C in 5% CO2. After incubation, the contents were centrifuged, and supernatants were collected and counted in a gamma counter. The percentage of specific release was calculated using the formula described by Shultz et al.12 Spontaneous release (S) was determined from wells with 51Cr SRBC-antibody conjugate in media, and total release (T) was determined from wells with 51Cr SRBC-antibody conjugates and 100 μL 2% sodium dodecyl sulfate: Percent Specific Release = [(X × S)/(T × S)] × 100.
IL-1 production from bone marrow cells
An assay of IL-1 production from bone marrow cells stimulated with IFN-γ and lipopolysaccharide (LPS) was performed as described previously.12 Bone marrow cells collected from femurs of mice were cultured with 500 U/mL human recombinant macrophage–colony-stimulating factor (rM-CSF) (Sigma), with and without 10 U/mL rat rIFN-γ (Genzyme, Cambridge, MA), and were cultured for 4 days at 37°C in 5% CO2. After 4 days, the medium was replaced with fresh medium alone or with medium containing 10 μg/mL Escherichia coli LPS (Life Technologies, Grand Island, NY). After an additional 24-hour incubation period, the culture supernatants were harvested and assayed for IL-1α levels using enzyme-linked immunosorbent assay (ELISA) kits (Amersham Pharmacia Biotech United Kingdom, Buckinghamshire, England). The amount of IL-1α in the supernatants was expressed as absorbance at 405 nm.
Human cell engraftment
Umbilical cord blood (CB) cells were collected during normal full-term deliveries after obtaining informed consent. Mononuclear cells (MNCs) were separated by Ficoll-Hypaque density-gradient centrifugation after depletion of phagocytes with Silica (Immuno Biological Laboratories, Fujioka, Japan). CD34+ cells were isolated using Dynabead M-450 CD34 (Dynal AS, Oslo, Norway) as described previously.20 Briefly, MNCs separated from CB were suspended at 4 × 107 cells/mL in phosphate-buffered saline (PBS) containing 2% bovine serum albumin (BSA), 0.6% citrate, and 100 IU/mL penicillin and streptomycin. The MNC suspension was incubated at 4°C for 30 minutes with Dynabead M-450 CD34 with a bead-cell ratio of 1:1. Beads with attached cells were collected using a magnetic particle concentrator (MPC; Dynal) and were incubated with Detach-a-bead CD34 (Dynal) at 37°C for 15 minutes to release the cells, and these were collected by MPC. Purity was evaluated by flow cytometric analysis. Approximately 95% of the cells were CD34+. To deplete NK cells, NOD/Shi-scid mice were intraperitoneally given 400 μL PBS containing 20 μL anti-asialo GM1 antiserum (Wako, Osaka, Japan) shortly before the transplantation of CB CD34+ cells and every 11th day thereafter.15 All mice were irradiated with 2.4 Gy using a cobalt radiation source shortly before cell transfer. CD34+cells (1 × 105 or 4 × 104) were intravenously inoculated into mice. After transplantation, mice were given sterile water containing prophylactic neomycin sulfate (Gibco BRL). Human peripheral blood mononuclear cells (PBMNCs) were collected from a disease-free donor using density-gradient centrifugation. Then 1 × 107 PBMNCs were intraperitoneally inoculated into nonirradiated mice with and without treatment using anti-asialo GM1 antibody, as described above. Two weeks after inoculation, the cells in ascites were recovered and analyzed using flow cytometry.
Flow cytometry
To detect human cells in mice, multicolor cytometric analysis was performed using FACScalibur (Becton Dickinson [BD], Franklin Lakes, NJ), according to the manufacturer's protocol but with a minor modification.13 Peripheral blood (PB) was taken from the retro-orbital venous plexus at 4, 8, and 12 weeks for comparison of the engraftment rate between NOD/SCID/γ and NOD/Shi-scid mice treated with anti-asialo GM1 antibodies or at 4, 11, and 20 weeks between NOD/SCID/γ and NOD/SCID/β2mnull mice after the transplantation under ether anesthesia. Blood was collected through heparinized calibrated pipettes (Drummond Scientific, Broomall, PA) and transferred to EDTA (ethylenediaminetetraacetic acid) 2Na containing Capiject (Terumo Medical, Somerset, NJ). A complete blood count was obtained using Celltac α (Nihon Kohden, Tokyo, Japan). At 4 or 5 months after transplantation, the mice were killed and the femurs and spleens were removed. Bone marrow (BM) and spleen cells were collected and subjected to flow cytometry. Samples were mixed with rabbit IgG to block nonspecific staining and were incubated with an appropriate volume of indicated antibodies for 30 minutes on ice. The mixture was depleted of erythrocytes and was fixed in Lysing Solution (BD PharMingen, San Diego, CA). Human white blood cells (WBCs) were examined by double staining with fluorescein isothiocyanate (FITC)–conjugated antihuman CD45 antibody (BD PharMingen) and allophycocyanin (APC)-conjugated antimouse CD45 antibody (BD PharMingen). The percentage of human CD45+ cells was calculated as follows: Percent Human CD45+ Cells = No. Human CD45+ Cells/(No. Human CD45+ Cells + No. Mouse CD45+Cells) × 100. To detect mouse NK and dendritic cells in the spleen, 2-color cytometric analysis was also performed using a flow cytometer (Cytron Absolute, Ortho-Clinical Diagnostics, Raritan, NJ). Antibodies used were biotin-conjugated antimouse pan NK (clone DX5), biotin-conjugated antimouse CD11b, and FITC-conjugated antimouse CD11c from BD PharMingen.
CB CD34+ cell transplantation in a limiting dose
To evaluate the efficiency of NOD/SCID/γ mice as hosts for human stem cells, CB CD34+ cells were transplanted in a limiting dose. The indicated doses of CB CD34+ cells were transplanted to NOD/SCID/γ mice, and engraftment levels were examined at more than 3 months after transplantation. We used flow cytometric analysis to determine human hematopoietic cell engraftment. A protocol similar to that described above was used. Briefly, BM cells were incubated with rabbit IgG and stained with antihuman CD45-FITC, antimouse CD45-APC, and Viaprobe (BD PharMingen). Successful engraftment was defined by the presence of at least 100 human CD45+ and mouse CD45− cells in 1 × 105 Viaprobe-negative (live) cells. There were few nonspecific dots with this method. However, to eliminate possible overestimation caused by nonspecific staining, we did not count mice with fewer than 100 positive human cells as engrafted. Results from 2 independent experiments on a total of 14 mice were analyzed.
Antigen preparation of Listeria monocytogenes
The L monocytogenes EGD strain was provided by Dr M. Mitsuyama (Kyoto University) and was maintained as described previously.21 In brief, bacteria were passed twice through C57BL/6J mice to maintain the virulence. Single-colony isolation was performed after plating the homogenate of spleens of infected mice on Tripto-soy broth (Eiken, Tokyo, Japan) agar plates and incubating overnight at 37°C. Suspended bacteria were grown overnight at 37°C in liquid Tripto-soy broth (Eiken) with vigorous shaking, harvested, and washed 3 times with PBS. Aliquots of bacterial suspension in PBS were used. Heat-killed bacteria were prepared by heating at 74°C for 90 minutes.
In vitro culture of spleen cells with L monocytogenesantigen
Spleen cells were separated from 4 or more mice in each group, as described previously.22 One milliliter of 1 mg/mL Collagenase D solution (Roche Diagnostics GmbH, Mannheim, Germany) was injected into the spleen through a syringe using a 25-gauge needle. After incubation for 30 minutes at 37°C, the spleen was minced with a scissors, mixed well with a Pasteur pipette, and passed through a nylon mesh. CD11c+ cells were depleted from spleen cells from NOD/Shi-scid mice treated with anti-asialo GM1 antiserum using anti-CD11c antibody-labeled magnetic beads by a magnetic cell sorter (MACS; Miltenyi Biotec GmbH, Gladbach, Germany), according to the manufacturer's protocol. Two hundred microliters cell suspension (5 × 106/mL) in RPMI supplemented with 10% fetal bovine serum, 10 mM mercaptoethanol, and streptomycin and penicillin (Life Technologies) was cocultured with 107heat-killed L monocytogenes in 96-well plates for 8 hours at 37°C. After incubation, the supernatants were kept at −80°C until ELISA.
Cytokine detection by ELISA
IFN-γ and IL-6 in culture supernatants were determined using OptEIA ELISA kits (BD PharMingen). All assays were performed in accordance with protocols recommended by the manufacturer.
Statistical analysis
The Student t test was used to determine statistical significance, and P < .05 was considered significant.
Results
Development of NOD/SCID/γ mice
NOD/SCID/γ mice were established by 8 backcrossings of C57BL/6J-γ mice with NOD/Shi-scid mice. To confirm the genetic background of the NOD/SCID/γ mouse, 46 DNA microsatellite markers were examined. Results with 17 of 46 markers, which were different between NOD/Shi-scid and C57BL/6J mice, showed the genetic background of the NOD/Shi strain but not that of the C57BL/6J strain except for the region linked with the Il2rg gene on the X chromosome. Immunodeficiency from homozygosity for the scidmutation was also confirmed by immunodiffusion for serum IgM. PCR analysis of γ revealed that NOD/SCID/γ mice were homologous with γ mice (data not shown).
Similarity of multiple immunological impairments between NOD/Shi-scid and NOD/LtSz-scid mice
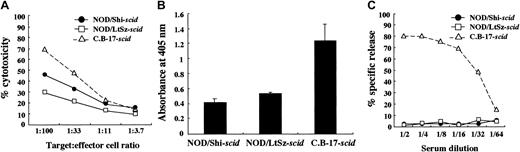
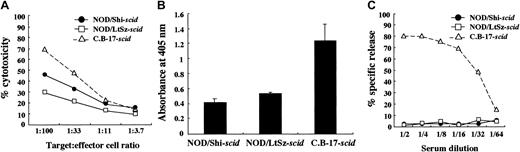
We used NOD/Shi-scid mice but not NOD/LtSz-scid mice for this study. Therefore, immunological evaluations were first made between these substrains of mice. For this purpose, we compared NK cell activity, IL-1 production after macrophage activation, and complement-dependent hemolytic activity. As shown in Figure 1A, NK cell activity was reduced in both substrains of mice compared with findings in CB-17-scid mice, though the activity in NOD/Shi-scid mice was slightly higher. IL-1 production by LPS-stimulated macrophages and complement-dependent hemolytic activity were also severely impaired in both substrains of mice (Figure 1B-C), suggesting that impairments of these immunological functions might be similar in both NOD strains of mice.
Comparison of immunologically multifunctional defects among C.B-17-
scid, NOD/Shi-scid, and NOD/LtSz-scid mice. (A) NK cell activity of poly I:C–stimulated spleen cells from 3 strains of mice. (B) IL-1 production of IFN-γ–stimulated bone marrow cells from 3 strains of mice. (C) Complement-dependent hemolytic activity in the sera from 3 strains of mice.
Comparison of immunologically multifunctional defects among C.B-17-
scid, NOD/Shi-scid, and NOD/LtSz-scid mice. (A) NK cell activity of poly I:C–stimulated spleen cells from 3 strains of mice. (B) IL-1 production of IFN-γ–stimulated bone marrow cells from 3 strains of mice. (C) Complement-dependent hemolytic activity in the sera from 3 strains of mice.
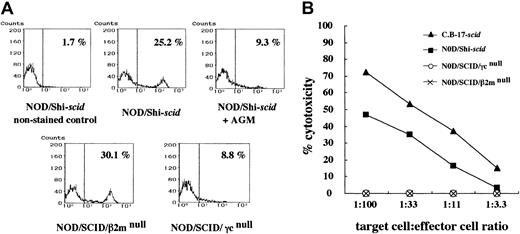
No NK cells and NK activities in NOD/SCID/γmice
Flow cytometric analyses confirmed the presence of NK cells in NOD/Shi-scid mice (25.2%) and NOD/SCID/β2mnull mice (30.1%), albeit at low levels in NOD/SCID/γ mice (8.8%) and NOD/Shi-scid mice treated with anti-asialo GM1 antibody (9.3%) (Figure 2A). Interestingly, NK cell activity itself was hardly detectable in NOD/SCID/γ and NOD/SCID/β2mnullmice in functional examinations stimulated by poly I:C (Figure 2B). Therefore, both strains of NOD/SCID/γ and NOD/SCID/β2mnull mice lacked NK cell activity.
NK cells and NK cell activity in NOD/Shi-
scid mice with or without anti-asialo GM1 antibody, NOD/SCID/γ, and NOD/SCID/β2mnull mice. (A) Spleen cells from mice were stained with streptavidin-FITC and biotin-labeled anti-pan NK cell antibody. No NK cells were observed in spleen cells from NOD/SCID/γ mice or from NOD/Shi-scidmice treated with anti-asialo GM1 antibody. (B) Spleen cells from mice treated with poly I:C 2 days before the assay were used. The formula for percentage cytotoxicity is described in “Materials and methods.”
NK cells and NK cell activity in NOD/Shi-
scid mice with or without anti-asialo GM1 antibody, NOD/SCID/γ, and NOD/SCID/β2mnull mice. (A) Spleen cells from mice were stained with streptavidin-FITC and biotin-labeled anti-pan NK cell antibody. No NK cells were observed in spleen cells from NOD/SCID/γ mice or from NOD/Shi-scidmice treated with anti-asialo GM1 antibody. (B) Spleen cells from mice treated with poly I:C 2 days before the assay were used. The formula for percentage cytotoxicity is described in “Materials and methods.”
Significantly high level of human cell reconstitution in NOD/SCID/γ mice
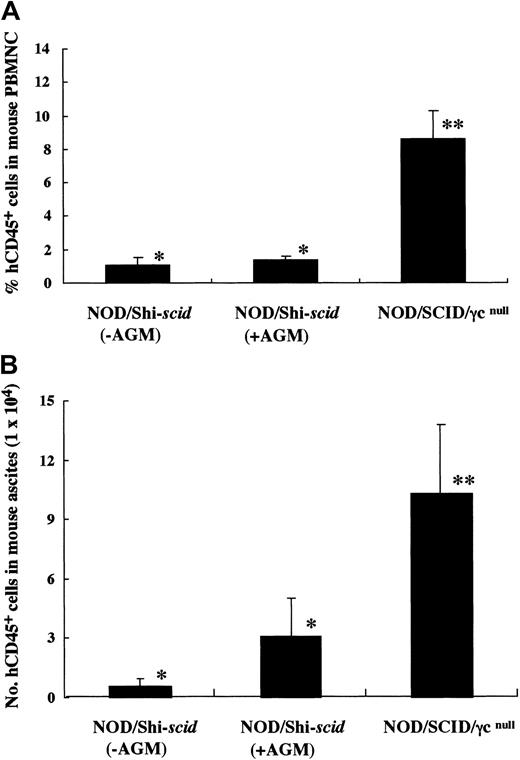
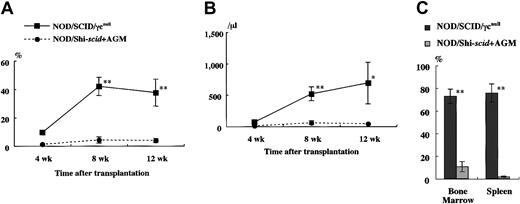
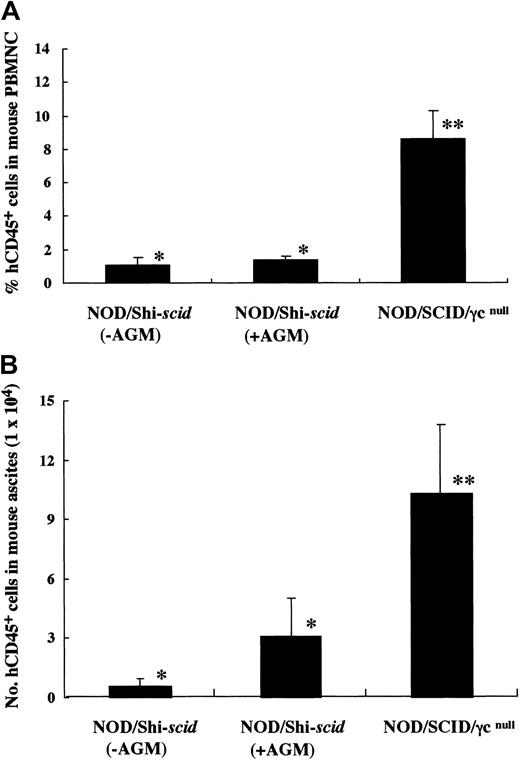
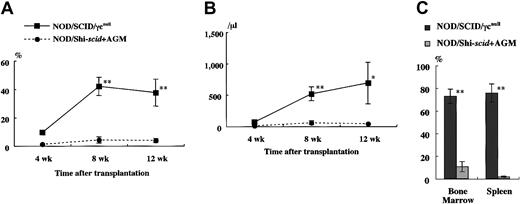
It has been reported that residual NK activity might hamper the engraftment level of xenogenic grafts, thereby resulting in decreased chimeric rates of human hematopoietic cells. In fact, the chimeric percentages of human CD45+ cells in peripheral WBCs from NOD/Shi-scid and NOD/LtSz-scid mice with anti-NK cell antibody treatment were 15.7% to 19.1% and 1.9% to 25.3%, respectively (data not shown). In contrast, only 0% to 2% and 0.3% to 7.1% of peripheral WBCs were human CD45+ cells in NOD/Shi-scid and NOD/LtSz-scid mice not given the treatment. No NK cell activity was observed in either NOD/SCID/γ mice or NOD/SCID/β2mnull mice, respectively. It is suggested that the NK-depleted NOD/SCID/γ mouse is a good recipient for human cell transplantation. To evaluate the growth potential of human cells in NOD/SCID/γ mice, human CB CD34+ cells (1 × 105 intravenously) and PBMNCs (1 × 107 intravenously) were transferred into NOD/SCID/γ or NOD/Shi-scid mice with or without anti-asialo–GM1 antibody (Figure3A-B). In both cases, significantly more growth of human cells was observed in NOD/SCID/γmice than in NOD/Shi-scid mice with or without antibody treatment. In the transplantation of PBMNCs, high growth of human cells was observed in spleen and peripheral blood as well as in ascites of NOD/SCID/γ mice (data not shown). When human CB CD34+ cells (1 × 105 intravenously) were transplanted into NOD/SCID/γ or NOD/Shi-scid mice with anti-asialo–GM1 antibody, a high engraftment rate was also observed in NOD/SCID/γmice. Figure 4A-B shows the chimeric rates and absolute numbers of human CD45+ cells in the peripheral blood of mice at 4, 8, and 12 weeks after cell transplantation. Approximately 4 months after transplantation, BM and spleen cells in both groups of mice were also examined (Figure 4C). At every time point, significantly more growth of human cells was observed in NOD/SCID/γ mice than in NOD/Shi-scid mice with antibody treatment (*P < .05; **P < .01). These results imply that unidentified factors other than NK activity, profitable for the engraftment of human cells, are present in NOD/SCID/γ mice.
High-engraftment efficiency of human cells in NOD/SCID/γ mice.
(A) Rates of human cells in peripheral blood from NOD/SCID/γ and NOD/Shi-scid mice with or without anti-asialo GM1 antibody 4 weeks after intravenous transfer of human CB CD34+ cells are shown. Some NOD/Shi-scid mice were treated with anti-asialo GM1 antibody immediately before cell transplantation. Percentage human CD45+ cells in mouse peripheral blood was assayed by flow cytometry. AGM indicates anti-asialo GM1 antibody. Asterisks indicate significant difference (P < .01) between data columns marked * and **. (B) Numbers of human cells in ascites from NOD/SCID/γ and NOD/Shi-scid mice with or without anti-asialo GM1 antibody 2 weeks after intraperitoneal transplantation of human PBMNCs are shown. Numbers of human CD45+ cells in ascites were calculated after flow cytometry. Antibody treatment was performed 1 day before cell transplantation in the same manner as CB CD34+ cell transplantation. Asterisks indicate significant difference (P < .01) between data columns marked * and **.
High-engraftment efficiency of human cells in NOD/SCID/γ mice.
(A) Rates of human cells in peripheral blood from NOD/SCID/γ and NOD/Shi-scid mice with or without anti-asialo GM1 antibody 4 weeks after intravenous transfer of human CB CD34+ cells are shown. Some NOD/Shi-scid mice were treated with anti-asialo GM1 antibody immediately before cell transplantation. Percentage human CD45+ cells in mouse peripheral blood was assayed by flow cytometry. AGM indicates anti-asialo GM1 antibody. Asterisks indicate significant difference (P < .01) between data columns marked * and **. (B) Numbers of human cells in ascites from NOD/SCID/γ and NOD/Shi-scid mice with or without anti-asialo GM1 antibody 2 weeks after intraperitoneal transplantation of human PBMNCs are shown. Numbers of human CD45+ cells in ascites were calculated after flow cytometry. Antibody treatment was performed 1 day before cell transplantation in the same manner as CB CD34+ cell transplantation. Asterisks indicate significant difference (P < .01) between data columns marked * and **.
High-engraftment efficiency of CB CD34+ cells in NOD/SCID/γ mice.
The rate of human CD45+ cells was examined sequentially at the indicated times after transplantation of 1 × 105 CB CD34+ cells into NOD/SCID/γ and NOD/Shi-scid mice (n = 8 each; total of 4 independent experiments). Percentage (A) and absolute number (B) of human CD45+ cells are shown. (C) Percentage human CD45+ cells in the BM and spleen of each mouse at approximately 4 months after transplantation. AGM indicates anti-asialo GM1 antibody. * P < .05;** P < .01.
High-engraftment efficiency of CB CD34+ cells in NOD/SCID/γ mice.
The rate of human CD45+ cells was examined sequentially at the indicated times after transplantation of 1 × 105 CB CD34+ cells into NOD/SCID/γ and NOD/Shi-scid mice (n = 8 each; total of 4 independent experiments). Percentage (A) and absolute number (B) of human CD45+ cells are shown. (C) Percentage human CD45+ cells in the BM and spleen of each mouse at approximately 4 months after transplantation. AGM indicates anti-asialo GM1 antibody. * P < .05;** P < .01.
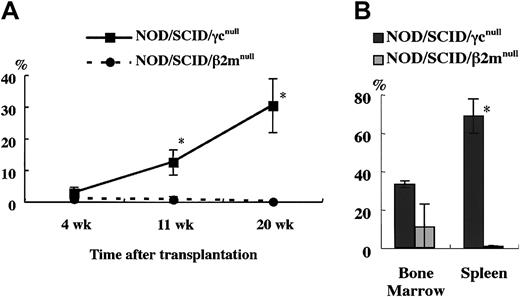
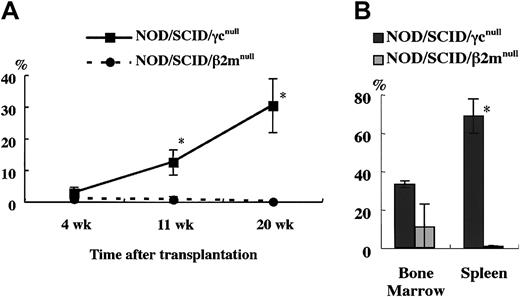
To confirm this, we compared the engraftment efficiency of NOD/SCID/γ mice and NOD/SCID/β2mnull mice, which were equally defective in NK activity as shown in Figure 2. At 4, 11, and 20 weeks after the transplantation of 4 × 104 human CB CD34+cells, significant differences in the engraftment rates of human cells were evident between these 2 strains (Figure5A). Examination of BM and spleen cells at 5 months after transplantation revealed significantly higher engraftment levels in NOD/SCID/γ mice than in NOD/SCID/β2mnull mice (Figure 5B). To evaluate the engraftment efficiency of NOD/SCID/γ mice that underwent transplantation with a low dose of CB CD34+cells, a limited dose of CB CD34+ cells was transplanted (Table 1). Surprisingly, all recipient mice of transplanted 1 × 103 CB CD34+ cells showed successful engraftment (more than 0.1%). Further, as few as 100 cells could be engrafted in 2 of 6 mice with multilineage differentiation (Figure 6). These results suggested that NOD/SCID/γ mice provide a suitable environment for settlement and proliferation of human hematopoietic cells.
Comparison of engraftment levels of human cells in NOD/SCID/γ and NOD/SCID/β2mnullmice.
(A) At the indicated times after 4 × 104CD34+ cell transplantation, human CD45+ cells in mouse peripheral blood were assayed by flow cytometry. (B) Percentage of human CD45+ cells in the BM and spleen in each mouse 5 months after transplantation was also examined (n = 3 each; total of 2 independent experiments). *P < .05.
Comparison of engraftment levels of human cells in NOD/SCID/γ and NOD/SCID/β2mnullmice.
(A) At the indicated times after 4 × 104CD34+ cell transplantation, human CD45+ cells in mouse peripheral blood were assayed by flow cytometry. (B) Percentage of human CD45+ cells in the BM and spleen in each mouse 5 months after transplantation was also examined (n = 3 each; total of 2 independent experiments). *P < .05.
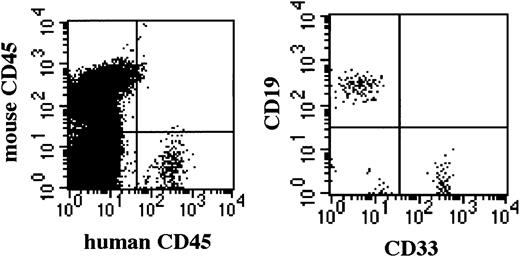
Successful engraftment by 1 × 102CD34+ cells in NOD/SCID/γcnull mice.
Successfully engrafted BM of NOD/SCID/γcnull mice shows multilineage human hematopoietic cells 5 months after transplantation.
Successful engraftment by 1 × 102CD34+ cells in NOD/SCID/γcnull mice.
Successfully engrafted BM of NOD/SCID/γcnull mice shows multilineage human hematopoietic cells 5 months after transplantation.
Multilineage differentiation of transferred CD34+cells in NOD/SCID/γ mice
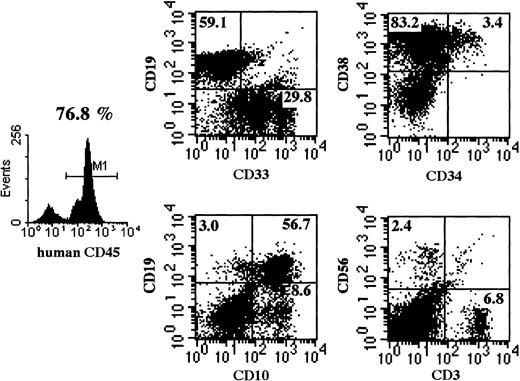
Multilineage differentiation of transferred CD34+cells was examined in the bone marrow of NOD/SCID/γ mice 4 months after transplantation. As shown in Figure 7, multilineage cells were observed in human CD45+ cells from bone marrow. CD19+ and CD33+ cells reached 59.1% and 29.8%, respectively. Surprisingly, CD3+ T cells were also observed. Multilineage differentiation including T cells was identified in all 8 mice that underwent transplantation with CB CD34+cells. These findings revealed that the differentiation of human CD34+ cells was well supported in NOD/SCID/γ mice.
Representative flow cytometric analysis of BM of mice that underwent transplantation.
Four months after CD34+ cell transplantation, BM cells were subjected to flow cytometry. NOD/SCID/γ mice show a significantly higher percentage of CD45+cells and of multilineage cells, including CD3+ T cells. Emergence of T cells was observed in all NOD/SCID/γ mice.
Representative flow cytometric analysis of BM of mice that underwent transplantation.
Four months after CD34+ cell transplantation, BM cells were subjected to flow cytometry. NOD/SCID/γ mice show a significantly higher percentage of CD45+cells and of multilineage cells, including CD3+ T cells. Emergence of T cells was observed in all NOD/SCID/γ mice.
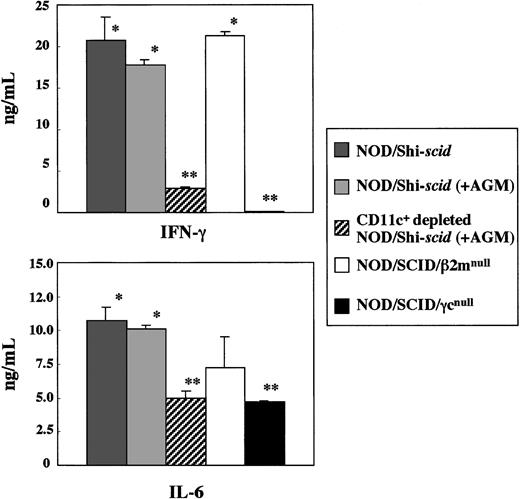
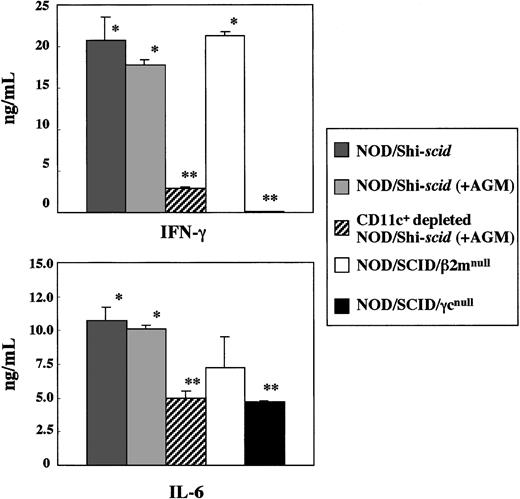
Impairment of cytokine production by spleen cells from NOD/SCID/γ mice
In vitro production of cytokines, IFN-γ, and IL-6 from spleen cells of 3 strains of NOD/Shi-scid, NOD/SCID/β2mnull, and NOD/SCID/γmice was investigated in the presence of the L monocytogenesantigen, which induces Th1-type cytokine production including IFN-γ (Figure 8). In NOD/SCID/γ mice, IFN-γ production was hardly detectable, whereas production in NOD/Shi-scid and NOD/SCID/β2mnull mice was evident. Administration of an anti-asialo GM1 antibody into NOD/Shi-scid mouse did not inhibit IFN-γ production. The amounts of IL-6 produced by spleen cells of NOD/SCID/γ mice were also significantly reduced compared with findings in NOD/Shi-scid mice, NOD/Shi-scid mice given antibody treatment, and NOD/SCID/β2mnull mice. Additional immunological dysfunction including cytokine production probably exists in NOD/SCID/γ mice.
In vitro cytokine production of
L monocytogenes–stimulated spleen cells from 3 strains of mice. Asterisks indicate a significant difference (* vs **: P < .01).
In vitro cytokine production of
L monocytogenes–stimulated spleen cells from 3 strains of mice. Asterisks indicate a significant difference (* vs **: P < .01).
To investigate the diminished production of cytokines in the NOD/SCID/γ mice, CD11c+-depleted spleen cells from antibody-treated NOD/Shi-scid mice were cocultured with L monocytogenes antigen. This depletion of CD11c+ dendritic cells markedly reduced the production of IFN-γ (data not shown), which suggests that the major source of IFN-γ production is CD11c+ dendritic cells. Paradoxically, flow cytometric analysis showed that the number of CD11c+ dendritic cells itself was detected in NOD/SCID/γ mice, which indicates functional impairment of CD11c+ dendritic cells in NOD/SCID/γ mice. These results imply the existence of additional immunological dysfunctions in the NOD/SCID/γ mice.
Discussion
Mice with SCID mutations have a high potential to grow and differentiate human cells after transplantation.3,4Various immunodeficient mice were developed by introducing thescid mutant gene to inbred strains7,10 or by combining it with other mutant genes.8,9 Among them, NOD/LtSz-scid mice, developed by Grenier et al11 and Shultz et al,12 were found to have superior potential for engraftment and differentiation of human hematopoietic cells, and these mice are suitable recipients for transplantation experiments using human hematopoietic cells.23,24 For further improvement, NOD/SCID/β2mnull (β2-microglobulin–deficient NOD/LtSz-scid) mice or NOD/LtSz-scidEmv30null mice16,25 were generated. We also developed NOD/Shi-scid mice independent of NOD/LtSz-scid mice and found them to have high levels of engraftment.13,14 We further prepared the newly developed NOD/SCID/γ mice, produced by backcrossing C57BL/6J-γ mice to NOD/Shi-scid mice, and the extremely high engraftment levels of human cells were confirmed in this strain. The NOD mouse was originally established in 1980 by Makino et al,26 and it is a good model to study diabetes in nonobese patients. NOD mice are now available worldwide, and this has led to a variety of NOD substrains. Shultz et al12 reported that the high engraftment of human cells in NOD/LtSz-scid mice is caused by reduced NK cell activity, macrophage function, and complement-dependent hemolytic activity originated from NOD/Lt mice. NOD/Shi with different genetic backgrounds used for the generation of NOD/Shi-scid mouse is an original strain maintained by Dr Makino.26 We first examined whether NOD/Shi-scid mice also have immunologically functional impairments similar to those of NOD/LtSz-scidmice. Our findings suggest that NOD/Shi-scid mice have the same multiple defects in immunological functions as NOD/LtSz-scid mice, and these defects may allow for the high engraftment of human cells. In NOD/Shi-scid and NOD/LtSz-scid mice, treatment with anti-NK cell antibodies, such as anti-asialo GM1 and anti–IL-2Rβ (TMβ-1) antibodies, enhances the engraftment rates of transplanted human cells,13,14 which means that NK cell activity has a crucial role in the rejection of xenotransplanted cells. Recently, Christianson et al16 developed NOD/SCID/β2mnull mice defective in NK cell activity. We developed NOD/SCID/γ mice by backcrossing C57BL/J-γ and NOD/Shi-scid mice because γ mice were completely defective in NK cell activity.18 When human PBMNCs and CD34+cells derived from human CB were transplanted into NOD/SCID/γ mice, we found a remarkably high engraftment rate compared with that in NOD/Shi-scid mice in the absence or presence of anti-NK cell antibody. Surprisingly, the engraftment level was significantly higher in NOD/SCID/γ mice than in NOD/SCID/β2mnull mice, though NK cell activity was lacking in these 2 mice.16 The lower engraftment rate in NOD/SCID/β2mnull observed here, compared with that reported by Kollet et al17 may be attributed to our low irradiation amount to mice, the number of transferred cells, or the difference of donors used for the resource of CD34+ cells. These results suggest that the higher engraftment of human cells in NOD/SCID/γ mice might be caused by factors other than defects in T and B cells, macrophage dysfunction, decreased NK cell activity, and absence of complement activity. In addition to the high engraftment rate of human cells, defects in undefined factors in NOD/SCID/γ mice also caused the multilineage differentiation of human repopulating cells in bone marrow. Furthermore, the findings of Dr K. Ando (Tokai University, Japan; personal communication) that CD34+ cells expressing green fluorescence protein (GFP) by lentivirus have been observed even in NOD/SCID/γ mice to undergo the third serial transplantation strongly suggest that these mice would have extremely high potential for xenotransplantation, especially for human stem cells. Another characteristic observation of great importance in NOD/SCID/γ mice is the emergence of CD3+ T cells. All NOD/SCID/γ mice that underwent transplantation with CB CD34+ cells developed CD3+ T cells in the BM and spleen when examined 4 months after transplantation. Although further studies are needed to reach a conclusion, this observation suggests the generation of CD3+ T cells from human CB CD34+ cells in a murine environment. Specific studies are under way to examine human T cells in these mice.
Ohteki et al27 reported that spleen cells from C57BL/6-Rag2null/γ mice, which lack NK cells, could not produce IFN-γ when stimulated with L monocytogenes antigens in vitro, though spleen cells from C57BL/6-Rag2null mice could produce it. However, the elimination of NK cells from spleen cells in C57BL/J-Rag2null mice by anti-asialo GM1 antibody did not decrease the production of IFN-γ. In the present study, spleen cells from NOD/SCID/γ mice also failed to produce IFN-γ by the stimulation with L monocytogenesantigens, though the cells from NOD/Shi-scid and NOD/SCID/β2mnull mice could produce a large amount of IFN-γ. Elimination of NK cells in NOD/Shi-scid mice by anti-asialo GM1 antibody did not affect IFN-γ production. Although both NOD/SCID/γ and NOD/SCID/β2mnull mice lacked NK cell activity, only NOD/SCID/γ mice had a defect in IFN-γ production. These results imply that NK cells are not a major source for IFN-γ and that defective IFN-γ production in NOD/SCID/γ mice might not reflect the lack of NK cells derived from γc mutation.
The depletion of CD11c+ dendritic cells from spleen cells of NOD/Shi-scid mice treated with anti-asialo GM1 antibody markedly reduced the production of IFN-γ, suggesting that the major source of IFN-γ production in these mice was CD11c+dendritic cells. Given that flow cytometric analysis showed the presence of CD11c+ dendritic cells in NOD/SCID/γ mice, functional impairment of CD11c+ dendritic cells may exist in NOD/SCID/γ mice. Taken together with the findings of C57BL/6-Rag2null/γmice,27 it has been suggested that CD11c+dendritic cells in NOD/SCID/γ mice became functionally defective as a result of γc mutation, resulting in the reduction of IFN-γ production. However, direct evidence that supports the involvement of impaired function of dendritic cells in NOD/SCID/γ mice for improved engraftment of human cells remains to be elucidated. The high engraftment rate of human cells in Rag2null/γ mice has been reported by Goldman et al.28 Dendritic cells are known to be a unique antigen-presenting cell to activate naive T cells.29Because T, B, and NK cells are defective in NOD/SCID/γ mice, dendritic cells may play a role in attacking xenografts directly or indirectly through macrophages. In this sense, NOD/SCID/γ mice may be an appropriate in vivo model to explain the role of dendritic cells in xenotransplantation tolerance.
In summary, newly developed NOD/SCID/γ mice showed extremely high engraftment rates using human hematopoietic cells. The reason for the high engraftment rates might be attributed to multiple immunological functional defects, including dendritic cells in addition to the absence of T, B, and NK cells. This mouse model may become an extremely useful tool for the analysis of differentiation and the growth of human hematopoietic stem cells in vivo and may pave the way for clarification of the immunological mechanisms concerning tolerance for xenotransplantation.
We thank Dr K. Ando (Tokai University, Isehara) and Dr M. Nakamura (Tokyo Medical and Dental University) for helpful discussions. We also thank Mrs Natsuko Eguchi and Michi Ebukuro (CIEA) for technical assistance.
Prepublished online as Blood First Edition Paper, July 5, 2002; DOI 10.1182/blood-2001-12-0207.
Supported in part by the Program for the Promotion of Fundamental Studies in Health Science, Organization for Pharmaceutical Safety and Research of Japan, and by Grant-in-Aid for Creative Scientific Research (13GS0009) and for Scientific Research (B) (13558100) from the Ministry of Education, Culture, Sports, Science and Technology of Japan.
M.I. and H.H. contributed equally to this work.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Mamoru Ito, Central Institute for Experimental Animals, 1430 Nogawa, Miyamae, Kawasaki, Kanagawa, 216-0001, Japan; e-mail: mito@ciea.or.jp.